GENERAL PAPERS Organized by G. Coimbatore
Symposia Papers Presented Before the Division of Environmental Chemistry American Chemical Society
Washington, DC August 28 - September 1, 2005
Effect of iron content on bimetallic iron-aluminum particles for dechlorination of carbon tetrachloride
Hsing-Lung Lien and Wen-Shan Lee
Department of Civil and Environmental Engineering, National University of Kaohsiung, 700 Kaohsiung University Rd., 811 Kaohsiung, Taiwan.
E-mail: lien.sam@nuk.edu.tw; Fax: 011886-7591-9376,
Abstract
In this paper, we present a novel material, bimetallic Fe/Al particles, for effective degradation of carbon tetrachloride. Bimetallic Fe/Al was prepared under acidic conditions where iron was readily deposited onto aluminum surface. The bimetallic Fe/Al is designed to take advantage of zero-valent aluminum for serving as an electron donor to prevent the formation of passive layer from iron corrosion and maintain the surface reactivity of iron. In this study, iron content ranging from 5% to 45% (by weight) was conducted. XRD analysis indicated a minimum full surface coverage of iron at the aluminum surface was determined when the iron to aluminum ratio was equal to 15%. Batch experiments, that were carried out to investigate the product distribution and reaction kinetics, indicated the iron to aluminum ratio of 15% exhibited optimum performance for bimetallic Fe/Al. Excessive or insufficient amounts of iron deteriorate the effectiveness of bimetallic Fe/Al.
Introduction
Zero-valent iron (ZVI) has been successfully used for the dechlorination of organic environmental contaminants in subsurface. However, the accumulation of hydroxide precipitates onto the iron surface resulting in the loss of iron reactivity over time has emerged as a critical issue that needs to be addressed. Recently, many novel materials such as zero-valent bimetals (e.g., Pd/Fe, Cu/Fe and Cu/Al), nanoscale iron
particles, and nanoscale bimetallic particles have been shown a better performance in remediation of chlorinated organic compounds compared to ZVI [e.g., 1-4]. We have reported the use of bimetallic Cu/Al for reductive degradation of chlorinated methanes [2]. Aluminum was selected because it is an effective electron donor (the standard reduction potential of -1.667 V) under both acidic and alkaline conditions. To prevent the formation of passive layer on the iron surface, we developed a novel material, bimetallic Fe/Al particles. Bimetallic Fe/Al particles consist of a core metal (aluminum) and a shell metal (iron). Unlike conventional ZVI technology that iron acts as an electron donor, bimetallic Fe/Al particles are designed to use aluminum as a source of electrons that may keep the iron surface from corrosion and maintain the surface reactivity of iron.
In this paper, the objective was aimed at investigating the feasibility of using zero-valent aluminum as the source of electrons to prevent the formation of iron oxide and maintain a fresh surface of iron so that the iron-mediated dechlorination can be effectively preceded without declining the reactivity of iron. The structure of Fe/Al was determined by X-ray diffraction (XRD). Effects of iron content on the effectiveness of Fe/Al for the degradation of carbon tetrachloride were examined.
Experimental Section
Preparation of bimetallic Fe/Al particles
Bimetallic Fe/Al particles were prepared in a fume hood under ambient temperature and pressure. All chemicals are analytic grade or better. Five sets of bimetallic Fe/Al particles at different iron content, 5%, 15%, 25% 35% and 45% by weight, were prepared. To prepare the iron to aluminum ratio of 25%, 2.4 g of aluminum powder was added into a 1000 mL glass beaker containing 2.9 g FeCl3•6H2O in 25 mL deionized water and the suspension was mixed with a magnetic stirrer. 10 mL of concentrated HCl was slowly added to the glass beaker. Immediate fume evolution was observed. 10 mL of distilled water was added quickly to dissipate heat for 30 seconds. Under acidic conditions, ferric ions were reduced to elemental iron and deposited onto the aluminum surface to for bimetallic Fe/Al.
0 0 3 3 Al Fe Al Fe (1)
Bimetallic Fe/Al particles were then washed by 2 L deionized water and harvested via vacuum filtration. Assuming that all the ferric ions were reductively precipitated onto the aluminum surface, the ratio of iron to aluminum was calculated to be 25% by weight. Identical procedures with different ferric ion concentrations were conducted to prepare bimetallic Fe/Al at different iron contents.
Batch experiments
Batch experiments were conducted in 150 mL serum bottles. In a typical experiment, the initial concentration was approximately 31.7 mg/L. The metal loading of bimetallic Fe/Al was 3 g/100 mL. The serum bottles were then capped with Teflon Mininert valves and mixed on a rotary shaker (30 rpm) at room temperature (22±1°C).
Analytic methods
Concentrations of carbon tetrachloride and its intermediates were measured by a headspace-gas chromatograph (GC) method. Headspace samples (50-µl gas aliquot) were analyzed by a HP4890 GC-FID equipped with a DB-624 capillary column (J&W, 30 m 0.32 mm). Temperature conditions were programmed as follows: oven temperature at 45°C for 5 minutes; injection port temperature at 250°C; and detector temperature at 300°C.
XRD Analysis
Solid phase analysis was conducted by a X-ray diffractometer (Rigaku Co.) at 40 keV and 20 mA. The instrument used copper target tube radiation (Cu Kα) to produce X-rays with a wavelength of 1.54056 Å . Samples were placed on a quartz plate and were scanned from 5° to 80° (2θ) at a rate of 5° 2θ/minute.
Kinetics Analysis
The rate of transformation for carbon tetrachloride in a batch system is described as a pseudo-first-order rate model:
C dt dC obs k (2)
Where C is the concentration of carbon tetrachloride (mg/L); kobs is the observed rate constant (h-1) and t is time (h).
Results and Discussion
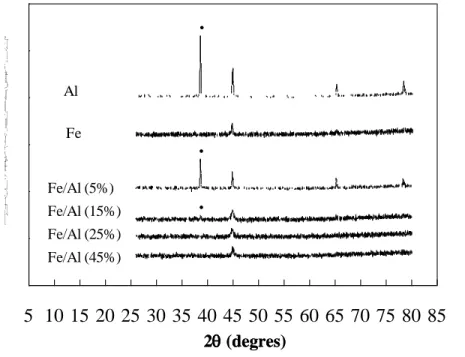
XRD measurements were conducted to examine the structure of bimetallic Fe/Al particles. Figure 1 illustrates the XRD patterns of zero-valent aluminum, zero-valent iron, and bimetallic Fe/Al at different iron content ranging from 5% to 45%. The characteristic peaks of aluminum appeared at 38.6°, 44.9°, 65.2°, and 78.5° where the main diffraction peak is near at the diffraction angle (2) of 38.6°. The peaks assigned to iron were at the positions at 44.7° and 65.0° (insignificant). It is apparent that both metals have similar XRD patterns at diffraction angle between 38° and 70°. Nevertheless, XRD analysis still provided useful insight into the structure of bimetallic Fe/Al. At the iron content of 15%, a minimum full surface coverage of iron on bimetallic Fe/Al particles was achieved. The main diffraction peak (38.6°) is nearly negligible and characteristic peaks of aluminum at 65.2° and 78.5° were not observed. This also provided clear evidence that the peak at near 44.8° is associated with the iron. Further, XRD measurements indicated when the iron to aluminum ratio was equal or more than 25%, the surface of bimetallic particles was completely dominated by iron because the main diffraction peak (38.6°) and other characteristic peaks of aluminum at 65.2° and 78.5° were not observed. Below 15% of iron content, the surface of bimetallic Fe/Al particles was consisted with both aluminum and iron. For example, the iron to aluminum ratio at 5%, XRD analysis clearly exhibited the characteristic peaks of aluminum at 38.5°, 44.7°, and 65.2°. It is worthy of note that neither aluminum oxide
nor iron oxide was formed on Fe/Al surface. This may be attributed to the acidic wash of aluminum during the preparation of bimetallic Fe/Al particles.
Figure 1. XRD patterns for iron, aluminum, and bimetallic Fe/Al at various iron content. The solid circle indicated the position of the main peak of aluminum.
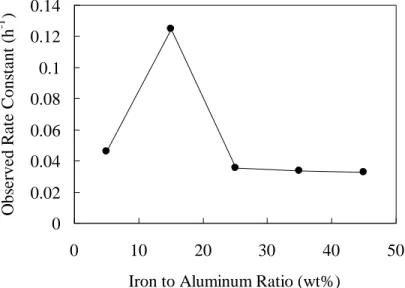
Effects of iron content on the effectiveness of Fe/Al for the degradation of carbon tetrachloride are given in Figure 2. The iron to aluminum ratio ranged from 5% to 45%. Kinetic analysis indicated the degradation of carbon tetrachloride by Fe/Al follows the behavior of first-order kinetics (R2 = 0.995). The maximum degradation rate was found at the iron to aluminum ratio of 15%, which was approximately 3-4 times greater than the rest of ratios. As discussed above, the minimum full surface coverage of iron was determined when the iron to aluminum ratio reached to 15%. Taken together, these results indicated that the bimetallic Fe/Al particles with a minimum full surface coverage of iron have the best dechlorination efficiency.
As shown in Figure 2, no significant difference between the ratio of 25% and 45% was observed in terms of the degradation rate. This indicated that the excessive amount of iron did not enhance the degradation rate and from application points of view, tended to increase costs. The degradation rates remained relatively constant at higher iron content could therefore be attributed to the formation of full coverage of iron at aluminum surface. Because the degradation of carbon tetrachloride is a surface-mediated reaction [5], electrons transferred from inner aluminum may be limited by the excessive iron. As a result, the performance of Fe/Al at higher iron content turned out to behave as iron alone. At the lower iron content (iron to aluminum ratio of 5%), the slow degradation of carbon tetrachloride by Fe/Al was also observed. Further, XRD measurement indicated the surface was partially covered by iron. Because the
2(degres) 2(degres) 0 500 1000 1500 2000 2500 3000 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 Al Fe Fe/Al (5%) Fe/Al (15%) Fe/Al (25%) Fe/Al (45%)
dechlorination reaction preferentially occurred at the Fe/Al surface, the outer surface of Fe/Al without the coverage of iron inevitably deteriorated the effectiveness.
Figure 2. Effects of iron content on the effectiveness of Fe/Al for the dechlorination of carbon tetrachloride.
Acknowledgements
The authors would like to thank National Science Council (NSC), ROC for finical supporting this work through NSC contract number 93-2211-E-390-006-.
References
1. Fennelly, J. P. and A. L. Roberts, “Reaction of 1,1,1-Trichloroethane with Zero-Valent Metals and Bimetallic Reductants,” Environ. Sci. Technol., 32, 1980-1988 (1998).
2. Lien, H-L. and W-X. Zhang, “Enhanced Dehalogenation of Halogenated Methanes by Bimetallic Cu/Al,” Chemosphere, 49, 371-378 (2002).
3. Lien, H-L. and W-X. Zhang, “Hydrodechlorination of Chlorinated Ethanes by Nanoscale Pd/Fe Bimetallic Particles,” J. Environ. Eng., 131, 4-10 (2005).
4. Boronina, T., K. J. Klabunde and G. Sergeev, “Destruction of Organohalides in Water Using Metal Particles: Carbon Tetrachloride/Water Reactions with Magnesium, Tin, and Zinc,” Environ. Sci. Technol., 29, 1511-1517 (1995).
5. Weber, E. J. “Iron-Mediated Reductive Transformations: Investigation of Reaction Mechanism,” Environ. Sci. Technol., 30, 716-719 (1996).
0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0 10 20 30 40 50
Iron to Aluminum Ratio (wt%)
O bs e rve d Ra te Cons ta nt (h -1 )