Corresponding author: Hua-Chang Fang, MD, 386 Ta-Chung 1st Rd., Kaohsiung 813, Taiwan, R.O.C. Tel: 3468090, Fax: +886-7-3455412, E-mail: hcfang@isca.vghks.gov.tw
Received: October 27, 2010; Accepted: March 7, 2011.
Acta Nephrologica 25(1): 1-4, 2011 1
Fenofibrate Reversibly Increases Serum
Creatinine Level in Chronic Kidney Disease
Patients by Reducing Glomerular
Filtration Rate
Yu-Ling Chen1, 3, Chih-Yang Hsu1, Wei-Chieh Huang1, Chien-Liang Chen1, 2, Po-Tsang Lee1, 2, Tsu-Yuan Chang1, Kang-Ju Chou1, 2, Hsiao-MinChung1, 2,
and Hua-Chang Fang1, 2
1Division of Nephrology, Kaohsiung Veterans General Hospital, Kaohsiung
2Department of Internal Medicine, National Yang-Ming University School of Medicine, Taipei 3Department of Medicine, China Medical University and Beigang Hospital, Yunlin, Taiwan, Republic of China
Abstract
BACKGROUND. Fenofibrate is a potent lipid-lowering agent that was found to increase serum creatinine, but the underlying mechanism remains controversial.
METHODS. Thirteen hypertriglyceridemic patients, composed of 9 males and 4 females with a mean age of 62 (51-76) presenting with an acute rise of serum creatinine 2 weeks to 6 months after starting fenofibrate (200 mg daily) treatment, were recruited. Other possible causes of acute renal failure were excluded based on clinical judgment. The serum creatinine level, BUN, creatinine clearance calculated using a 24-h urine collection, and estimation of glomerular filtration rate using the Cockroft-Gault formula were obtained on fenofibrate treatment. The same tests were repeated 2 weeks after discontinuing the treatment.
RESULTS. The data showed serum creatinine rose from 1.8 (1.3-4.9) mg/dL before the treatment to 3.0 (1.8-5.0) mg/dL (P < 0.05) and BUN from 27 (18-40) to 38 (27-58) mg/dL (P < 0.05) 2 weeks to 6 months after the treatment. After discontinuing fenofibrate, serum creatinine declined from 3.0 (2.0-4.5) to 2.1 (1.7-4.1) mg/dL (P < 0.05), BUN from 34 (52-26) to 24 (19-39) mg/dL (P < 0.05), Cockroft-Gault estimated GFR increased from 32 (23-49) to 47 (31-65) mL/min (P < 0.05) and creatinine clearance increased from 37 (25-49) to 54 (39-67) mL/min (P < 0.05). However, daily urine creatinine excretion did not significantly change, from 18.1 (15.1-24.5) to 19.3 (13.2-22.7) mg/kg/day.
CONCLUSION. Our study shows fenofibrate reversibly increased the serum creatinine level with simultaneous BUN changes, but there was no change in creatinine production. The results suggest a reduction of glomerular filtration rate is responsible for an elevation of serum creatinine. (Acta Nephrologica 2011; 25: 1-4)
KEY WORDS: fenofibrate, serum creatinine, glomerular filtration rate
Introduction
Lipid metabolism abnormality is common in chronic kidney disease (CKD) patients that are at greater risk of developing accelerated atherosclerosis and cardiovascular events (1, 2). Fenofibrate is a lipid-modifying agent that is mainly used to reduce
low-density lipoprotein cholesterol and triglyceride levels, and to increase high-density lipoprotein cholesterol levels (3). Fibrate exerts its therapeutic effects through activating the peroxisome proliferator-activated receptor α (PPARα), nuclear receptors. These nuclear receptors, once bound by fibrates, down-regulate the expression of the inducible cyclooxygenase-2
2 Chen, Hsu, Huang, Chen, Lee, Chang, Chou, Chung and Fang
2) enzyme, which may be critical for the maintenance of vasodilatory prostaglandins within the kidneys (4-6). Fenofibrate treatment is generally well tolerated and adverse effects include myalgia, rhabdomyolysis, liver enzymes elevation, and gastrointestinal upset (7). In combination with statins, rhabdomyolysis-associated acute renal failure has been reported in some patients (8). However, there are few reports addressing the influence of renal function in patients without rhab-domyolysis. In our clinical observation, many patients on fenofibrate for treating hypertriglyceridemia de-veloped a reversible elevation of their serum creatinine levels. This study examined the changes of renal function parameters in this clinical setting.
Methods
Thirteen hypertriglyceridemic patients present-ing with an acute rise of serum creatinine 2 weeks to 6 months after beginning fenofibrate at a dose of 200 mg daily were recruited. The patients were referred to nephrology outpatient clinics for assessing an acute rise in serum creatinine of unknown cause. Medical records and medication history were carefully studied. None of these patients had a history of myositis, con-current use of nephrotoxic agents such as aminogly-coside, or other lipid-lowering agents such as statins. All patients had a normal serum creatinine phospho-kinase level. Other possible causes of acute renal failure were excluded based on clinical judgment. Their baseline median serum creatinine was 1.8 (1.3-4.9) mg/dL and BUN 27 (18-40) mg/dL. All the
patients had received fenofibrate treatment, 200 mg per day, from 2 weeks to 6 months for hypertrig-lyceridemia before recruitment. Their serum crea-tinine level, serum creatine phosphokinase level, BUN, creatinine clearance calculated using a 24-h urine collection, and estimation of glomerular filtration rate using the Cockroft-Gault formula were obtained during fenofibrate treatment and two weeks after cessation of treatment. The biochemistry data were measured using an Express Plus Chemistry Analyzer (Chiron Corp, Boston, MA, USA). The Human Re-search Review Committee of Kaohsiung Veterans General Hospital approved the protocol and informed written consent was obtained from each patient. Statistics
Data are expressed as medians (range). Non-parametric paired t-tests were used for comparisons during use and after discontinuing fenofibrate. A P value < 0.05 was set as the criterion for significance in all comparisons.
Results
Table 1 shows the demographic data of the thirteen hypertriglyceridemia patients. The median age is 62 (51-76) years old, with 9 males and 4 females. Their baseline median serum creatinine of 1.8 (1.3-4.9) mg/ dL and BUN 27 (18-40) mg/dL rose to 3.0 (1.8-5.0) mg/dL and 38 (27-58) mg/dL, respectively (P < 0.05). All the patients had received fenofibrate treatment,
Table 1. Demographic data of thirteen patients with fenofibrate-associated hypercreatininemia
Patient number 13
Age (years) 62 (51–76)
Sex (male/female) 9/4
Height (cm) 165 (157–172)
Weight (kg) 68 (49–88)
Serum creatine phosphokinase (IU/L) 134 (110–203)
Serum creatinine (mg/dL)
before treatment 1.8 (1.3–4.9)
during treatment 3.0 (1.8–5.0)
Blood urea nitrogen (mg/dL)
before treatment 27 (18–40)
during treatment 38 (27–58)
Underlying disease
Normal 3
Chronic interstitial nephritis 4
Nephroslerosis 2
Chronic glomerulonephritis 2
Diabetes nephropathy 1
Fenofibrate Effect on Renal Function 3
200 mg per day, from 2 weeks to 6 months for hy-pertriglyceridemia on recruitment. The serum levels of creatine phosphokinase remained normal in all patients with a mean value of 134 (110-203) IU/L during fenofibrate treatment. Except for three patients with normal renal function, there were four cases of chronic interstitial nephritis, two of nephrosclerosis, two of diabetes nephropathy, one of chronic glomer-ulonephritis, and one of right nephrectomy.
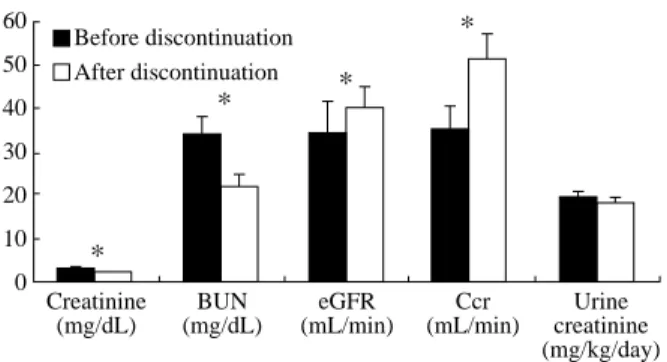
Fig. 1 shows the values of serum creatinine, BUN, estimated GFR, 24-h urine creatinine clearance, and daily urine creatinine excretion before and 2 weeks after discontinuing fenofibrate treatment. Ex-cept for the daily urine creatinine excretion, those values changed significantly after discontinuing the treatment before vs. 2 weeks after discontinuation: serum creatinine, 3.0 (2.0-4.5) vs. 2.1 (1.7-4.1) mg/ dL; BUN, 34 (52-26) vs. 24 (19-39) mg/dL; estimated GFR, 32 (23-49) vs. 47 (31-65) mL/min; 24-h urine creatinine clearance, 37 (25-49) vs. 54 (39-67) mL/ min; daily urine creatinine excretion, 18.1 (15.1-24.5) vs. 19.3 (13.2-22.7) mg/kg/day.
Discussion
Hypertriglyceridemia is one of the most important risk factors of cardiovascular morbidity and mortality. Fenofibrate is a potent triglyceride-lowering agent that might cause rhabdomyolysis with or without acute renal failure. In this study, there was no laboratory evidence of rhabdomyolysis in our patients. Patients on fenofibrate treatment without rhabdomyolysis have previously been reported as showing a reversible increase in serum creatinine levels in some previous studies in normal healthy subjects and chronic kidney disease patients (9-11). The mechanism underlying the rise of serum creatinine remains controversial. Possible causes of an increased serum creatinine include over-production of creatinine from damaged muscles (8, 9), laboratory interference of serum creatinine levels, inhibition of tubular secretion of creatinine (10), and a decreased
glomerular filtration rate.
There is a previous report suggesting fenofibrate might induce elevation of serum creatinine without influencing the glomerular filtration rate, but with increased daily urine creatinine excretion. It has been proposed increased creatinine production is re-sponsible for the creatinine rise (9). Ansquer et al. demonstrated a decline of creatinine clearance, un-changed urine creatinine excretion, and a constant glomerular filtration rate based on inulin clearance calculation, suggesting an inhibition of creatinine secretion (10). In our study, the daily urine creatinine excretion before and after discontinuing fenofibrate did not change significantly, suggesting an increased production of creatinine did not occur in our patients. Further, the simultaneous rise of BUN and serum creatinine level also made both laboratory interference and inhibition of creatinine secretion less likely causes. Our data suggest a glomerular filtration rate reduction is the major cause of the elevation of serum creatinine in patients for fenofibrate treatment.
A possible mechanism is fibrate lowers the glomerular filtration rate to bind the peroxisome pro-liferator-activated receptors and downregulate the expression of the inducible COX-2 enzyme. The enzyme is critical for maintaining vasodilatory pro-staglandin action in kidneys (4, 5). Activation of per-oxisome proliferator-activated receptors (PPARs) might also attenuate angiotensin II-mediated vascular remodeling (12).
Some previous reports have demonstrated fenofi-brate has a long-term beneficial effect in slowing renal progression through a presumed lipid modifying mechanism. Among them, two previous animal studies demonstrated a beneficial effect of fenofibrate on reduc-ing albuminuria and renal injury in diabetes rats without significant changes in plasma creatinine, but with an increased BUN (13, 14). Further, in a study to assess the effect of fenofibrate on cardiovascular disease events in 9795 diabetes patients, the results showed plasma creatinine remained at an average of 0.11-0.14 mg/dL higher in the fenofibrate group compared to the placebo group (15). Some patients in the investigation were restudied 8 weeks after ceasing medications at the end of the trial. The plasma creatinine declined from a median of 1.04 to 0.87 mg/dL in the fenofibrate group. In the latter study, it also showed more patients in the fenofibrate group had less progression of albu-minuria. Therefore, the short-term reversible effect on plasma creatinine and BUN observed in those studies is similar to the four findings.
There are some major limitations to our study. First, the small sample size and retrospective design may not have enough power to detect the underlying mechanisms of the elevation of serum creatinine. Second, the duration of treatment varied among the
Fig. 1. Parameters before and 2 weeks after discontinuation of fenofibrate treatment. *P < 0.05. * * * * 60 50 40 30 20 10 0 Creatinine (mg/dL) BUN (mg/dL) eGFR (mL/min) Ccr (mL/min) Urine creatinine (mg/kg/day) Before discontinuation After discontinuation
4 Chen, Hsu, Huang, Chen, Lee, Chang, Chou, Chung and Fang
patients, so other causes of kidney injury might have confounded the investigation results. Third, the reli-ability of glomerular filtration rate assessment used in our study is not as accurate as inulin clearance.
In conclusion, fenofibrate is a potent lipid-lowering agent, widely used in patients with renal impairment. Physicians taking care of CKD patients should consider its effects on the serum creatinine level. Our study suggests fenofibrate reversibly increased serum creatinine levels in CKD patients by reducing the glomerular filtration rate. However, different mechanisms were reported. Further clarification of the underlying mechanisms requires studies with a large sample size and measurements of creatinine dynamics and accurate renal function tests such as inulin clearance.
References
1. Attman PO, Alaupovic P, Travella M, Knight-Gibson C. Abnormal lipid and apoprotein composition of the major lipoprotein density classes in patients with chronic renal failure. Nephrol Dial
Trans-plant 11: 63-69, 1996.
2. Grutzmacher P, Marz W, Peschke B, Gross W, Schoeppe W. Lipoproteins and apolipoprtoeins during the progression of renal disease. Nephron 50: 103-111, 1988.
3. Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et
al. Helsinki heart study: primary-prevention trial with gemfibrozil
in middle-aged men with dyslipidemia. N Engl J Med 317: 1237-1245, 1987.
4. Wilson MW, Lar LT, Chow CK, Tai H, Robertson LW, Glauert HP. Altered hepatic eicosanoid concentration in rats treated with the peroxisome proliferators ciprofibrate and perfluorodecanoic acid.
Arch Toxicol 69: 491-497, 1995.
5. Ledwith BJ, Pauley CJ, Wagner LK, Rokos CL, Alberts DW, Manam S. Induction of cyclooxygenase-2 expression by peroxi-some proliferators and non-tetradecanoylphorbol 12, 13-myristate-type tumor promoters in immortalized mouse liver cells. J Biol
Chem 272: 3707-3714, 1997.
6. Yoshinari M, Asano T, Kaori S, Shi AH, Wakisaka M, Iwase M, et
al. Effect of gemfibrozil on serum levels of prostacyclin and
precursor fatty acids in hyperlipidemic patients with Type 2 diabetes.
Diabetes Res Clin Pract 42: 149-154, 1998.
7. Blane GF. Comparative toxicity and safety profile of fenofibrate and other fibric acid derivatives. Am J Med 83: 26-36, 1987. 8. Oldemeyer JB, Lund RJ, Koch M, Meares AJ, Dunlay R.
Rhab-domyolysis and acute renal failure after changing statin-fibrate combinations. Cardiology 94: 127-128, 2000.
9. Hottelart C, EI Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron 92: 536-541, 2002.
10. Ansquer JC, Dalton RN, Causse E, Crimet D, Le Malicot K, Foucher C. Effect of fenofibrate on kidney function: a 6-week ran-domized crossover trial in healthy people. Am J Kidney Dis 51: 904-913, 2008.
11. Lipscombe J, Lewis GF, Cattran D, Bargman JM. Deterioration in renal function associated with fibrate therapy. Clin Nephrol 55: 39-44, 2001.
12. Schiffrin EL. Peroxisome proliferator-activated receptors and car-diovascular remodeling. Am Physiol Heart Cir Physiol 288: H1037-H1043, 2005.
13. Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, et al. PPARα agonist fenofibrate improves diabetic nephropathy in db/db mice.
Kidney Int 69: 1511-1517, 2006.
14. Chen LL, Zhang JY, Wang BP. Renoprotective effects of fenofibrate in diabetic rats are achieved by suppressing kidney plasminogen activator inhibitor-1. Vascul Pharmacol 44: 309-315, 2006. 15. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et
al. Effects of long-term fenofibrate therapy on cardiovascular
events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 366: 1849-1861, 2005.