Variation in molecular stacking resulting from the diVerent
polarity of liquid crystalline molecules: synthesis and study of azo
dye compounds
LONG-LI LAI*, ESHIN WANGDepartment of Applied Chemistry, National Chi Nan University, Puli, Taiwan 545, ROC
YI-HUNG LIU and YU WANG
Instrumentation Center and Department of Chemistry, National Taiwan University, Taipei, Taiwan 106, ROC
(Received 28 December 2001; accepted 7 February 2002)
To investigate the eVect of molecular polarity on the packing of liquid crystalline molecules, two liquid crystals,N,N-disubstituted aminophenylazo-4-alkylbenzenes, were synthesized and studied by single crystal structure determination. A comparison of the resulting molecular stacking with that ofN,N-disubstituted aminophenylazo-4-butylbenzoate was made.
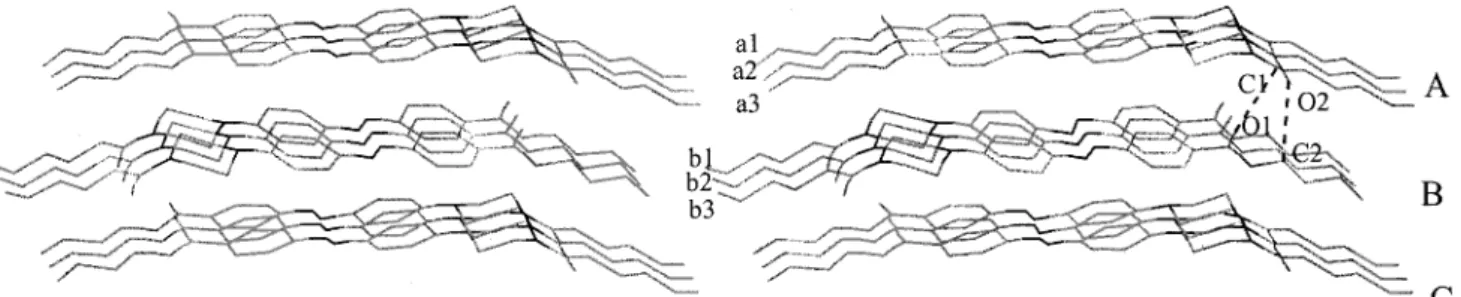
1. Introduction stacking of compound 1 is shown in gure 1. Molecules in the same layer are directed head-to-head and tail-Supramolecular aggregatio n by molecular self-assembly
is an important issue in the eld of structural chemistry to-tail (for example, molecules a1–a3 in the layer A), but molecules in adjacent layers are directed by a head-[1]. In addition to electrostatic interaction, non-covalent
forces also play a signi cant role in determining the to-tail arrangement (for example, molecules a1–a3 in layer A and molecules b1–b3 in layer B) [5a]. Apparently,
structural stacking and properties of molecular assemblies
[2]. The interaction between the functional molecules the carboxylate and amido groups in compound 1 are both strongly polar moieties. For greatest molecular is found to be critical for molecular packing in
crystal-lization [3]; furthermore the stability and phase behaviour attraction and minimum dipole moment in the solid state, the carboxylate group of one molecule is directed of mesogenic molecules have been reported to arise
therefrom [4]. Previously, we studied two liquid crystals, toward the amido group of another molecule in an adjacent layer. The closest distance between diVerent azo dyes 1 and 2, by single crystal structure
deter-mination [5]. During exadeter-mination of their molecular layers (not including hydrogen atoms) thus appears in the vicinity of these groups, and the distances of the stacking, we found that the polarity of the liquid crystals
aVects the molecular arrangement during crystallization. C1–O1 and C2–O2 between A and B layers are 3.48 and 3.25 AÃ , respectively. For a better understanding of On the basis of crystallographi c study, the molecular
Figure 1. The molecular stacking of azo dye 1. *Author for correspondence; e-mail: lilai@ncnu.edu.tw
L iquid Crystals ISSN 0267-829 2 print/ISSN 1366-585 5 online © 2002 Taylor & Francis Ltd http://www.tandf.co.uk/journals
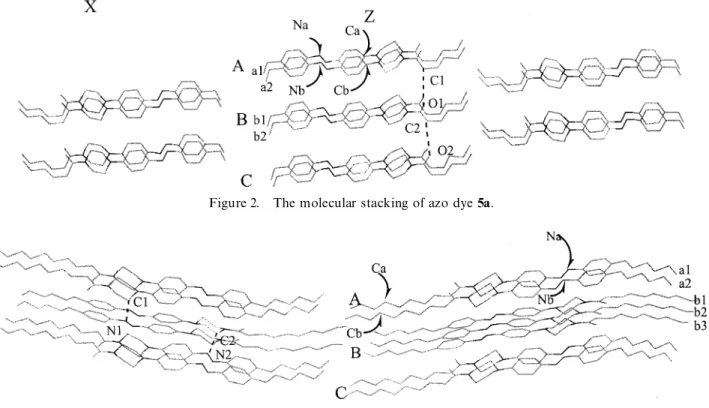
Figure 2. The molecular stacking of azo dye 5a.
Figure 3. The molecular stacking of azo dye 5b.
the molecular stacking of liquid crystals with diVerent 392.2576; found 392.2578. 5b. 16.5%.1H NMR d (CDCl 3) 0.78–0.88 (m, 6H, 2Ö CH3), 1.23–1.69 (m, 22H, 11Ö CH2), polar moieties, a similar azo dye compound without the
carboxylate group has been synthesized and studied by 2.35 (t, 2H, CH2), 2.65 (t, 2H, CH2), 3.34 (s, b, 4H, 2CH2), 3.67 (s, b, 2H, CH2), 3.80 (s, b, 2H, CH2), 7.01 single crystal structure determination. As the driving
force for the molecular stacking could be diVerent from the (d, 2H, J5 8.7 Hz, 2Ö Ar–H ), 7.27 (d, 2H, J5 8.1 Hz, 2Ö Ar–H), 7.78 (d, 2H, J5 8.7 Hz, 2Ö Ar–H), 7.92 case of azo dye 1 which contains amido and carboxylate
groups, compounds 5a and 5b containing only an amido (d, 2H, J5 8.1 Hz, 2Ö Ar–H). HRMS for C32H48N4O: 504.3828; found 504.3830.
group were accordingly prepared and their molecular stackings and mesogenic behaviours investigated. We
now wish to report preliminary results. 3. Characterization and results
Compounds 5a and 5b were studied by single crystal structure determination†, and their correspond-2. Synthesis
Compounds 5a and 5b were synthesized according to ing molecular stackings are shown in gures 2 and 3, respectively. In gure 2, molecules of 5a in the same the scheme. Compound 3 was rst obtained by reaction of
N-phenylpiperazin e with alkanoyl chloride in dichloro- column are directed regularly head-to-head and
tail-to-tail (for example, molecules a1–a2 and b1–b2 in methane in an almost quantitative yield. The diazonium
salt 4 was then prepared according to the literature column Z). Furthermore, molecules are found to be method [6]; compounds 5a and 5b were subsequently
obtained in about 15% yields, and characterized to be the correct compounds by NMR ( Varian 300) and high
† Crystal data, 5a: C24H32N4O, crystal dimension 0.45Ö resolution mass spectroscopy ( VG70-250; EI, 70ev). 0.25Ö 0.10 mm3,P2
1, monoclinic,a5 7.602(2), b 5 5.7559(12), 5a. 17.1%. 1H NMR: d (CDCl
3) 0.94 (t, 3H, CH3), c5 49.912(10 ) AÃ, b 5 90.19(3)ß , Z 5 4, rcalcd5 1.194 g cmÕ 3, re ections measured5 12334, re ections used 5 3830 ((Rint)5 1.33 (t, 3H, CH3), 1.69–1.78 (m, 6H, 3Ö CH2), 2.41
(0.0390) ),R15 0.0873, [I>2s(I)], R15 0.1083 (all data), GOF (t, 2H, CH2), 2.75 (quart, 2H, CH2), 3.38 (s, b, 4H, onF25 1.158. 5b: C 32H48N4O, crystal dimension 0.35Ö 0.25Ö 2CH2), 3.68 (t, 2H, CH2), 3.82 (t, 2H, CH2), 7.01 0.02 mm3, P2 12121, orthorhomic,a5 5.724(2), b 5 7.364(2), (d, 2H, J5 8.7 Hz, 2Ö Ar–H), 7.34 (d, 2H, J5 8.1 Hz, c5 67.76(2) AÃ, Z5 4, r
calcd5 1.174 g cmÕ 3, re ections measured5
2Ö Ar–H), 7.82 (d, 2H, J5 8.7 Hz, 2Ö Ar–H), 7.91 8198, re ections used5 4980 ((R
int)5 (0.0843 )), R15 0.0953, [I>2s(I)], R15 0.1760 (all data), GOF on F25 1.003. (d, 2H, J5 8.1 Hz, 2Ö Ar–H ). HRMS for C24H32N4O:
Scheme.
parallel to the adjacent molecules in the same layer (for plane containing diazobenzene moieties of the molecule in layer A is approximately parallel to the corresponding example, molecules a1–a2 in layer A) and the distances
between two corresponding atoms in the same layer (for plane in layer B and the distance between two parallel planes is about 3.8 AÃ , which is half of the distance between example, Na–Nb and Ca–Cb) are all about 7.6 AÃ . The
Table. Phase transition temperature (ß C) and corresponding enthalpies (J gÕ 1), in parentheses, of 5. The phase transition temperatures and corresponding enthalpies of 5 were determined by 2nd DSC scans using a Perkin Elmer DSC-6 at heating and cooling rates of 10ß C minÕ 1 between 50 and 220ß C. Abbreviations: Cr5 crystalline, SmX 5 unidenti ed smectic phase, SmX¾5 unidenti ed smectic phase, SmA 5 smectic A phase, I 5 isotropic liquid.
5a, n5 2 Cr1 , GGG ) 125.7(40.1) 56.6 SmX , GGG ) 138.6 63.0b SmX¾ , GGG ) 141.1a 137.6(3.6) SmA , GGG ) 187.4(11.6) 180.0(12.8) I R5 C5H11 5b, n5 6 Cr1 , GGGG ) 104.1(16.0) 56.6 SmX , GGGG ) 154.1(6.8) 153.1(5.4) SmA , GGGG ) 195.1(15.5) 192.7(14.5) I R5 C9H19
aThe peaks are overlapped and their total enthalpy is 4.5 J gÕ 1. bThe peaks are overlapped and their total enthalpy is 26.5 J gÕ 1.
corresponding atoms in the same layer. The closest the crystallizing process and is believed to be the major contribution to stabilization of the molecular stacking distance between two adjacent layers (not including
hydrogens) is 3.88 AÃ , found, for example, at the C1–O1 of compound 5b.
Based on the previous results, we may reasonabl y regard between layers A and B as well as at C2–O2 between
layers B and C. The distances O1–H (at C1) and O2–H that for a bi-polar compound such as azo dye 1, the polarity of functional groups determines the orientation (at C2) are both 3.01 AÃ , which are in the range of a
normal hydrogen bond [7a]. After careful examination of the molecular arrangement in the crystal structure. However, for mono-polar compounds such as azo dyes of molecular stacking, we do not nd that the interatomic
distance in diVerent molecules is closer than this value. 5a and 5b, the intermolecular hydrogen bonding inter-action is most in uential for the molecular stacking. We thus believe that the driving force for the stabilization
of molecular stacking of azo dye 5a mostly arises from Additionally, it is interesting to note that molecule 5a is in a linear arrangement, but there is a slight bending intermolecular hydrogen bonding.
In gure 3, molecules of 5b are directed regularly head- between the piperazine and decanoyl moieties in structure 5b. The hexyl tail at the other end of molecule 5b makes to-head and tail-to-tail in the same layers (for example,
molecule a1–a2 in layer A). However, molecules in this bending more pronounced and thus the whole molecule is arranged in a slightly curved shape. Compounds 5a adjacent layers are arranged head-to-tail (for example,
molecules a1–a2 in layer A and molecules b1–b3 in and 5b both show a SmA phase in thermal cycling, characterized by a focal-conic texture under polarizing layer B). In the same layer, the molecules are found to
be parallel to adjacent molecules and the distances optical microscopy (see the table), which is diVerent from the mesogenic behaviour of azo dye 1 which shows a between two corresponding atoms in the same layer
(for example, Na–Nb and Ca–Cb) are all about 5.72 AÃ . SmC phase [5a].
Based on the crystallographi c study, the extended Molecules in diVerent layers are not parallel to each
other. The intercept angle between the plane containing molecular lengths of azo dyes 5a and 5b are calculated to be 24.08 and 32.83 AÃ , respectively, which are quite diazobenzene moieties of the molecules in layer A and
the corresponding plane in layer B is estimated at about close to the corresponding d-spacing distances of azo
dyes 5a and 5b in the SmA phase, obtained from powder 40ß on the basis of crystallographic data. The closest
distance between two adjacent layers (not including X-ray diVraction (XRD)‡. Although the amido moiety hydrogens) is 3.37 AÃ , which is, for example, found at the
C1–N1 between A and B layers, as well as C2–N2 ‡ The d-spacings of compound 5a are 24.95, 25.09, 25.09, 25.11, 25.24 AÃ at 180, 170, 160, 150, 140ß C, respectively, during between B and C layers. The distances of N1–H (at C1)
cooling; thed-spacings of compound 5b are 32.88, 32.86, 32.70, and N2–H (at C2) are both 2.82 AÃ which is comparable
2.45, 32.41 A´ at 188, 185, 175, 165, 155ß C, respectively, during to the sum of the van der Waals radii of H and N cooling. Powder XRD patterns were obtained from a Siemens (Bondi radius: H 1.20, N 1.55) [7b]. The H-bond D-5000 X-ray DiVractometer equipped with a TTK 450
temperature controller. interaction arising therefrom should be in uential in
of compounds 5a and 5b may vibrate up and down We thank the National Chi Nan University and the National Science Council (NSC 90-2113-M-260-00 2 ) during the thermal process, the extended length of the
for nancial support. The National Center of High-molecules may not change much as a result. According
Performing Computing and the Institute of Chemistry, to the literatures, the N-substituent of the piperazine
Academia Sinica are also highly appreciated for pro-favours equatorial conformation, and the energy barrier
viding the Beilstein database system, as well as a most between the equatorial and axial conformations is only
helpful library service and the XRD apparatus , respectively. about 1.9–4 kcal molÕ 1 [8]. As we have shown in the
case of azo dye 1 [5a], the N-substituent in either
References equatorial or axial conformation does not signi cantly
[1] (a) Lehn, J.-M., 1995, Supramolecula r Chemistry (Weinheim: change the extended molecular length. Thus thed-spacing
VCH ); (b) Hamilton, A. D., 1996, Perspectives in of the molecules will not vary much during thermal
pro-Supramolecular Chemistry (John Wiley). cessing; this is consistent with the observations obtained
[2] (a) Hunter, C. A., 1993, Angew. Chem. int. Ed. Engl., 32, from powder XRD. 1584; (b) Hunter, C. A., 1994, Chem. Soc. Rev., 23, 101.
[3] (a) Desiraju, G. R., 1989, Crystal Engineering: T he Design of Organic Solids (New York: Elsevier); (b) Desiraju, G. R., 1997, Chem. Commun., 1475.
4. Conclusion
[4] (a) Lee, K. M., Lee, C. K., and Lin, I. J. B., 1997, Angew. While exploring crystal stacking with varying molecular
Chem. int. Ed. Engl., 36, 1850; (b) Chang, J., and polarity, compounds 5a and 5b were investigated by
Moore, J. S., 1994,J. Am. chem. Soc., 116, 2655. crystallography and the study of mesogenic behaviour. [5] (a) Lai. L. L., Lee, L. J., Lee, G. H., Wang, Y., Lu, K. L., As mentioned previously, for the bi-polar azo dye 1, the and Lee, S. J., 2001, L iq. Cryst., 28, 1513; (b) Lai, L. L., Lin, Y. J., Ho, C. H., Wang, E., Liu, Y. H., Wang, Y., mutual attraction from amido and carboxylate groups
Lin, Y. C., and Cheng, K. L., 2001, Helv. Chim. Acta of diVerent molecules aVects the molecular stacking in
(in the press). the crystal structure. For the mono-polar azo dyes 5a
[6] McNab, H., and Monahan, L. C., 1989, J. chem. Soc. and 5b, intermolecular hydrogen bonding is the major Perkin T rans. 1, 419.
contribution to stabilization of the molecular stacking. [7] Allen, F. H., Lommerse, J. M. P., Hoy, V. J., Howard, J. A. K., and Desiraju, G. R., 1996, Acta These results provide not only a better understanding
Cryst., B52, 734; (b) Bondi, A., 1964, J. phys. Chem., of the eVect of the functional group and hydrogen
68, 441.
bonding on the stacking of liquid crystals in the solid [8] (a) Baker, V. J., Ferguson, I. J., Katritzky, A. R., and state, but also provide a useful guide for the future Patel, R., 1976, T etrahedron. L ett., 4735; (b) Niemeyer,
H. M., 1979, J. mol. Struct., 57, 241. design of desired materials.