行政院國家科學委員會專題研究計畫 成果報告
全基因體化分析克雷白氏肺炎桿菌 CG43 的雙分子調控系統
計畫類別: 個別型計畫 計畫編號: NSC93-2311-B-009-005- 執行期間: 93 年 08 月 01 日至 94 年 07 月 31 日 執行單位: 國立交通大學生物科技研究所 計畫主持人: 彭慧玲 計畫參與人員: 林靖婷, 鄭新耀 報告類型: 精簡報告 報告附件: 出席國際會議研究心得報告及發表論文 處理方式: 本計畫可公開查詢中 華 民 國 94 年 10 月 27 日
行政院國家科學委員會補助專題研究計畫
■ 成 果 報 告
□期中進度報告
全基因體化分析克雷白氏肺炎桿菌 CG43 的雙分子調控系統
A genome-wide analysis of the two-component regulatory systems in Klebsiella
pneumoniae CG43
計畫類別:■ 個別型計畫
□ 整合型計畫
計畫編號:NSC 93-2311-B-009-005-
執行期間:93 年 8 月 1 日至 94 年 7 月 31 日
計畫主持人:彭慧玲
共同主持人:
計畫參與人員: 林靖婷
鄭新耀
成果報告類型(依經費核定清單規定繳交)
:□精簡報告
■完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、列
管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:國立交通大學生物科技研究所
中
華
民
國
94 年
10 月
19 日
一、 中文摘要(Abstract)
關鍵詞:克雷白氏肺炎菌株,莢膜多醣生合成,雙分子調控系統,調控蛋白突變株,建立 啟動子-LacZ報告系統,雙分子調控系統網路。
在感染人體時,細菌面臨與外界迥異的環境,例如溫度、氧氣含量、細胞內外離子濃度差、 宿主免疫系統攻擊等。為了生存,細菌必須感應外在環境的變化,並藉由各種訊息傳遞系統調 控、啟動或抑制基因表現。雙分子調控系統(Two component systems, TCSs)為細菌中普遍存 在的訊息傳遞系統,在細菌適應環境、產生抗藥性、抵抗免疫系統攻擊等作用上,扮演著重要 的角色。當細胞膜上感應蛋白(sensor)察覺環境變化並自我磷酸化後,外界訊息得以轉化為 蛋白質間磷酸根的傳遞,細胞質內的反應調控蛋白(response regulator)在接受磷酸根後,改 變構形成為活化的轉錄因子(transcription factor),藉由磷酸根程度的不同,下游各種基因表 現得以適當調控。克雷白氏肺炎桿菌為常見的伺機性病原菌,常造成各種院內感染,在台灣其 造成糖尿病病人肝膿瘍的比率之高,使其成為臨床研究的重要對象。然而,近年來抗生素的濫 用使抗藥性菌株快速散佈,往往造成治療上的隱憂。我們希望藉由對其訊息傳遞系統的研究, 找尋新的藥物標的,並希望此研究能對當前治療對策有所幫助。藉由日本 KEGG 資料庫 (http://www.genome.ad.jp/kegg/pathway/)雙分子系統的分類,我們分析華盛頓大學基因體中 心(Genome Sequencing Center, St. Louis, Missouri, USA)克雷白氏肺炎桿菌 MGH78578 的序 列(99 % 定序完成),發現了 29 個感應蛋白及 26 個反應調控蛋白的基因,原計畫作全面 性的分析,而在過去一年中,我們先挑選因應逆境生存相關的雙分子系統:PhoPQ、PmrAB、 RstAB,建立其啟動子(promoter)報導系統及調控蛋白基因缺損株,進而發現 rstA 以及 pmrA 基因缺損株菌落明顯變小,且克雷白氏菌重要的致病因子:莢膜(capsular polysaccharides, CPS) 生成大幅減少,並使其容易被巨噬細胞吞噬;進一步分析其莢膜型態,發現在 rstA 缺損株中 莢膜聚合體(CPS polymer)較野生株減少最多,暗示 RstAB 可能調控與醣類合成相關基因。 這三組雙分子系統所感應的外在訊息、交互作用,將是未來研究的重點。
二、 英文摘要(Abstract)
Key words: Klebsiella pneumoniae; two-component signaling systems (TCSs); capsular polysaccharides (CPS) biosynthesis; stress-related Response Regulator mutants and TCS regulatory network;
Two-component system (TCS), consisting of a sensory histidine kinase and a response regulator, allows bacteria to sense and respond rapidly to environmental changes through a specific gene activation or repression. During infection, bacterial TCS acts to recognize and respond to the surrounding stress signals and therefore play an important role in bacteria pathogenesis. Signal binding to the periplasmic domain of a sensor protein results in its autophosphorylation, and subsequent phosphorus transfer to the cytoplasmic response regulator ends in a conformational change. The activated response regulator exhibits different levels of affinity to its target promoter, hence proper responses could be controlled in an coordinated manner. Klebsiella pneumoniae, an opportunistic pathogen, often causes nosocomial infections in immuno-compromised patients. In
Taiwan, the high infection rate of liver abscess in diabetic patients draws in researchable attentions in clinical microbiological field. Due to the widespread of antibiotics-resistant K. pneumoniae in recent years, specific efforts have been made in search for improvement of current therapies and new drug targets. Evaluation of bacterial TCSs as drug targets have been reviewed and we hope to perform a genome-wide study on TCSs in K. pneumoniae pathogenesis in order to find better targets for treatments. Through the analysis of KEGG database(http://www.genome.ad.jp/kegg/pathway/), the stress related TCSs, PmrAB, PhoPQ and RstAB, were selected as the main subject in this study. Interestingly, we find that deletion mutants of each gene encoding the response regulators displayed decreasing levels of capsular polysaccharides, a prominent virulence factor of K. pneumoniae species. Quantification of CPS further confirmed the involvement of the TCSs in regulation of CPS production, as also reflected by the increasing number of the bacteria engulfed by macrophages. In addition, analysis of CPS pattern highlights the role of RstAB in CPS polymer formation. The signals and interaction of these TCSs, as well as the target genes, may shed light on the mechanism by which a dramatic change in CPS is observed.
三、 報告內容(Content) 引言(Introduction)
Bacterial two component systems(TCSs),consisting of a sensor histidine kinase and a response regulator, act to cope with the capricious environments(2, 12, 13). More than 10 TCS genes are generally present in bacteria and it is believed that they form regulatory networks to show dependencies and regulatory hierarchies. For instance, some of the TCS sensors have been shown to be able to conditionally transfer the phosphoryl group to their cognate response regulators, as well as to non-cognate response regulators (8). In addition, many TCS proteins including the paralogues have been shown to perform similar or identical function(1). These together indicate that some interacting regulations have to be present for proper and efficient control of the TCS systems. We have recently used the TCS templates classified by the KEGG database ( http://www.genome.ad.jp/kegg/pathway/ ) to analyze the 99% completed genome of K.
pneumoniae MGH 78578(Genome Sequencing Center, St. Louis, Missouri, USA)and revealed the
presence of 29 HKs and 26 RRs(NSC92-2311-B-009-001)encoding genes respectively.
Previously two unorthodox TCS systems KvgAS-orfQ-orfR and KvhAS isolated by genomic subtraction were chosen in order to set up a genome-wide strategy to identify their functional roles. A LacZ reporter system including placZ15, a lacZ reporter in pYC016, and lacZ16, a K. pneumoniae CG43 lacZ deletion mutant have already been constructed. Moreover, we have set up 2D-PAGE technology for a comparative analysis of the protein patterns among the parental strain, kvgA and
kvhA mutants(NSC92-2311-B-009-001; manuscripts in preparation). Preliminary results revealed
that both the LacZ reporter system and the proteomic strategy are promising for us to accomplish the proposal. However, except for kvgAS and kvhAS, here we focus on other three stress-related TCSs, PhoPQ, PmrAB, and RstAB, to verify the functional roles and interacting regulation.
PhoPQ in Escherichia coli and Salmonella enterica, has been reported to govern the magnesium regulon(3, 9, 16). PhoQ protein is a sensor for extracytoplasmic magnesium that modifies the phosphorylated state of the DNA-binding protein PhoP, which has been demonstrated to control the expression of a large number of genes that mediate adaptation to low magnesium environments and/or virulence in S. enterica, Shigella flexneri, Yersinia pestis, Erwinia carotovora,
Neisseria meningitidis, Photorhabdus luminescens and E. coli(3). Another TCS, PmrAB, can be
activated either by magnesium through PhoPQ or by Fe3+, is independent of PhoPQ(6). PmrAB is responsible for modification of the lipopolysaccharide(LPS)layer and therefore conferred resistance to the cationic antibiotic polypeptide polymyxin and intra-macrophage survival in S. enterica(4, 5). Interestingly, the PmrAB system also confers resistance to Fe3+-mediated killing(7). Under low Mg2+ condition, pmrD, a PhoP-activated gene, is required to activate the response regulator PmrA (7). Paradoxically, the pmrD expression was found also to be negatively regulated by PmrAB system under conditions independent of the PhoPQ system(6). The complicated regulation of PhoPQ, PmrAB and PmrD allowed bacteria to mount resistances to host defense upon stimulating by different signals. This emphasized the importance of a coordinated regulation of the TCS network (6). The TCS RstAB has not been characterized except for its regulation by PhoPQ (9), and hence engaged us for the functional role of RstAB in K. pneumoniae PhoP regulon.
研究目的(Specific aims)
We herein propose a genome-wide study to draw an interacting map of the TCSs in K.
pneumoniae CG43, a highly virulent strain of K2 serotype, using both LacZ reporter system and the
proteomic technology. The specific aims proposed to be finished in the past year are:
1) Isolate the putative promoters of the stress related TCSs, PmrAB, PhoPQ and RstAB, from
K. pneumoniae CG43 and generate a promoter-lacZ fusion to monitor the promoter
activities.
2) construct the TCS-RR-deletion mutants by crossover PCR and the mutation effects analyzed
研究材料與方法(Materials and methods)
Allelic exchange strategy for generating gene-specific mutants. The two 1-kb regions flanking the
gene of interest were PCR-amplified, ligated, and subsequently cloned into the suicide vector pKAS46. The resulting plasmid was then mobilized into K. pneumoniae CG43S3 by conjugation for first crossover. A kanamycin-resistant colony of the transconjugants was picked up and refreshed in LB broth. After the event of second crossover, the mutant was serial-diluted, plated, and tested for streptomycin resistance. A kanamycin-sensitive, streptomycin-resistant colony was picked for PCR and Southern blot analysis and designated a mutant.
CPS quantification. CPS is extracted by the hot-phenol method described previously (14) and
carbazole assay exactly as described
Macrophage engulfment assay. Anti-phagocytosis assay is done as previously described (10). Briefly,
0.5 ml of THP-1 cells (ATCC TIB202) (106/ml) were plated onto per well of 24-well tissue culture plates and treated with phorbol 12-myristate 13-acetate (PMA) for 48h to induce differentiation into macrophage-like cells. Bacterial culture is washed twice with PBS and adjusted to 6×108CFU/ml. 50 micro liter of bacterial culture was added to each well containing THP cells that were washed twice with PBS and refreshed in RPMI without fetal bovine serum. After incubation for 2h, well were washed twice and added with fresh RPMI with gentamycin (100g/well) to eliminate extracellular bacteria. After 2h incubation, cells were washed twice with PBS and permeablized with addition of 500lof Triton X-100. Internalized bacteria were determined by plating the cell lysate on LB agar plates.
Determination of CPS pattern. CPS pattern is determined by electrophoresis on 5 % or 10 %
DOC-PAGE and then transferred to a PVDF membrane for immunoblotting and then detected either by anti-K2 polyclonal antiserum or with alcian blue-silver stain as described (11).
結果與討論(Result and discussion)
In this year, we have generated a series of mutant containing the deletions in the genes encoding response regulators, PhoP, PmrA, and RstA, in K. pneumoniae CG43. Phenotypes of these mutants were characterized and some likely roles of the three TCSs have been proposed.
1. Construction and phenotype characterization of the response regulator mutants in K.
pneumoniae CG43
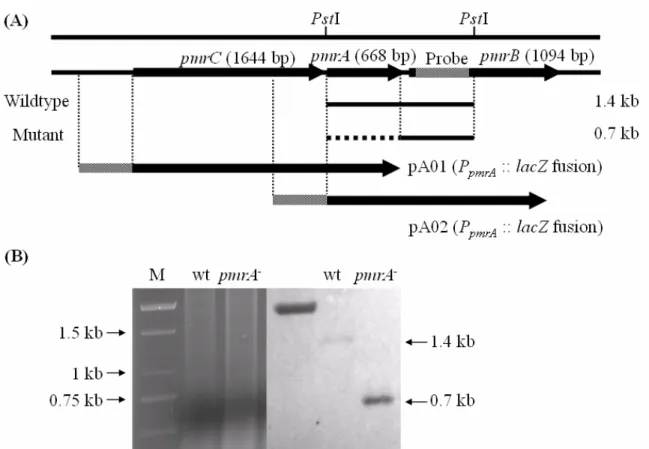
The deletion mutant of K. pneumoniae CG43 was generated by the allelic exchange strategy and confirmed by Southern blot analysis(Fig. 1, 2, and 3). All the mutants exhibited changes in colony morphology and lost viscid consistency, as reflected by failure to form a string(Table 1). In addition, when subjected to low-rate centrifugation, the mutants readily formed a pellet while the parental strain remained in suspension(Fig. 4A). It was suspected that the CPS structure was altered in each mutant. However, the TCS PmrAB was reported in Salmonella to modify the LPS lipid A portion but not CPS structure, so further evidences will be needed to support our hypothesis.
2. Determination of the CPS contents and anti-phagocytic properties of each mutant
The CPS core structure of K. pneumoniae K2 serotype hasbeen determined as[ →)4-Glc-(1→3)-α-Glc-(1→4)-β-Man-(3←1)-α-GlcA)-(1→]n, in which glucuronic acid is the core
component. We therefore utilized glucuronic acid contents as the measurement of CPS amount. Subsequent quantification showed that when compared to the wild type, all the mutants exhibited a significant reduction of CPS and a higher susceptibility to engulfment by the human macrophage THP-1(Table 1). The mannose content which determines the degree of phagocyte engulfment via macrophage mannose receptor was found to be relatively low in K2 CPS, and thus the high
resistance of the wild type strain may result from the shielding effect of CPS. In contrast, the increased susceptibility to lectino-phagocytosis in the mutants may result from decreasing amounts of CPS. The CPS amounts in phoP and pmrA mutants are approximately the same, but the pmrA mutant not only forms a smaller colony but reveals about three-fold susceptibility to macrophage engulfment than the phoP mutant, indicating an important role in regulation of CPS production that has not been found.
3. Dissection of CPS structure of pmrA and rstA mutants.
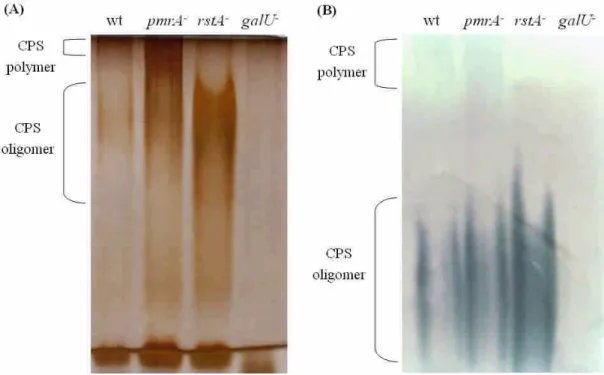
The models for assembly and translocation of CPS oligomers into polymers have been proposed in E. coli, in which the Group I CPS model may be applied in K. pneumoniae. Analysis of the CPS composition in the CPS-deficient mutants may provide cues in the functional role of the gene deleted. Since the colony morphology of pmrA mutants resembles that of rstA mutant while both mutants show different levels of CPS contents, we use alcian-blue silver stain and Western blot to dissect their CPS patterns. As Figure 5 shows, a significant increase of CPS oligomers were found in the
rstA mutant; while in the pmrA mutant only a slight increase was observed. In addition, the CPS
polymers, as revealed by Western blot, were retained in both the wild-type strain and pmrA mutant but lost in the rstA and galU mutant, which is deficient in both CPS and LPS. Such difference in CPS patterns imply the role of RstAB in regulation of enzymes required for oligosaccharides to assemble into polymers or enzymes that are required for translocation of CPS oligomers into periplasmic space for further polymerization.
Figure 1. Demonstration of construction of an rstA deletion mutant and the promoter region used in reporter assays. (A) Gene organization. (B) Southern blot analysis.
Figure 2. Demonstration of construction of a pmrA deletion mutant and the promoter region used in reporter assays. (A) Gene organization. (B) Southern blot analysis.
Figure 3. Demonstration of construction of a phoP deletion mutant and the promoter region used in reporter assays. (A) Gene organization. (B) Southern blot analysis.
Figure. 4 Phenotype analysis of the respective response regulator mutants. (A) Sedimentation test (B) Comparison of CPS contents in wild-type and the mutants.
Figure 5. CPS pattern of the pmrA-and rstA-mutants, and the wild-type strain. The bacterial CPS was extracted by the hot phenol method from the overnight culture and subjected to alcian-blue silver stain (A) or Western blot analysis (B) using anti-K2 CPS polyclonal antibody.
參考文獻(Reference)
1. Chen YT, Chang HY, Lu CL, Peng HL. 2004. Evolutionary analysis of the two-component systems in Pseudomonas aeruginosa PAO1. J Mol Evol. 59(6):725-37.
2. Cotter, P. A, and J. F. Miller. 1998. In vivo and ex vivo regulation of bacterial virulence gene expression. Current Opinion in Microbiology 1:17-26.
3. Groisman E. A., 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J
Bacteriol. 183(6):1835-42.
4. Gunn JS, Miller SI. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a
two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 178(23):6857-64.
5. Heithoff DM, Conner CP, Hanna PC, Julio SM, Hentschel U, Mahan MJ. 1997. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci U S A. 94(3):934-9. 6. Kato A, Latifi T, Groisman EA. 2003. Closing the loop: the PmrA/PmrB two-component
system negatively controls expression of its posttranscriptional activator PmrD. Proc Natl Acad
Sci U S A. 100(8):4706-11. Epub 2003 Apr 3.
7. Kox LF, Wosten MM, Groisman EA. 2000. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 19(8):1861-72.
8. Majdalani N., Heck M., Stout V., Gottesman S. 2005. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J Bacteriol. 187(19):6770-8.
Utsumi R. 2003. Identification and molecular characterization of the Mg2+ stimulon of
Escherichia coli. J Bacteriol. 185(13):3696-702
10. Perez-Perez GI, Shepherd VL, Morrow JD, Blaser MJ. 1995. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect Immun. 63(4):1183-7.
11. Rahn, A., and C. Whitfield. 2003. Transcriptional organization and regulation of the
Escherichia coli K30 group 1 capsule biosynthesis (cps) gene cluster. Mol. Microbiol.
47:1045–1060.
12. Soncini, F. C, and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801.
13. Stock, J. B, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53: 450-490.
14. Whitfield C, Perry MB, MacLean LL, Yu SH. 1992. Structural analysis of the O-antigen side chain polysaccharides in the lipopolysaccharides of Klebsiella serotypes O2(2a), O2(2a,2b), and O2(2a,2c). J Bacteriol. 174(15):4913-9.
15. Wosten MM, Kox LF, Chamnongpol S, Soncini FC, Groisman EA. 2000. A signal transduction system that responds to extracellular iron. Cell. 103(1):113-25.
16. Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F, Hare JM, Huang H, Groisman EA. 2005. Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica.
Proc Natl Acad Sci U S A. 102(8):2862-7.
四、 計畫成果自評
In this year, the specific goals have been achieved 100% as expected which include isolation of the genes encoding PhoPQ, PmrAB, and RstAB TCSs from K. pneumoniae CG43, generation of the respective response regulator mutants and also the promoter-lacZ fusion constructs. In addition, a manuscript concerning interactions between PhoPQ and RstAB is now in preparation. Most interestingly, phenotypic analysis of the three mutants including growth, CPS content and pattern, capability of antiphagocytosis, biofilm formation, and polymyxin susceptibility revealed that the TCSs PhoPQ, PmrAB, and RstAB most likely form an interacting circuit. Molecular signals for each of the TCS and the interacting pathway of the three TCSs are being determined and hopefully in the near future we will be able to build up the interacting network.