From the 1Department of Neurology, Taipei Medical University; 2College of Nursing, Taipei Medical University; 3Department of Psychosomatic Medicine, Taipei City Psychiatric Center, Taipei City Hospital; 4Sleep Center, Taipei Medical University Hospital; 5School of Medical Technology, Taipei Medical University, Taipei, Taiwan; 6Department of Neurology, Taipei Medical University, Shuang Ho Hospital, Taiwan.
Received March 25, 2008. Revised May 14, 2008. Accepted October 6, 2008.
Reprint requests and correspondence to: Chaur-Jong Hu, MD. Department of Neurology and Sleep Center, Taipei Medical University Hospital, No. 252, Wu-Hsing Street, Taipei 110, Taiwan.
E-mail: chaurjongh@tmu.edu.tw
Low Sleep Efficiency in Patients with Cognitive Impairment
Jia-Ming Yu
1, Ing-Jy Tseng
2, Rey-Yue Yuan
2, Jau-Jiuan Sheu
2, Hsing-Cheng Liu
3,
and Chaur-Jong Hu
1,4,5,6Abstract- Alzheimer’s disease (AD) is the most common cause of dementias. Mild cognitive impairment
(MCI) indicates the situation that a person has memory complaints and mild objective cognitive impairment but no evidence of dementia. Sleep disturbance, one of the behavioral and psychological symptoms of dementia (BPSD), frequently occurs in patients with AD or MCI. The alteration of sleep architectures in AD patients remains inconclusive. In this study, we conducted the polysomnography (PSG) examination among patients with mild AD with cholinesterase inhibitors (N=10) or MCI (N=12) and age-matched non-demented controls (N=13). The results showed sleep efficiency, which was one of the important parameters for sleep quality was significantly lower in patients with MCI and AD (N=22), 79.14Ų11.06 % vs. 67.07Ų 19.10 %, p=0.046. There were no statistic differences of sleep architecture but a trend of REM insufficiency in patients with MCI or AD. The mean scores of geriatric depression score (GDS) and Epworth sleepiness scale (ESS) did not differ among the three groups. Our study implicated maintenance of sleep was impaired in patients with cognitive impairment and it was independent with depressive symptoms.
Key Words: Dementia, Alzheimer’s disease, Mild cognitive impairment, Sleep, Polysomnography
Acta Neurol Taiwan 2009;18:91-97
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease, characterized by insidious onset, memory deter-ioration, cognitive functions impairment, behavioral dis-turbances and various kinds of psychiatric manifesta-tions(1)
. AD is the most common cause of dementias.
The etiology of AD is still obscure. The neuropathologi-cal landmarks of AD include neuritic senile plaques, neurofibrillary tangles, and neuronal loss. Only a few drugs are approved for AD therapy. One group is cholinesterase inhibitor and the other group is N-methyl D-aspartate (NMDA) receptor inhibitor. Unfortunately, these medications only could slow down the progression
of AD so their cost-effective is still controversial(2)
. Mild cognitive impairment (MCI) usually defines a transitional stage between normal ageing and dementia, usually in terms of AD. MCI indicates the clinical situa-tion of subjective memory complaints and objective evi-dence of mild cognitive impairment but no evievi-dence of dementia(3)
. The disease validity of MCI has been sup-ported by conversion rates to AD of about 12% annually and 80% at 6 years follow-up(4,5)
. MCI could be the pre-clinical or very early stage of AD. But, the diagnosis cri-teria for MCI are still controversial(6)
.
Sleep disturbance and circadian disarrangement are both common manifestations of behavioral and psycho-logical symptoms of dementia (BPSD)(7). BPSD also
commonly occurs in patients with MCI(8)
. During sleep, there usually are one or few cycles containing rapid eye movement (REM) sleep and non-REM sleep in each cycle. REM sleep is linked to dreaming and it is with characteristic physiological presentations, including rapid eye movement, generalized hypotonia and alter-ation of autonomic nervous tone(9)
. Recent researches have raised the association between REM sleep and some specif ic forms of memory, especially in sleep deprivation studies(10)
. Sleep disturbance could be an indicator for poor outcome of dementia and it usually causes a heavy burden for care-givers of dementia(8,11)
. In AD animal models, sleep disturbance was found to be associated with the brainstem cholinergic neurons degeneration. These implicate that sleep disturbance is an important manifestation of AD(12,13)
. The compelling evidence supports memory consolidation during sleep. Especially, rapid eye movement (REM) stage is crucial for some visual-spatial memory and specific task-learn-ing(14)
. The initiation of REM sleep is mainly acetyl-choline-dependent(15)
. Cholinesterase inhibitors which can increase intracerebral acetylcholine levels have become the standard therapy of Alzheimer’s disease (AD) for improving cognitive function(16)
. Therefore, the-oretically, REM sleep should be insufficient in AD or MCI patients. However, the changes of sleep architec-tures among AD or MCI patients are still inconclusive (17-19)
. In this study, we conducted polysomnography exami-nations for age-matched controls and patients with
cog-nitive impairment, in terms of AD and MCI to explore whether sleep disturbed in the patients with cognitive impairment.
METHODS
Patients and controls
Ten AD patients with mild dementia, whose mini-mental status examination (MMSE) scores between 18 and 22, 12 MCI patients with MMSE scores 27-29 and 13 controls without cognitive impairment were recruited (Table). This study was approved by the Interstitial Review Board of Taipei Medical University Hospital (TMUH) and all the participants gave written informed consents. The diagnosis of AD is according to the crite-ria of NINCDS-ADRDA for probable AD(20)
. All the AD patients were taking cholinesterase inhibitors. None of them had sleep complaints at the study time. The diagno-sis of MCI was made by the consensus of neurologists (H.C. Liu and C.J. Hu) according to the diagnostic crite-ria including: (1) the person is neither normal nor demented; (2) there is evidence of cognitive deterioration shown by either objectively measured decline over time and/or subjective report of decline by self and/or infor-mant in conjunction with objective cognitive deficits; and (3) activities of daily living are preserved and com-plex instrumental functions are either intact or minimally impaired(21)
. In summary, the MCI patients were diag-nosed if their Clinical Dementia Rating (CDR) was 0.5 (memory box 0.5-1) and minimal mental status examina-tion (MMSE) ≥24. All the AD and MCI patients took laboratory tests to exclude other dementia causes, including complete blood count, folic acid, vitamin B12, VDRL, thyroid function test and CT of brain(4)
. Thirteen healthy volunteers who received routine health examina-tions at TMUH were subjects for controls. Each control was given clinical, mental, and neurological examina-tions, and none of them showed any cognitive defects or sleep complaints. None of them was taking hypnotics.
Questionnaires associated with day time sleepiness and depression
situ-ation, the questionnaires of Geriatric depression scale (GDS) and Epworth sleepiness scale (ESS) were per-formed before the polysomnography(22,23)
.
Polysomnography (PSG)
All the participants took PSG in the Sleep Center of TMUH by use of Sandman Elite (Tyco Healthcare, Canada). The scoring of PSG examination was based on the Rechtschaffen & Kales rules(24)
.
Statistical analyses
SPSS 13.0 for Windows was used for all statistical analyses. Descriptive statistics were expressed as mean Ųstandard deviations as well as frequencies in the AD, MCI and control groups. Multiple comparisons among AD, MCI and control groups were examined using the Kruskal-Wallis test. AD and MCI groups are merged into a group of cognitive impairment to compare with control group by using two-tailed values of the Mann-Whitney U test. P values below 0.05 were considered statistically significant.
RESULTS
Demographics
A total of 22 patients with cognitive impairment (AD Ŷ10, MCIŶ12) and 13 healthy controls were included in this study. Mean age of the patients was 77.14Ų8.93 years (rangeĈ63-90 years). Controls were age-matched with the mean age of 76.38Ų8.31 years. Further charac-teristics of the patients are listed in Tables.
Polysomnographic results
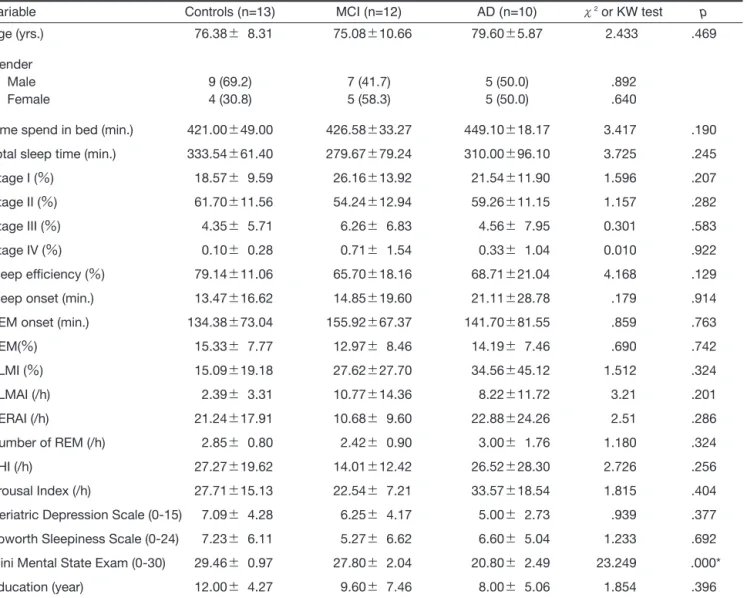
The data of total sleep time, time in bed, sleep effi-ciency, sleep onset (latency), REM onset (latency), per-centage of REM, periodic limbs movement index (PLMI), and number of REM obtained from PSG and subjective questionnaire data, including ESS and GDS are summarized in Tables. The PSG parameters in the three groups, in terms of controls, MCI and AD, are not statistically different from each other (Table 1). The patient group showed a disturbed sleep efficiency (67.07 Ų19.10Ʀ). Time in bed was 436.82Ų29.21 minutes. Total sleep time was 293.45Ų86.52 minutes. Sleep onset
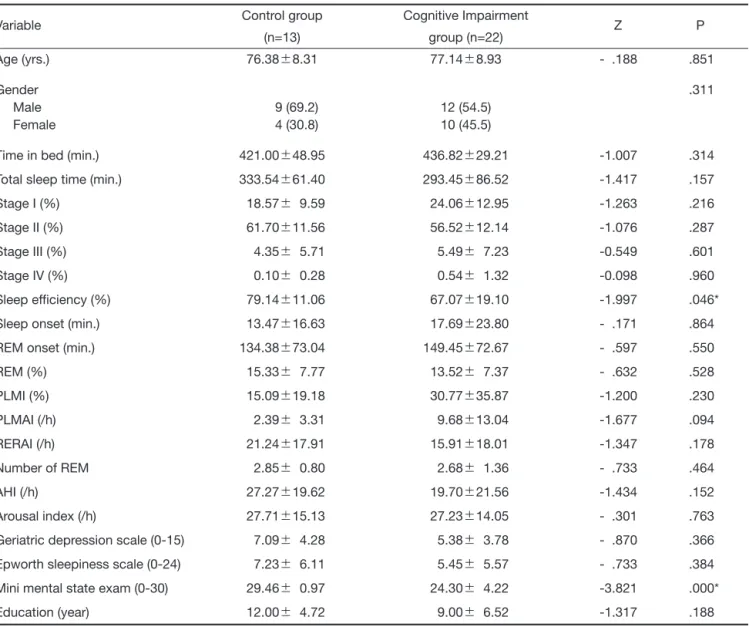
was 1061.68Ų1428.00 seconds. REM onset was 149.45 Ų72.67 minutes. The percentage of REM sleep was 13.52Ų7.37Ʀ. PLMI was 30.77Ų35.87/h. Number of REM sleep was 2.68Ų1.36. In the Control group, sleep efficiency was 79.14Ų11.06 %. Time in bed was 421.00 Ų48.95 minutes. Total sleep time was 333.54Ų61.40 minutes. Sleep onset was 803.31Ų997.65 seconds. REM onset was 134.38Ų73.04 minutes. The percentage of REM sleep was 15.33Ų7.77Ʀ. PLMI was 30.77Ų 35.87/h. Number of REM sleep was 2.85Ų0.80. Only the sleep efficiency was significantly different among all the sleep variables between cognitive impairment group and control group. There were no statistically significant differences in the scores on the ESS and GDS.
There were no differences in sleep architecture between controls and patients with cognitive impairment, in terms of MCI and mild AD. Sleep efficiency, which was one of the important parameters for sleep quality was significantly lower in patients with cognitive impair-ment than in controls. Low sleep eff iciency usually results from poor maintenance of sleep and it was proba-bly associated with depression neurosis. However, the geriatric depression scores (GDS) were not different between controls and patients. These results implicate maintenance of sleep was impaired in patients with cog-nitive impairment and it was independent from depres-sive symptoms.
DISCUSSION
In a meta-analysis of quantitative sleep parameters, among adults, total sleep time, sleep efficiency, percent-age of slow-wave sleep, percentpercent-age of REM sleep, and REM latency significantly decreased with age. Sleep latency, percentage of stage 1 sleep, percentage of stage 2 sleep, and wake after sleep onset signif icantly increased with age. Only sleep efficiency continued to significantly decrease after 60 years of age(25)
. Sleep effi-ciency which represents the ratio of total sleep time to the total time in bed, is a parameter of sleep mainte-nance. Sleep efficiency of lower than 85% usually indi-cates one of the diagnostic criteria of insomnia(26).
Although low sleep efficiency has been associated with excessive day time sleepiness in Parkinson’s disease, the
clinical consequence of low sleep efficiency in other neurodegenerative diseases still lacks detailed studies(27)
. On the other hand, decreased sleep efficiency has been found in many mental diseases, such as depression, psy-chosis and alcoholism(28-30)
. In this study, sleep efficiency of patients with cognitive impairment was significantly lower than that of controls. There was no increase of day time sleepiness based on the Epworth sleepiness scale. These results implicates that sleep maintenance might be impaired in the patient group but the impairment did not
impact their day time sleepiness. Since depression is common in patients with cognitive impairment, geriatric depression scale questionnaire (GDS), which is feasible even in mild dementia population, was performed to explore the role of mood in the decrease of sleep effi-ciency(7,31)
. The study found depression was not associat-ed with the decrease of sleep efficiency. Therefore, the causes and the consequences of decreased sleep efficien-cy in patients with cognitive impairment need further investigation.
Table 1. Comparison of means of sleep variables between MCI, AD and control group
Variable Controls (n=13) MCI (n=12) AD (n=10) ɬ2
or KW test p
Age (yrs.) 76.38Ų 8.31 75.08Ų10.66 79.60Ų5.87 2.433 .469
Gender
Male 9 (69.2) 7 (41.7) 5 (50.0) .892
Female 4 (30.8) 5 (58.3) 5 (50.0) .640
Time spend in bed (min.) 421.00Ų49.00 426.58Ų33.27 449.10Ų18.17 3.417 .190 Total sleep time (min.) 333.54Ų61.40 279.67Ų79.24 310.00Ų96.10 3.725 .245
Stage I (Ʀ) 18.57Ų 9.59 26.16Ų13.92 21.54Ų11.90 1.596 .207
Stage II (Ʀ) 61.70Ų11.56 54.24Ų12.94 59.26Ų11.15 1.157 .282
Stage III (Ʀ) 4.35Ų 5.71 6.26Ų 6.83 4.56Ų 7.95 0.301 .583
Stage IV (Ʀ) 0.10Ų 0.28 0.71Ų 1.54 0.33Ų 1.04 0.010 .922
Sleep efficiency (Ʀ) 79.14Ų11.06 65.70Ų18.16 68.71Ų21.04 4.168 .129
Sleep onset (min.) 13.47Ų16.62 14.85Ų19.60 21.11Ų28.78 .179 .914
REM onset (min.) 134.38Ų73.04 155.92Ų67.37 141.70Ų81.55 .859 .763
REM(Ʀ) 15.33Ų 7.77 12.97Ų 8.46 14.19Ų 7.46 .690 .742 PLMI (Ʀ) 15.09Ų19.18 27.62Ų27.70 34.56Ų45.12 1.512 .324 PLMAI (/h) 2.39Ų 3.31 10.77Ų14.36 8.22Ų11.72 3.21 .201 RERAI (/h) 21.24Ų17.91 10.68Ų 9.60 22.88Ų24.26 2.51 .286 Number of REM (/h) 2.85Ų 0.80 2.42Ų 0.90 3.00Ų 1.76 1.180 .324 AHI (/h) 27.27Ų19.62 14.01Ų12.42 26.52Ų28.30 2.726 .256 Arousal Index (/h) 27.71Ų15.13 22.54Ų 7.21 33.57Ų18.54 1.815 .404
Geriatric Depression Scale (0-15) 7.09Ų 4.28 6.25Ų 4.17 5.00Ų 2.73 .939 .377 Epworth Sleepiness Scale (0-24) 7.23Ų 6.11 5.27Ų 6.62 6.60Ų 5.04 1.233 .692 Mini Mental State Exam (0-30) 29.46Ų 0.97 27.80Ų 2.04 20.80Ų 2.49 23.249 .000*
Education (year) 12.00Ų 4.27 9.60Ų 7.46 8.00Ų 5.06 1.854 .396
Data are presented as mean Ų Standard deviation (SD). Significance was determined by nonparametric statistics (Kruskal-Wallis (KW) test for three groups). PLMI: Periodic limbs movement index; AHI: Apnea hypopnea index; PLMA: PLM arousal index; RERAI: Respiratory arousal index. *P < 0.05.
There have been many polysomnographic (PSG) reports about changes of sleep architectures in AD patients with or without cholinesterase inhibitors(17-19)
. The initiation of REM sleep depends on the activation of cholinergic neurons in lateral dorsal tegmental nuclei (LDT) and pedunculo-pontine tegmental nuclei (PPT)(13,15,32). The acetylcholine (ACh) levels are relatively
low in patients with AD and deficiency of ACh is con-sidered to be symptomatic for AD(33)
. Based on these
findings, it is speculated that REM sleep is suppressed in AD. This study did show a tendency that the number or percentage of REM decreased and REM latency increased in patients with cognitive impairment but not reaching the significance. These results might support the defects of acetylcholine-REM axis in AD. Because of the consideration of ethical issue and the compliance of PSG examination, the AD participants of this study were all at mild degree of severity and all of them were
Table 2. Comparison of means of sleep cariables between cognitive impairment group and control group
Variable Control group Cognitive Impairment Z P
(n=13) group (n=22)
Age (yrs.) 76.38Ų8.31 77.14Ų8.93 - .188 .851
Gender .311
Male 9 (69.2) 12 (54.5)
Female 4 (30.8) 10 (45.5)
Time in bed (min.) 421.00Ų48.95 436.82Ų29.21 -1.007 .314
Total sleep time (min.) 333.54Ų61.40 293.45Ų86.52 -1.417 .157
Stage I (%) 18.57Ų 9.59 24.06Ų12.95 -1.263 .216
Stage II (%) 61.70Ų11.56 56.52Ų12.14 -1.076 .287
Stage III (%) 4.35Ų 5.71 5.49Ų 7.23 -0.549 .601
Stage IV (%) 0.10Ų 0.28 0.54Ų 1.32 -0.098 .960
Sleep efficiency (%) 79.14Ų11.06 67.07Ų19.10 -1.997 .046*
Sleep onset (min.) 13.47Ų16.63 17.69Ų23.80 - .171 .864
REM onset (min.) 134.38Ų73.04 149.45Ų72.67 - .597 .550
REM (%) 15.33Ų 7.77 13.52Ų 7.37 - .632 .528 PLMI (%) 15.09Ų19.18 30.77Ų35.87 -1.200 .230 PLMAI (/h) 2.39Ų 3.31 9.68Ų13.04 -1.677 .094 RERAI (/h) 21.24Ų17.91 15.91Ų18.01 -1.347 .178 Number of REM 2.85Ų 0.80 2.68Ų 1.36 - .733 .464 AHI (/h) 27.27Ų19.62 19.70Ų21.56 -1.434 .152 Arousal index (/h) 27.71Ų15.13 27.23Ų14.05 - .301 .763
Geriatric depression scale (0-15) 7.09Ų 4.28 5.38Ų 3.78 - .870 .366 Epworth sleepiness scale (0-24) 7.23Ų 6.11 5.45Ų 5.57 - .733 .384 Mini mental state exam (0-30) 29.46Ų 0.97 24.30Ų 4.22 -3.821 .000*
Education (year) 12.00Ų 4.72 9.00Ų 6.52 -1.317 .188
Data are presented as mean Ų Standard deviation (SD). Significance was determined by nonparametric statistics (Mann-Whitney U test for two groups) as a result of data that were not normally distributed. PLMI: Periodic limbs movement index; AHI: Apnea hypopnea index; PLMA: PLM arousal index; RERAI: Respiratory arousal index. *P < 0.05.
taking cholinesterase inhibitors. Those factors might mask or underestimate the REM changes in cognitive-impaired patients.
One-night PSG is always challenged by the first night effect. Although it has been questioned and the studies showed no difference of sleep efficiency between the first night and second night, the first night effect obviously is a limitation of this study(34)
. In AD, sleep disordered breathing (SDB) occurred less frequently than in non-demented elderly subjects. The causal rela-tionship between AD and SDB remains controversial(35)
. The mean apnea-hyponea-indexes (AHI) were 27.27Ų 19.62, 19.70Ų21.56 in control, and cognitive impair-ment groups respectively (p=0.152). These results demonstrate SDB is a common disorder among the aged population. The gender distributions in the controls and cognitive impairment group were not the same in this study. However, the differences did not reach statistic significance and the gender effect on sleep architecture, especially in the elder populations, is largely unknown(36)
. Arousal index and causes of arousals roughly reflex the impacts of SDB, periodic limb movement and arousal status on the sleep. The arousal patterns in controls and cognitive impairment group need further analysis. Without statistic significance, the mean education years in controls were higher than it in the cognitive impair-ment group. This phenomenon might result from that the people with higher education year are easier to become a volunteer for the clinical study. There was a tendency that the PLMI of cognitive impairment group was higher than it of the control group. High PLMI usually reflexes the deficiency of dopamine levels in the brain and that could occur in the AD brain(37,38)
. PLMI might be a pre-dictor of total sleep time in cognitive impaired elders. Therefore, further investigation by larger sample size might be warranted.
In summary, the number and percentage of REM decreased and REM latency increased at borderline degree in patients of cognitive impairment. That might reflect the low brain ACh levels in these patients. The sleep efficiency significantly reduced but was without significant impacts on day time sleepiness and that was not associated with depressive symptoms. These results might imply that the patients with cognitive impairment
could be under the hyper-arousal status. The causes and consequences of low sleep efficiency among patients with cognitive impairment need further investigation.
REFERENCES
1. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989;39:1159-65.
2. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991;41:479-86. 3. Petersen RC, Doody R, Kurz A, et al. Current concepts in
mild cognitive impairment. Arch Neurol 2001;58:1985-92. 4. Morris JC, Storandt M, Miller JP, et al. Mild cognitive
impairment represents early-stage Alzheimer disease. Arch Neurol 2001;58:397-405.
5. Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002;59:1594-9. 6. Chong MS, Sahadevan S. Preclinical Alzheimer’s disease:
diagnosis and prediction of progression. Lancet Neurol 2005;4:576-9.
7. Chiu MJ, Chen TF, Yip PK, et al. Behavioral and psycho-logic symptoms in different types of dementia. J Formos Med Assoc 2006;105:556-62.
8. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neu-ropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288:1475-83.
9. Markov D, Goldman M. Normal sleep and circadian rhythms: neurobiologic mechanisms underlying sleep and wakefulness. Psychiatr Clin North Am 2006;29:841-53. 10. Fogel SM, Smith CT, Cote KA. Dissociable
learning-dependent changes in REM and non-REM sleep in declara-tive and procedural memory systems. Behav Brain Res 2007;180:48-61.
11. Fuh JL, Wang SJ, Liu HC, et al. The caregiving burden scale among Chinese caregivers of Alzheimer patients. Dement Geriatr Cogn Disord 1999;10:186-91.
12. Vloeberghs E, Van Dam D, Engelborghs S, et al. Altered circadian locomotor activity in APP23 mice: a model for
BPSD disturbances. Eur J Neurosci 2004;20:2757-66. 13. Zhang B, Veasey SC, Wood MA, et al. Impaired rapid eye
movement sleep in the Tg2576 APP murine model of Alzheimer’s disease with injury to pedunculopontine cholinergic neurons. Am J Pathol 2005;167:1361-9. 14. Stickgold R, Walker MP. Sleep-dependent memory
consoli-dation and reconsoliconsoli-dation. Sleep Med 2007;8:331-43. 15. Hobson JA. Sleep and dreaming: induction and mediation
of REM sleep by cholinergic mechanisms. Curr Opin Neurobiol 1992;2:759-63.
16. Fillit H, Hill J. Economics of dementia and pharmacoeco-nomics of dementia therapy. Am J Geriatr Pharmacother 2005;3:39-49.
17. Bahro M, Riemann D, Stadtmüller G, et al. REM sleep parameters in the discrimination of probable Alzheimer’s disease from old-age depression. Biol Psychiatry 1993; 34:482-6.
18. Dykierek P, Stadtmüller G, Schramm P, et al. The value of REM sleep parameters in differentiating Alzheimer’s dis-ease from old-age depression and normal aging. J Psychiatr Res 1998;32:1-9.
19. Cooke JR, Loredo JS, Liu L, et al. Acetylcholinesterase inhibitors and sleep architecture in patients with Alzheimer’s disease. Drugs Aging 2006;23:503-11. 20. McKhann G, Drachman D, Folstein M, et al. Clinical
diag-nosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939-44.
21. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240-6. 22. Chan AC. Clinical validation of the Geriatric Depression
Scale (GDS): Chinese version. J Aging Health 1996;8:238-53.
23. Chen NH, Johns MW, Li HY, et al. Validation of a Chinese version of the Epworth sleepiness scale. Qual Life Res 2002;11:817-21.
24. Rechtschaffen A, Kales A. A manual of standardized termi-nology, techniques and scoring system for sleep stages of human sleep. Los Angeles: Brain Information Service/
Brain Research Institute, UCLA, 1968.
25. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 2004;27:1255-73. 26. Morin CM, Hauri PJ, Espie CA, et al. Nonpharmacologic
treatment of chronic insomnia. An american Academy of Sleep Medicine review. Sleep 1999;22:1134-56.
27. Shpirer I, Miniovitz A, Klein C, et al. Excessive daytime sleepiness in patients with Parkinson’s disease: a polysomnography study. Mov Disord 2006;21:1432-8. 28. Argyropoulos SV, Wilson SJ. Sleep disturbances in
depres-sion and the effects of antidepressants. Int Rev Psychiatry 2005;17:237-45.
29. Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev 2003;7:523-39.
30. Benson KL. Sleep in schizophrenia: impairments, corre-lates, and treatment. Psychiatr Clin North Am 2006;29: 1033-45.
31. Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. Int Psychogeriatr 1996;8:103-12.
32. Stenberg D. Neuroanatomy and neurochemistry of sleep. Cell Mol Life Sci 2007;64:1187-204.
33. Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s dis-ease. J Neural Transm 2006;113:1625-44.
34. Kader GA, Griffin PT. Reevaluation of the phenomena of the first night effect. Sleep 1983;6:67-71.
35. Janssens JP, Pautex S, Hilleret H, et al. Sleep disordered breathing in the elderly. Aging (Milano) 2000;12:417-29. 36. Sakakibara S, Kohsaka M, Kobayashi R, et al. Gender
dif-ferences in self-evaluated sleep quality and activity of mid-dle-aged and aged subjects. Psychiatry Clin Neurosci 1998;52:184-6.
37. Becker PM, Jamieson AO, Brown WD. Dopaminergic agents in restless legs syndrome and periodic limb move-ments of sleep: response and complications of extended treatment in 49 cases. Sleep 1993;16:713-6.
38. Rossor MN. Neurotransmitters and CNS disease. Dementia. Lancet 1982;2:1200-4.