Case-Control Study
Yu-Mei Hsueh, PhD,
1Chi-Jung Chung, MSc,
2Horng-Sheng Shiue, MD,
3Jin-Bor Chen, MD,
4Shou-Shan Chiang, MD,
5Mo-Hsiung Yang, PhD,
6Cheng-Wei Tai, MSc,
2and Chien-Tien Su, MD
7Background: Inorganic arsenic has been linked to decreased kidney function through oxidative
damage. Arsenic methylation is believed to be a pathway for arsenic metabolism. Lycopene is an antioxidant that reduces oxidative stress; however, the association between urinary arsenic species, plasma lycopene level, and chronic kidney disease (CKD) has seldom been evaluated.
Study Design: Case-control study.
Setting & Participants: 125 patients with CKD and 229 controls were recruited from a hospital-based
pool.
Predictor: Urinary arsenic species and plasma lycopene level.
Outcomes & Measurements: CKD was defined as estimated glomerular filtration rate (eGFR) less
than 60 mL/min/1.73 m2, calculated by using the Modification of Diet in Renal Disease Study equation. Plasma lycopene was measured by means of high-performance liquid chromatography. Urinary arsenic species, including arsenite, arsenate, monomethylarsonic acid, and dimethylarsinic acid, were deter-mined by means of high-performance liquid chromatography and hydride generator–atomic absorption spectrometry.
Results: Lycopene level was associated positively with eGFR, and participants with a high serum
lycopene level had a significant, inverse association with CKD (odds ratio, 0.41; 95% confidence interval, 0.21 to 0.81). Total arsenic level was associated significantly with CKD in a dose-response relationship, especially in participants with a total arsenic level greater than 20.74 compared with 11.78 g/g creatinine or less (odds ratio, 4.34; 95% confidence interval, 1.94 to 9.69). Furthermore, participants with a high urinary total arsenic level or participants with a low percentage of dimethylarsinic acid had a positive association with CKD when their plasma lycopene level was low.
Limitations: Because of the single spot evaluation of plasma antioxidants and urinary arsenic
species and the small sample size, statistical significance should be interpreted with caution.
Conclusions: This study shows that high urinary total arsenic or low plasma lycopene level is
associated positively with CKD. Results suggest that the capacity for arsenic methylation may be associated with CKD in individuals who ingest low arsenic levels in drinking water and also have a low plasma lycopene level.
Am J Kidney Dis 54:859-870. © 2009 by the National Kidney Foundation, Inc.
INDEX WORDS: Arsenic; arsenic methylation capacity; lycopene; chronic kidney disease.
C
hronic kidney disease (CKD) now is
recog-nized as a common condition that
in-creases the risk of cardiovascular disease.
1The
national prevalence of CKD in Taiwanese
pa-tients with an estimated glomerular filtration rate
(eGFR) less than 60 mL/min/1.73 m
2is 11.93%,
but only 3.54% of participants are aware of their
disorder.
2CKD is an important public issue
because Taiwan ranks first in the world in the
incidence of end-stage renal disease.
3Epidemio-logical and clinical evidence have shown a link
between hypertension, diabetes, obesity, and
met-abolic syndrome and the onset and progression
of CKD.
4,5The metalloid arsenic is a naturally occurring
element in soil, food, and water. Humans are
exposed to inorganic arsenic from mining and
smelting metal ores, pesticide manufacturing,
From the1
Department of Public Health, School of Medi-cine; 2
School of Public Health; 3
Department of Chinese Medicine, Chang Gung Memorial Hospital, and Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei;4
Nephrology Division, Chang Gung Memorial Hospital-Kaohsiung Medical Center, Chang Gung University College of Medicine, Kaohsiung;5
Depart-ment of Internal Medicine/Nephrology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei; 6
Department of Nuclear Sci-ence, National Tsing-Hua University, Hsinchu; and7
Depart-ment of Family Medicine, Taipei Medical University
Hospi-tal, Taipei, Taiwan.
Received December 17, 2008. Accepted in revised form June 5, 2009. Originally published online asdoi: 10.1053/ j.ajkd.2009.06.016on August 17, 2009.
Address correspondence to Yu-Mei Hsueh, PhD, Depart-ment of Public Health, School of Medicine, Taipei Medical University, No. 250 Wu-Hsing St, Taipei 110, Taiwan. E-mail:
ymhsueh@tmu.edu.tw
© 2009 by the National Kidney Foundation, Inc.
0272-6386/09/5405-0011$36.00/0 doi:10.1053/j.ajkd.2009.06.016
wood preservatives, and medicines. Food may
contain both organic and inorganic arsenic,
whereas drinking water contains primarily
inor-ganic arsenic. Long-term exposure to inorinor-ganic
arsenic has been related to risk of cancer in the
skin, bladder, liver, kidney, and lung.
6Histori-cally, the arsenic concentration permitted in
pub-lic water supplies in Taiwan was 50
g/L.
How-ever, in 2000, a new standard of 10
g/L was
announced. Our recent study showed that
indi-viduals with an unfavorable urinary arsenic
pro-file had increased risk of urothelial carcinoma,
even at low levels of exposure.
7We do not know
whether a urinary arsenic profile within a low
allowable range affects the risk of CKD.
In comparison to other metals, such as lead
and cadmium, studies of arsenic-induced
nephro-toxicity are rare. However, a report is available
for arsenic-induced kidney damage.
8A recent
study from Michigan also has shown an
in-creased rate of kidney disease in people exposed
to arsenic-contaminated drinking water.
9The
mechanisms underlying arsenic-induced kidney
toxicity are complex.
Absorbed arsenic undergoes complicated
biomethylation to form monomethylarsonic
acid (MMA
V[the superscript indicates an
oxi-dation number of 5 for arsenic]) and
dimethyl-arsinic acid (DMA
V), which are excreted by
the kidneys into urine.
10The presumed arsenic
methylation pathway in the human body is
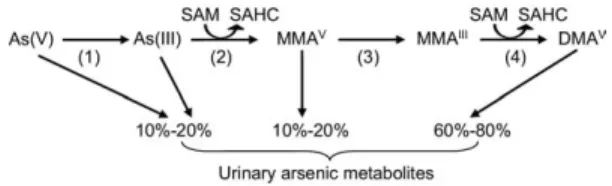
shown in
Fig 1
.
11-15A previously published
report suggests that excessive generation of
reactive oxygen species (ROS) by various
met-als may cause kidney damage.
16Because
ar-senic also generates ROS during the metabolic
activation process,
17whether arsenic
metabo-lites are part of the mechanism for
arsenic-induced nephrotoxicity remains to be
deter-mined.
Lycopene is a potent carotenoid antioxidant.
Lycopene most likely is involved in the
scaveng-ing ROS that contribute to defense against lipid
peroxidation.
18A recent study has shown that
lycopene is able to protect against mercuric
chlo-ride–induced nephrotoxicity in rats,
19as well as
cisplastin-induced decreased kidney function and
oxidative stress in rats.
20Low plasma lycopene
levels and arsenic exposure may be a risk factor
for CKD. Therefore, the primary goal of the
present study is to examine the association
be-tween the capacity for arsenic methylation,
lyco-pene level, and CKD and the interaction between
the capacity for arsenic methylation and
lyco-pene level in affecting CKD.
METHODS
Study Participants, Interview
Process, and Measurements
On a weekly basis from September 2005 and December 2007, patients (age range, 22 to 88 years) with clinical evidence of CKD based on urine sample collection were recruited from the Department of Internal Medicine/Nephrol-ogy of Shin Kong Wu Ho-Su Memorial Hospital in Taipei, Taiwan, resulting in 125 participants. eGFR traditionally is considered the best overall index of kidney function in health and disease. We used the 4-variable equation from the Modification of Diet in Renal Disease (MDRD) Study1to
estimate eGFR as 186.3 ⫻ (serum creatinine)⫺1.154 ⫻ (age)⫺0.203⫻ (0.742 for female) and defined the 5 stages of CKD according to the relevant Kidney Disease Outcomes Quality Initiative guidelines from the National Kidney Foun-dation. In this study, participants who were in stages 3 to 5 (eGFR⬍ 60 mL/min/1.73 m2) for 3 months were defined as
having CKD. Age frequency–matched control participants with no evidence of CKD (eGFRⱖ 60 mL/min/1.73 m2) in a
2:1 ratio of controls to cases were accrued weekly from a hospital-based pool, including those receiving senior citizen health examinations at Taipei Medical University Hospital and those receiving adult health examinations at Taipei Municipal Wan Fang Hospital. A total of 229 control partici-pants was obtained, and a urine sample was collected from each.
Well-trained personnel carried out standardized personal interviews based on a structured questionnaire. The informa-tion collected included demographic and socioeconomic characteristics and potential risk factors for CKD, such as lifestyle, alcohol consumption, cigarette smoking, exposure to potential occupational and environmental carcinogens (hair dyes and pesticides), medication history, consumption of conventional and alternative medicines, and personal and family histories of hypertension, diabetes, and CKD.
Figure 1. The presumed arsenic methylation pathway in the human body. The numbered steps are catalyzed by the following enzymes: (1) arsenate reductase or purine nucleoside phosphorylase (PNP), (2) arsenite methyl trans-ferase (As3MT), (3) glutathione S-transtrans-ferase omega 1 or 2 (GSTO1, GSTO2), and (4) arsenite methyl transferase (As3MT). Abbreviations: DMA, dimethylarsinic acid; MMA, monomethylarsonic acid; SAHC, S-adenosylhomocys-teine; SAM, S-adenosylmethionine.
The Research Ethics Committee of Taipei Medical Univer-sity (Taipei, Taiwan) approved the study. All patients pro-vided informed consent forms before sample and data collec-tion. The study was consistent with the World Medical Association Declaration of Helsinki.
A 10-mL blood sample was collected from participants on recruitment by use of EDTA-treated vacuum syringes and disposable needles. Plasma samples were centrifuged at 3,000 rpm for 15 minutes at room temperature, separated into aliquots, and stored at⫺80°C until used. Spot urine samples also were collected from all participants and imme-diately transferred to a⫺20°C freezer until further use for urinary arsenic species analysis.
Determination of Urinary Arsenic Species
It has been shown that urinary arsenic species are stable for at least 6 months when preserved at⫺20°C.21Therefore,
the urine assay was performed within 6 months after collec-tion. Frozen urine samples were thawed at room tempera-ture, dispersed by using ultrasonication, filtered through a Sep-Pak C18column (Mallinckrodt Baker Inc, Phillipsburg,
NJ) and levels of arsenite (As[III]), arsenate (As[V]), MMAV,
and DMAVwere determined. A urine aliquot of 200L was
used for determination of arsenic species by using high-performance liquid chromatography (HPLC; Waters 501; Waters Associates, Milford, MA) with columns obtained from Phenomenex (Nucleosil, Torrance, CA). Inorganic ar-senic and its metabolites were quantified by using hydride generator–atomic absorption spectrometry.22A standard
so-lution of 4 arsenic species was prepared in our laboratory; the sample and sample-spiked standard solution were deter-mined by using online HPLC–hydride generator–atomic absorption spectrometry. Recovery rates of the 4 arsenic species were calculated by using the following formula: [(sample-spiked standard solution concentration⫺ sample concentration)/(standard solution concentration)] ⫻ 100. Recovery rates for As(III), DMAV, MMAV, and As(V)
ranged between 93.8% and 102.2%, with detection limits of 0.02, 0.06, 0.07, and 0.10g/L, respectively. The urinary concentration of the sum of inorganic arsenic, MMAV, and
DMAV was normalized against urinary creatinine levels
(micrograms per gram of creatinine). The colorimetric assay automatically determined by the Roche Modular P800 instru-ment (Roche Inc, Mannheim, Germany) was used to calcu-late creatinine level by measuring the creatinine–picric acid complex formed by the reaction of creatinine and picric acid. The standard reference material, SRM 2670, contains 480⫾ 100g/L of inorganic arsenic and was obtained from the National Institute of Standards and Technology (Gaithers-burg, MD). SRM 2670 was used as a quality standard and analyzed along with urine samples. The mean value of SRM 2670 determined by our system was 507⫾ 17g/L (n ⫽ 4). The arsenic methylation indices were assessed by the percent-ages of various urinary arsenic species present in the sum of inorganic arsenic, MMAV, and DMAV. The primary
methyl-ation index was defined as the ratio of MMAVto levels of
inorganic arsenic, ie, As(III)⫹ As(V), and the secondary methylation index was defined as the ratio of DMAV to
MMAV.23
Determination of Plasma Antioxidant
Micronutrient Level
Levels of-carotene, lycopene, ␣-tocopherol, and retinol in plasma samples were measured by using HPLC according to the procedure described previously.24Analysis was
car-ried out by using reversed-phase HPLC (Hitachi Inc, Tokyo, Japan) with a mobile phase consisting of methanol:acetoni-trite:chloroform (47:47:6) and multiwave length monitoring. Retinol was detected at 325 nm;␣-tocopherol, at 280 nm; and lycopene and-carotene, at 466 nm. Plasma samples for each case and control set were thawed from⫺80°C in dim light at room temperature and assayed on the same day to ensure that temporal variability in laboratory assays would affect cases and controls equally. All laboratory personnel were unaware of the disease status of participants from whom plasma samples were tested. Recovery rates for -carotene, lycopene, ␣-tocopherol, and retinol were 90% to 100% at the highest concentration and 90% to 107% at the lowest concentration of the standard solution. The precision (coefficient of variance) of-carotene, lycopene, ␣-tocoph-erol, and retinol was 1.0% to 6.0%. We also used an internal control (␣-tocopherol acetate) to reduce systematic error; the coefficient of variance for␣-tocopherol acetate was 2.5%.
Statistical Analysis
Continuous variables are expressed as mean⫾ SE. Stu-dent t test was used to compare differences in urinary arsenic profiles between case participants and controls. Analysis of variance and Scheffe multiple comparison correction were applied to compare urinary arsenic profiles between the varied exposure strata. Unconditional logistic regression models were used to estimate multivariate-adjusted odds ratio (OR) and 95% confidence interval (CI). Cutoff values for continuous variables were the respective tertiles of controls. Significance tests for linear trend among ORs across exposure strata were calculated by categorizing expo-sure variables and treating scored variables as continuous. For joint-effect analysis, cutoff values for plasma lycopene, urinary arsenic species percentage, or arsenic methylation indices were the respective medians of the controls. The synergy index proposed by Rothman25 was computed to
assess the additive interaction relationship between lyco-pene levels and urinary arsenic species percentages or ar-senic methylation indices on CKD risk. An observed syn-ergy index value that departs substantially from the expected additive null, ie, a synergy index not equal to 1, suggests an additive interaction effect. ORs and variance covariance matrixes then were used to calculate values for synergy index and 95% CIs.26
RESULTS
Participants who had higher educational levels
had a significantly lower risk of CKD than those
with lower educational levels. Participants with
diabetes or hypertension had a significantly greater
CKD risk than those without diabetes (OR, 4.00;
95% CI, 2.04 to 7.76) or those with normal blood
pressure (OR, 2.23; 95% CI, 1.34 to 3.70).
Alco-hol consumption was related to a significantly lower
CKD risk than for nondrinkers. Cigarette smoking
was not associated with CKD risk. A significantly
greater risk was shown in analgesic users than
nonusers; however, analgesic use on an as-needed
basis had a significantly lower CKD risk than in
nonusers (
Table 1
). Coffee consumption, pesticide
exposure, and paint or dye use did not affect risk
of CKD (data not shown).
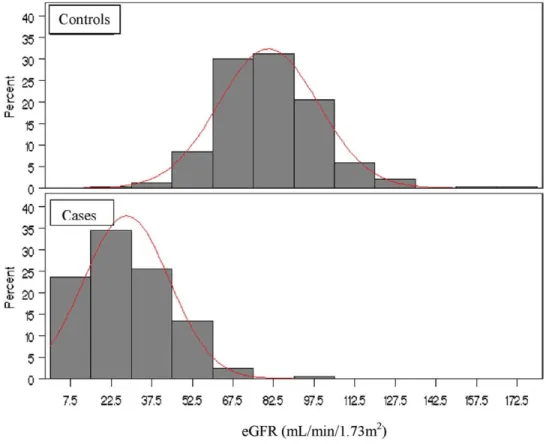
The CKD group had a significantly lower
eGFR (28.40
⫾ 1.41 mL/min/1.73 m
2; n
⫽ 125)
than controls (80.17
⫾ 1.21 mL/min/1.73 m
2;
n
⫽ 229; P ⬍ 0.001;
Fig 2
). Patients with CKD
had a significantly greater urinary total arsenic
level, greater MMA
Vpercentage, lower DMA
Vpercentage, and lower plasma lycopene level
than controls (
Table 2
).
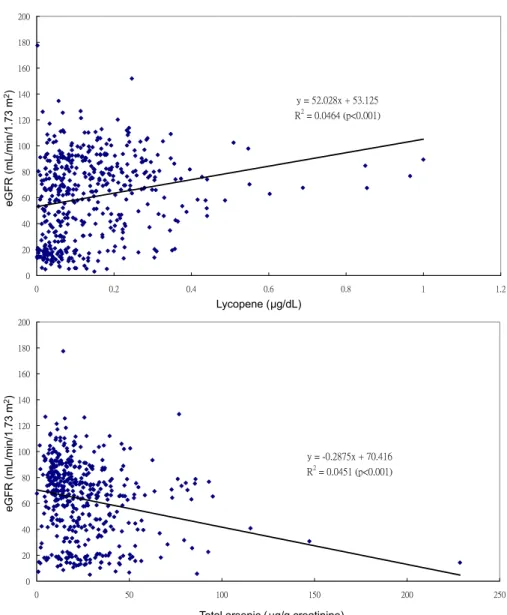
Plasma lycopene level was positively
associ-ated and urinary total arsenic level was
nega-tively associated with eGFR (both associations
were statistically significant;
Fig 3
), whether
adjusted for age and sex or multiple covariates.
When eGFR was adjusted for multiple
covari-ates, greater MMA
Vpercentages correlated with
significantly lower eGFRs (ie, inverse
correla-tion), and greater DMA
Vpercentages correlated
with significantly greater eGFRs (data not
shown).
Compared with men, women had lower
MMA
Vpercentages, but significantly greater
to-tal arsenic levels. Cigarette smoking, alcohol
consumption, and habitual analgesic use did not
influence the arsenic profile (
Table 3
).
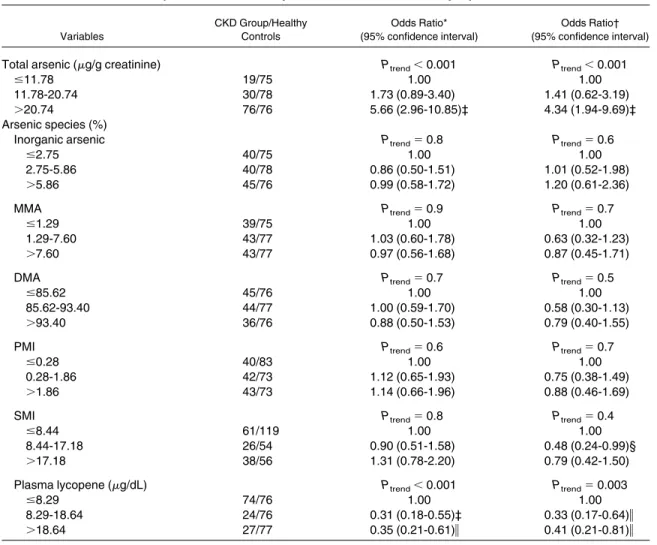
By performing trend analysis on urinary total
arsenic level, percentage of arsenic species, or
plasma lycopene strata in tertiles, total urinary
arsenic level was associated significantly with the
CKD OR in a dose-response relationship, as listed
in
Table 4
. This was especially true in participants
with a total arsenic level greater than 20.74
g/g
creatinine, in whom the OR of CKD was increased
4-fold compared with those with a total arsenic
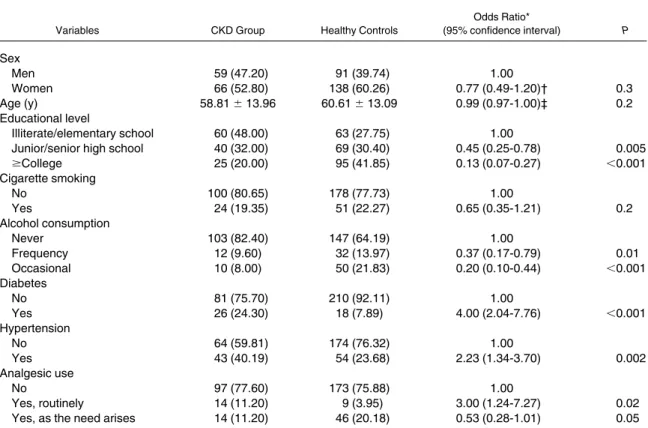
Table 1. Sociodemographic Characteristics of the CKD Group and Healthy ControlsVariables CKD Group Healthy Controls
Odds Ratio* (95% confidence interval) P Sex Men 59 (47.20) 91 (39.74) 1.00 Women 66 (52.80) 138 (60.26) 0.77 (0.49-1.20)† 0.3 Age (y) 58.81⫾ 13.96 60.61⫾ 13.09 0.99 (0.97-1.00)‡ 0.2 Educational level Illiterate/elementary school 60 (48.00) 63 (27.75) 1.00
Junior/senior high school 40 (32.00) 69 (30.40) 0.45 (0.25-0.78) 0.005
ⱖCollege 25 (20.00) 95 (41.85) 0.13 (0.07-0.27) ⬍0.001 Cigarette smoking No 100 (80.65) 178 (77.73) 1.00 Yes 24 (19.35) 51 (22.27) 0.65 (0.35-1.21) 0.2 Alcohol consumption Never 103 (82.40) 147 (64.19) 1.00 Frequency 12 (9.60) 32 (13.97) 0.37 (0.17-0.79) 0.01 Occasional 10 (8.00) 50 (21.83) 0.20 (0.10-0.44) ⬍0.001 Diabetes No 81 (75.70) 210 (92.11) 1.00 Yes 26 (24.30) 18 (7.89) 4.00 (2.04-7.76) ⬍0.001 Hypertension No 64 (59.81) 174 (76.32) 1.00 Yes 43 (40.19) 54 (23.68) 2.23 (1.34-3.70) 0.002 Analgesic use No 97 (77.60) 173 (75.88) 1.00 Yes, routinely 14 (11.20) 9 (3.95) 3.00 (1.24-7.27) 0.02
Yes, as the need arises 14 (11.20) 46 (20.18) 0.53 (0.28-1.01) 0.05
Note: Values expressed as number (percent) or mean⫾ SE unless noted otherwise.
Abbreviation: CKD, chronic kidney disease. *Adjusted for age and sex, except where indicated. †Adjusted only for age.
level of 11.78
g/g creatinine or less. Other arsenic
species indices were not related to the CKD OR.
Plasma lycopene level was related inversely to
CKD in a dose-response relationship (participants
with a plasma lycopene level
⬎ 18.64
g/dL
com-pared with
ⱕ 8.29
g/dL; OR, 0.41; 95% CI, 0.21
to 0.81). Plasma retinol level was associated
signifi-cantly with CKD risk (data not shown), whereas
other micronutrients were not related to CKD (data
not shown).
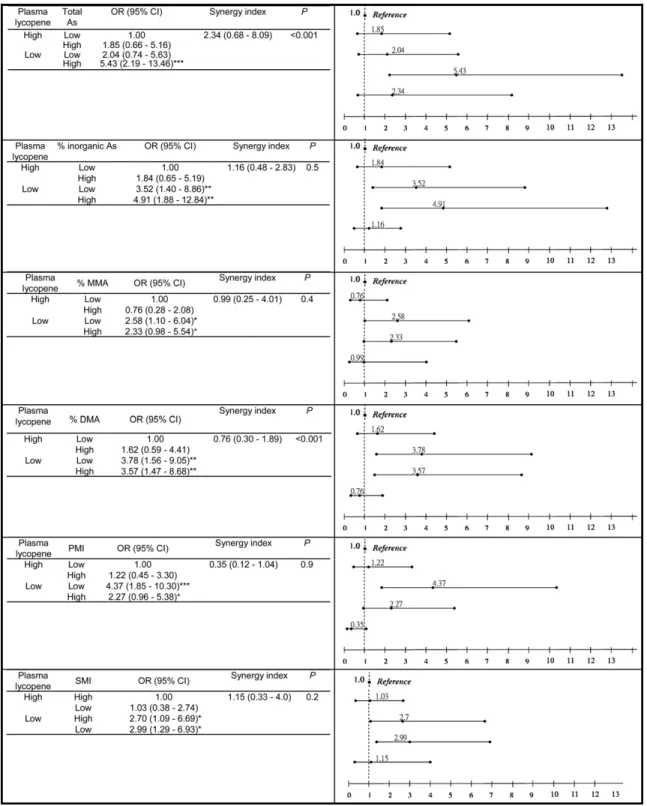
Additional analyses were carried out to assess
the joint effects of the following pairs of factors
on CKD risk: lycopene and total arsenic levels,
lycopene level and percentage of arsenic species,
or lycopene level and arsenic methylation
indi-ces (
Fig 4
). Trend analysis showed progressively
Figure 2. The distribution of estimated glomerular filtration rate (eGFR) in the chronic kidney disease group and controls.Table 2. Differences in Urinary Total Arsenic, Percentages of Arsenic Species, and Arsenic Methylation Indices Between the CKD Group and Healthy Controls
Variables
CKD Group Healthy Controls
P
No. Tested Value No. Tested Value
Total arsenic (g/g creatinine) 124 31.95⫾ 2.59 229 20.71⫾ 1.10 ⬍0.001
Arsenic species (%)
Inorganic arsenic 125 7.50⫾ 1.04 229 6.67⫾ 0.62 0.5
DMA 125 82.02⫾ 2.05 229 87.04⫾ 0.83 0.03
MMA 125 10.49⫾ 1.77 229 6.29⫾ 0.49 0.02
Primary methylation index 120 2.97⫾ 0.55 219 2.37⫾ 0.42 0.4
Secondary methylation index 97 29.68⫾ 5.58 164 26.03⫾ 3.63 0.6
Lycopene (g/dL) 125 6.22⫾ 1.43 229 10.40⫾ 0.89 ⬍0.001
Note: Values expressed as mean⫾ SE. Total arsenic indicates inorganic arsenic ⫹ MMA ⫹DMA. Abbreviations: CKD, chronic kidney disease; DMA, dimethylarsinic acid; MMA, monomethylarsonic acid.
increased risks through exposure to no risk
factor, 1 risk factor, or both 2 risk factors.
Although plasma lycopene level tended to
in-teract additively with total urinary arsenic level,
percentage of inorganic arsenic, primary
meth-ylation index, and secondary methmeth-ylation
in-dex in modifying CKD risk, the interactions
were all statistically insignificant, as shown by
the absence of a substantial deviation from 1 in
the synergy index. We also assessed the
inter-action as a departure from joint multiplicative
effects by using the product term of 2 risk
factors and showed that total arsenic level and
DMA percentage significantly interacted with
lycopene level (
Fig 4
).
DISCUSSION
The present study showed that patients with
CKD compared with control individuals had a
significantly greater total urinary arsenic level,
greater MMA
Vpercentage, and lower DMA
Vpercentage, indicating a less efficient capacity to
methylate inorganic arsenic to DMA
V. In
addi-tion, it was found that only total urinary arsenic
level was related to CKD risk in a dose-response
̌ʳːʳˈ˅ˁ˃˅ˋ̋ʳʾʳˈˆˁ˄˅ˈ ˥˅ʳːʳ˃ˁ˃ˇˉˇʳʻ̃ˏ˃ˁ˃˃˄ʼ ˃ ˅˃ ˇ˃ ˉ˃ ˋ˃ ˄˃˃ ˄˅˃ ˄ˇ˃ ˄ˉ˃ ˄ˋ˃ ˅˃˃ ˃ ˃ˁ˅ ˃ˁˇ ˃ˁˉ ˃ˁˋ ˄ ˄ˁ˅ ̌ʳːʳˀ˃ˁ˅ˋˊˈ̋ʳʾʳˊ˃ˁˇ˄ˉ ˥˅ʳːʳ˃ˁ˃ˇˈ˄ʳʻ̃ˏ˃ˁ˃˃˄ʼ ˃ ˅˃ ˇ˃ ˉ˃ ˋ˃ ˄˃˃ ˄˅˃ ˄ˇ˃ ˄ˉ˃ ˄ˋ˃ ˅˃˃ ˃ ˈ ˅ ˃ ˃ ˅ ˃ ˈ ˄ ˃ ˃ ˄ ˃ ˈ ˃ Lycopene (μg/dL)
Total arsenic ( μg/g creatinine)
eGFR (m L/min/1.73 m 2) eGFR (m L/min/1.73 m 2)
Figure 3. The association between estimated glomerular filtration rate (eGFR) and plasma lycopene or urinary total arsenic level.
relationship adjusted for age and sex or
sepa-rately adjusted for multiple risk factors. Patients
with CKD had significantly lower plasma
lyco-pene levels, indicating lower antioxidant
capabili-ties than controls.
Upon entering the body, arsenic targets
ubiqui-tous enzyme reactions and affects nearly all
organ systems.
27Several trace elements,
includ-ing arsenic, cadmium, lead, and mercury, have
been implicated in the decrease in kidney
func-tion.
28A study in Utah has shown increased rates
of nephritis and nephrosis in people drinking
arsenic-contaminated well water.
29According to
animal studies, vacuolation of renal tubular
epi-thelium was observed in a case of low-dose
arsenic exposure, whereas pathologically
moder-ate glomerular sclerosis and severe tubular
necro-sis were shown in the case of exposure to high
doses of arsenic.
30However, a case report by
Prasad and Rossi
31showed that tubulointerstitial
nephritis is associated with increased urinary
arsenic concentration.
According to the Taipei Water Department of
the Taipei City Government, average arsenic
concentration in Taipei tap water is 0.7
g/L and
ranges from undetectable to 4.0
g/L. However,
the concentration range of urinary arsenic of
study participants of approximately 20 to 30
g/g creatinine in this study possibly resulted
from exposure to some foods. Although our
study participants drank tap water with no
evi-dence of arsenic contamination, we also found
that total urinary arsenic level and MMA
Vper-centage were associated significantly with
de-creased eGFR in this study. However, the precise
mechanism of arsenic-induced nephrotoxicity
may be difficult to assess because of the complex
biological chemistry associated with arsenic.
32Absorbed arsenic is excreted mainly through
urine, suggesting that the kidney is a primary
target for arsenic toxicity. Kidney arsenic
toxic-ity may be complicated by methylation of
inor-ganic arsenic to the less toxic MMA
Vand DMA
V,
which are excreted rapidly by the kidney.
10MMA
IIIand DMA
IIIhave been identified in
human urine.
33,34Many studies have shown that
these trivalent methylated arsenic species are
more toxic than inorganic compounds.
35,36 How-Table 3. Distribution of Urinary Total Arsenic, Percentages of Arsenic Species, and Arsenic Methylation IndexAccording to Sex, Cigarette Smoking, Alcohol Consumption and Analgesic Use
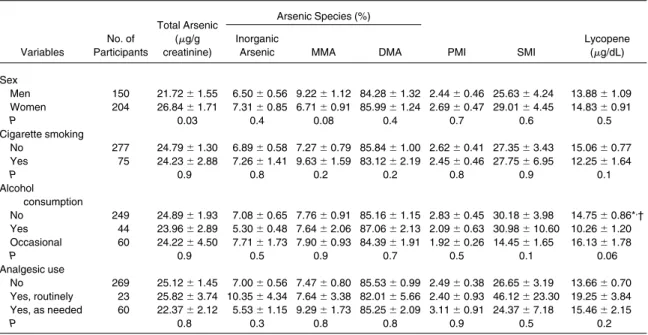
Variables No. of Participants Total Arsenic (g/g creatinine) Arsenic Species (%) PMI SMI Lycopene (g/dL) Inorganic
Arsenic MMA DMA
Sex Men 150 21.72⫾ 1.55 6.50⫾ 0.56 9.22 ⫾ 1.12 84.28 ⫾ 1.32 2.44 ⫾ 0.46 25.63 ⫾ 4.24 13.88⫾ 1.09 Women 204 26.84⫾ 1.71 7.31⫾ 0.85 6.71 ⫾ 0.91 85.99 ⫾ 1.24 2.69 ⫾ 0.47 29.01 ⫾ 4.45 14.83⫾ 0.91 P 0.03 0.4 0.08 0.4 0.7 0.6 0.5 Cigarette smoking No 277 24.79⫾ 1.30 6.89⫾ 0.58 7.27 ⫾ 0.79 85.84 ⫾ 1.00 2.62 ⫾ 0.41 27.35 ⫾ 3.43 15.06⫾ 0.77 Yes 75 24.23⫾ 2.88 7.26⫾ 1.41 9.63 ⫾ 1.59 83.12 ⫾ 2.19 2.45 ⫾ 0.46 27.75 ⫾ 6.95 12.25⫾ 1.64 P 0.9 0.8 0.2 0.2 0.8 0.9 0.1 Alcohol consumption No 249 24.89⫾ 1.93 7.08⫾ 0.65 7.76 ⫾ 0.91 85.16 ⫾ 1.15 2.83 ⫾ 0.45 30.18 ⫾ 3.98 14.75⫾ 0.86*, † Yes 44 23.96⫾ 2.89 5.30⫾ 0.48 7.64 ⫾ 2.06 87.06 ⫾ 2.13 2.09 ⫾ 0.63 30.98 ⫾ 10.60 10.26 ⫾ 1.20 Occasional 60 24.22⫾ 4.50 7.71⫾ 1.73 7.90 ⫾ 0.93 84.39 ⫾ 1.91 1.92 ⫾ 0.26 14.45 ⫾ 1.65 16.13⫾ 1.78 P 0.9 0.5 0.9 0.7 0.5 0.1 0.06 Analgesic use No 269 25.12⫾ 1.45 7.00⫾ 0.56 7.47 ⫾ 0.80 85.53 ⫾ 0.99 2.49 ⫾ 0.38 26.65 ⫾ 3.19 13.66⫾ 0.70 Yes, routinely 23 25.82⫾ 3.74 10.35 ⫾ 4.34 7.64 ⫾ 3.38 82.01 ⫾ 5.66 2.40 ⫾ 0.93 46.12 ⫾ 23.30 19.25 ⫾ 3.84 Yes, as needed 60 22.37⫾ 2.12 5.53⫾ 1.15 9.29 ⫾ 1.73 85.25 ⫾ 2.09 3.11 ⫾ 0.91 24.37 ⫾ 7.18 15.46⫾ 2.15 P 0.8 0.3 0.8 0.8 0.9 0.5 0.2
Note: Values expressed as mean⫾ SE. Total arsenic indicates inorganic arsenic ⫹ MMA ⫹ DMA. Cigarette smoking
history and analgesic use data were unavailable for 1 and 2 participants, respectively.
Abbreviations: CKD, chronic kidney disease; DMA, dimethylarsinic acid; MMA, monomethylarsonic acid; PMI, primary methylation index; SMI, secondary methylation index.
*Significantly different from those who consume alcohol, P⬍ 0.05. †Significantly different from occasional drinker, P⬍ 0.05.
ever, trivalent methylated arsenic metabolites
have a short half-life. Whether they can be
de-tected depends on the conditions and
tempera-ture of sample storage and concentrations in
urine. The reason we did not observe trivalent
methylated metabolites in the study is that the
analytical method used lacks the requisite
speci-ficity. In general, arsenic methylation is
consid-ered a detoxification process in which MMA
Vand DMA
Vgenerally are considered nontoxic.
Few studies have examined arsenic metabolism
on decreased kidney function in humans. One
study reported that the main detectable species
were the relatively nontoxic compounds
arseno-betaine and DMA, whereas levels of such toxic
inorganic arsenic compounds as arsenite and
arsenate were less than the detection limit in
serum.
37In the present study, we found that
patients with CKD had significantly greater
uri-nary total arsenic levels, greater MMA
Vpercent-ages, and lower DMA
Vpercentages than
con-trols. Of these variables, only total arsenic level
Table 4. Dose-Response Relationship Between CKD Risk and Urinary Total Arsenic, Percentages of ArsenicSpecies, Arsenic Methylation Indices, and Plasma Lycopene
Variables CKD Group/Healthy Controls Odds Ratio* (95% confidence interval) Odds Ratio† (95% confidence interval)
Total arsenic (g/g creatinine) Ptrend⬍ 0.001 Ptrend⬍ 0.001
ⱕ11.78 19/75 1.00 1.00
11.78-20.74 30/78 1.73 (0.89-3.40) 1.41 (0.62-3.19)
⬎20.74 76/76 5.66 (2.96-10.85)‡ 4.34 (1.94-9.69)‡
Arsenic species (%)
Inorganic arsenic Ptrend⫽ 0.8 Ptrend⫽ 0.6
ⱕ2.75 40/75 1.00 1.00
2.75-5.86 40/78 0.86 (0.50-1.51) 1.01 (0.52-1.98)
⬎5.86 45/76 0.99 (0.58-1.72) 1.20 (0.61-2.36)
MMA Ptrend⫽ 0.9 Ptrend⫽ 0.7
ⱕ1.29 39/75 1.00 1.00
1.29-7.60 43/77 1.03 (0.60-1.78) 0.63 (0.32-1.23)
⬎7.60 43/77 0.97 (0.56-1.68) 0.87 (0.45-1.71)
DMA Ptrend⫽ 0.7 Ptrend⫽ 0.5
ⱕ85.62 45/76 1.00 1.00
85.62-93.40 44/77 1.00 (0.59-1.70) 0.58 (0.30-1.13)
⬎93.40 36/76 0.88 (0.50-1.53) 0.79 (0.40-1.55)
PMI Ptrend⫽ 0.6 Ptrend⫽ 0.7
ⱕ0.28 40/83 1.00 1.00
0.28-1.86 42/73 1.12 (0.65-1.93) 0.75 (0.38-1.49)
⬎1.86 43/73 1.14 (0.66-1.96) 0.88 (0.46-1.69)
SMI Ptrend⫽ 0.8 Ptrend⫽ 0.4
ⱕ8.44 61/119 1.00 1.00
8.44-17.18 26/54 0.90 (0.51-1.58) 0.48 (0.24-0.99)§
⬎17.18 38/56 1.31 (0.78-2.20) 0.79 (0.42-1.50)
Plasma lycopene (g/dL) Ptrend⬍ 0.001 Ptrend⫽ 0.003
ⱕ8.29 74/76 1.00 1.00
8.29-18.64 24/76 0.31 (0.18-0.55)‡ 0.33 (0.17-0.64)储
⬎18.64 27/77 0.35 (0.21-0.61)储 0.41 (0.21-0.81)储
Note: Total arsenic indicates inorganic arsenic⫹ MMA ⫹ DMA.
Abbreviations: CKD, chronic kidney disease; DMA, dimethylarsinic acid; MMA, monomethylarsonic acid; PMI, primary methylation index; SMI, secondary methylation index.
*Adjusted for age and sex.
†Adjusted for age, sex, educational level, paternal and maternal ethnicity, cigarette smoking, coffee drinking, analgesic use, hypertension, and diabetes history.
‡P⬍ 0.001. §P⬍ 0.05. 储P ⬍ 0.01.
Figure 4. Multiple logistical regression analysis of the combination of urinary total arsenic, arsenic species percentage, and plasma lycopene on chronic kidney disease. * P⬍ 0.05; ** P ⬍ 0.01; *** P ⬍ 0.001. The unit of total arsenic isg/g creatinine. The relative proportion of each arsenic species (% inorganic As, % MMA and % DMA) was calculated by dividing the levels of each species by the total arsenic level. Abbreviations: PMI, primary methylation index; SMI, secondary methylation index. High is defined as a value greater than the median; low, as a value equal to the median or less. Odds ratios (ORs) based on analyses adjusted for age, sex, educational level, paternal and maternal ethnicity, cigarette smoking, coffee drinking, hypertension, and diabetes history. P represents statistical interaction as a departure from joint multiplicative effects.
was associated significantly with CKD risk.
Whether the capacity of arsenic methylation is
related to patients with CKD when they ingest
low arsenic levels in drinking water needs
fur-ther investigation.
Inorganic arsenic-induced oxidative damage
results in chronic kidney pathological states
in-volving ROS production,
reduction/oxidation-related gene expression, and cytotoxicity.
38How-ever, oxidative stress has been identified as an
important mechanism in arsenic-induced
de-creased kidney function through accumulation of
arsenic in kidney tissue; increased levels of
se-rum urea nitrogen, creatinine, and lipid
peroxida-tion end products; and reduced glutathione in a
mouse model.
39Our recent study showed that
arsenic methylation species were associated with
oxidative damage assessed by using urinary
8-hy-droxy-2=-deoxyguanosine,
40suggesting that
ar-senic metabolites are related to oxidative stress.
Antioxidants could be considered an
alterna-tive approach to mitigate arsenic-induced
oxida-tive damage.
41In our previous study, a
signifi-cant inverse dose-response relationship was
observed between arsenic-related ischemic heart
disease and serum
␣- and -carotene levels.
42Our study also showed that serum
-carotene
level was related negatively to arsenic-induced
skin cancer.
43In the present study, we found that
participants with high plasma lycopene levels
had a significantly decreased risk of CKD
com-pared with patients with low plasma lycopene
levels. Additionally, participants with low plasma
lycopene levels were at greater risk of having
CKD when they presented with at least 1 of high
total arsenic level or low DMA
Vpercentage.
Although these data suggest that participants
with low antioxidant capacity may not easily
mitigate oxidative stress produced by arsenic
metabolites and therefore may be at risk of CKD,
these findings need additional study.
Our study had some important limitations that
need to be considered when interpreting results.
First, there is the possibility of selection bias
because cases and controls were recruited from 2
different hospitals; however, bias was minimized
because these hospitals both belonged to medical
centers and were located in Taipei. Furthermore,
the majority of cases and controls lived in Taipei
and were similar in age and sex distribution
(
Table 1
) with respect to demographic
character-istics. Possible selection bias may have occurred
because the recruited CKD cases more often had
an elementary school education than controls.
However, in a large-scale screening program, it
has been reported that participants with a high
level of education had lower CKD risk than those
with a low level of education in Taiwan.
2Sec-ond, the accuracy of a single spot evaluation of
plasma antioxidants and urinary arsenic species
may be in doubt. However, the values might be
reliable because all participants had no change in
lifestyle and appeared to maintain their
homeo-static metabolism. Third, because of the small
sample size, statistical significance should be
interpreted with caution. Fourth, CKD cases were
recruited in this study; however, we cannot
ex-clude that the findings of an association between
lycopene or arsenic and its various metabolites
and CKD might be the result and not the cause of
CKD.
In conclusion, this is the first study showing
that high urinary total arsenic levels or low
plasma lycopene levels are associated positively
with CKD. Similarly, our data suggest that the
capacity for arsenic methylation may be
associ-ated with CKD in individuals who also had low
plasma lycopene levels when they ingested low
arsenic levels in drinking water.
ACKNOWLEDGEMENTS
Support: The study was supported by grants
SKH-TMU-95-23, NSC- 96-2314-B038-003 and NSC 97-2314-B-038-015-MY3 from Shin Kong Wu Ho-Su Memorial Hospital in Taipei, Taiwan and National Science Council of the ROC, Taiwan.
Financial Disclosure: None.
REFERENCES
1. National Kidney Foundation: K/DOQI Clinical Prac-tice Guidelines for Chronic Kidney Disease: Evaluation, classification, and stratification. Am J Kidney Dis 39:S1-S266, 2002 (suppl 1)
2. Wen CP, Cheng TY, Tsai MK, et al: All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371:2173-2182, 2008
3. US Renal Data System: USRDS 2007 Annual Data Report. Available athttp://www.usrds.org/.Accessed Decem-ber 31, 2007
4. Haroun MK, Jaar BG, Hoffman SC, et al: Risk factors for chronic kidney disease: A prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14:2934-2941, 2003
5. Higashikuni Y, Ishizaka N, Ishizaka Y, et al: Relation-ship between blood pressure and chronic kidney disease in
the Japanese population: The lower the better even in individuals without hypertension? Hypertens Res 31:213-219, 2008
6. Chen CJ, Kuo TL, Wu MM: Arsenic and cancers. Lancet 1:414-415, 1988
7. Pu YS, Yang SM, Huang YK, et al: Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol Appl Pharmacol 218:99-106, 2007 8. Hong F, Jin T, Zhang A: Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals 17:573-580, 2004
9. Meliker JR, Wahl RL, Cameron LL, et al: Arsenic in drinking water and cerebrovascular disease, diabetes melli-tus, and kidney disease in Michigan: A standardized mortal-ity ratio analysis. Environ Health 6:4, 2007
10. Thompson DJ: A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact 88:89-114, 1993
11. Aposhian HV, Zakharyan RA, Avram MD, et al: A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicol Appl Pharmacol 198:327-335, 2004
12. Aposhian HV, Aposhian MM: Arsenic toxicology: Five questions. Chem Res Toxicol 19:1-15, 2006
13. De CS, Ghosh P, Sarma N, et al: Genetic variants associated with arsenic susceptibility: Study of purine nucleo-side phosphorylase, arsenic (⫹3) methyltransferase, and glutathione S-transferase omega genes. Environ Health Per-spect 116:501-505, 2008
14. Kitchin KT: Recent advances in arsenic carcinogen-esis: Modes of action, animal model systems, and methyl-ated arsenic metabolites. Toxicol Appl Pharmacol 172:249-261, 2001
15. Schmuck EM, Board PG, Whitbread AK, et al: Char-acterization of the monomethylarsonate reductase and dehy-droascorbate reductase activities of omega class glutathione transferase variants: Implications for arsenic metabolism and the age-at-onset of Alzheimer’s and Parkinson’s dis-eases. Pharmacogenet Genomics 15:493-501, 2005
16. Scibior A, Zaporowska H: Effects of vanadium(V) and/or chromium(III) onL-ascorbic acid and glutathione as well as iron, zinc, and copper levels in rat liver and kidney. J Toxicol Environ Health A 70:696-704, 2007
17. Nesnow S, Roop BC, Lambert G, et al: DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem Res Toxicol 15:1627-1634, 2002
18. Stahl W, Sies H: Antioxidant activity of carotenoids. Mol Aspects Med 24:345-351, 2003
19. Augusti PR, Conterato GM, Somacal S, et al: Effect of lycopene on nephrotoxicity induced by mercuric chloride in rats. Basic Clin Pharmacol Toxicol 100:398-402, 2007
20. Atessahin A, Ceribasi AO, Yilmaz S: Lycopene, a carotenoid, attenuates cyclosporine-induced renal dysfunc-tion and oxidative stress in rats. Basic Clin Pharmacol Toxicol 100:372-376, 2007
21. Chen YC, Amarasiriwardena CJ, Hsueh YM, et al: Stability of arsenic species and insoluble arsenic in human
urine. Cancer Epidemiol Biomarkers Prev 11:1427-1433, 2002
22. Hsueh YM, Huang YL, Huang CC, et al: Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J Toxicol Environ Health A 54:431-444, 1998
23. Tseng CH, Huang YK, Huang YL, et al: Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Tai-wan. Toxicol Appl Pharmacol 206:299-308, 2005
24. Miller KW, Lorr NA, Yang CS: Simultaneous deter-mination of plasma retinol, alpha-tocopherol, lycopene, al-pha-carotene, and beta-carotene by high-performance liquid chromatography. Anal Biochem 138:340-345, 1984
25. Rothman KJ: Modern Epidemiology. Boston, MA, Little Brown, 1986
26. Hosmer DW, Lemeshow S: Confidence interval esti-mation of interaction. Epidemiology 3:452-456, 1992
27. Ratnaike RN: Acute and chronic arsenic toxicity. Postgrad Med J 79:391-396, 2003
28. Vanholder R, Cornelis R, Dhondt A, et al: The role of trace elements in uraemic toxicity. Nephrol Dial Transplant 17:S2-S8, 2002 (suppl 2)
29. Lewis DR, Southwick JW, Ouellet-Hellstrom R, et al: Drinking water arsenic in Utah: A cohort mortality study. Environ Health Perspect 107:359-365, 1999
30. Tsukamoto H, Parker HR, Gribble DH, et al: Nephro-toxicity of sodium arsenate in dogs. Am J Vet Res 44:2324-2330, 1983
31. Prasad GV, Rossi NF: Arsenic intoxication associated with tubulointerstitial nephritis. Am J Kidney Dis 26:373-376, 1995
32. Fowler BA: Mechanisms of kidney cell injury from metals. Environ Health Perspect 100:57-63, 1993
33. Le XC, Ma M, Cullen WR, et al: Determination of monomethylarsonous acid, a key arsenic methylation inter-mediate, in human urine. Environ Health Perspect 108:1015-1018, 2000
34. Mandal BK, Ogra Y, Suzuki KT: Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem Res Toxicol 14:371-378, 2001
35. Mass MJ, Tennant A, Roop BC, et al: Methylated trivalent arsenic species are genotoxic. Chem Res Toxicol 14:355-361, 2001
36. Petrick JS, Ayala-Fierro F, Cullen WR, et al: Mono-methylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163: 203-207, 2000
37. Zhang X, Cornelis R, De KJ, et al: Accumulation of arsenic species in serum of patients with chronic renal disease. Clin Chem 42:1231-1237, 1996
38. Sasaki A, Oshima Y, Fujimura A: An approach to elucidate potential mechanism of renal toxicity of arsenic trioxide. Exp Hematol 35:252-262, 2007
39. Sinha M, Manna P, Sil PC: Arjunolic acid attenuates arsenic-induced nephrotoxicity. Pathophysiology 15:147-156, 2008
40. Chung CJ, Huang CJ, Pu YS, et al: Urinary 8-hy-droxydeoxyguanosine and urothelial carcinoma risk in low
arsenic exposure area. Toxicol Appl Pharmacol 226:14-21, 2008
41. Bongiovanni GA, Soria EA, Eynard AR: Effects of the plant flavonoids silymarin and quercetin on arsenite-induced oxidative stress in CHO-K1 cells. Food Chem Toxicol 45:971-976, 2007
42. Hsueh YM, Wu WL, Huang YL, et al: Low serum
carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis 141: 249-257, 1998
43. Hsueh YM, Chiou HY, Huang YL, et al: Serum beta-carotene level, arsenic methylation capability, and inci-dence of skin cancer. Cancer Epidemiol Biomarkers Prev 6:589-596, 1997