ELSEVIER PII SO142-9612 (97) 00016-l
Biomoteriak 18 (1997) 915-921 0 1997 Elsevier Science Limited Printed in Great Britain. All rights reserved
0142-9612/97/$17.00
Degradation behaviour of a new
bioceramic: Ca2P207 with addition of
Feng-Huei Lin* , Chun-Jen Liao’, Kao Shao Chen$, Jui-Sheng Smt
and Haw-Chang Liut
‘Centre for Biomedical Engineering and +Department of Orthopedic Surgery, College of Medicine, National Taiwan University, Taipei, Taiwan, ROC; bepartment of Materials Engineering, Jatung institute of Technology, Taipei, Taiwan, ROC
A newly produced bioceramic, /I-Ca2P207 with addition of Na4P20,.10H20 (SDCP), has been implanted into the femoral condyle of rabbits. Within 6 weeks after implantation, most of the bioceramic is replaced by new woven bone. On the contrary, block form hydroxyapatite (HA) and /3-tricalcium phosphate (/?-TCP), which are osteoconductible, do not resorb within a short period of time. We believe that the biodegradable behaviour of SDCP may occur in two steps. The first and most important step is the digestion of particles and migration of the particles by phagocytosis. The object of this study is to examine the change in morphologies, chemical compositions and crystal structure of SDCP after soaking in distilled water for a certain period of time. The SDCP ceramic was also co- cultured with leucocytes to observe how the SDCP particles were digested by the leucocytes, so that the mechanism of biodegradable behaviour of SDCP ceramic in viva might be clarified. Four types of sintered calcium phosphate ceramics were tested in the experiment: SDCP, pure b-Ca2P207 (DCP), HA and /?-TCP. They were soaked in distilled water at 37°C for up to 30 days. The microstructure and morphology of crystals deposited on the surface were observed using scanning electron microscopy. Sodium, calcium and phosphorus ion contents in the supernatant solution were detected by atomic absorption analysis and ion coupled plasma. In summary, HA and DCP showed no significant evidence of dissolution in distilled water. In static distilled water, calcium ions may be released from P-TCP into solution during the initial 7 days and then converted into HA by reprecipitation. The results showed that the SDCP was firstly dissolved into small grains or fragments by the solution. The small fragments should be so small as to be digested by the phagocytes in a physiological environment. 0 1997 Elsevier Science Limited. All rights reserved
Keywords: Bioceramics, degradation, leucocytes Received 10 July 1996; accepted 6 January 1997
Early studies on the use of calcium phosphates as hard tissue implants resolved around the theory that local release of calcium ion would stimulate osteogenesis1-3. The results of these early studies, which used locally placed, high-surface-area powders of varying composition, were equivocal, with some investigators reporting accelerated healing, while others observed little or no increase in the bone healing rate334. It has been reported that the use of block forms of hydroxy- apatite (HA) to reconstruct atrophic residual mandibular ridges results in an unacceptably high failure rate in human clinical applications5. The use of granular instead of block forms of HA has therefore been suggested. Whichever form is used, HA lacks biodegradability. As a result, /?-tricalcium phosphate (j?-TCP) bioceramic has been developed as a biodegrad- able bone replacement”*7. However, when used as substitute for larger condyle defects, the rate of Correspondence to Professor Jui-Sheng Sun.
bioabsorption of block form /?-TCP has been shown to be too slow, even without significant evidence of absorptionaVg.
The ultimate goal of implantation of biomaterials in skeletal tissue is to reach full integration of the non- living implant with living bone%ll. The material could be used, much as a bone graft, as a material itself resorbs or dissolves as bone growth occurs, and the end result is new remoulded bone. The requirements centre on resorbability and the lack of toxicity or other harmful effects arising from the release and metabolism of the degeneration products. In a previous study”, calcium pyrophosphate with addition of sodium phosphate ceramic was developed as a bioresorbable bone substitute. The results of the quantitative assessment and histological evaluation showed significant evidence of the new bone growing readily into the macropores of the materials, the implant gradually dissolving in the in vivo environment and
being progressively replaced by the regenerated bone. 915 Biomaterials 1997. Vol. 16 No. 13
916 Degradation behaviour of bioceramics: Feng-Huei Lin et al.
The factors concerning the bioresorption of calcium phosphate ceramics have not been completely elucidated4*‘1. The chemical composition, physical characteristics and crystal structures certainly play an important role in the biological behaviour of calcium
phosphates’3-15. Block forms of calcium
pyrophosphate, HA and /?-TCP were not dissolved or biodegradable in the physiological environment. However, block forms of calcium pyrophosphate with addition of sodium phosphate ceramic could be dissolved or degraded in the same conditions. The objective of this study was to examine the change in the composition, morphologies and crystal structure of calcium pyrophosphate with sodium phosphate after incubation in simulated body fluid for a period of time. From the results, we discuss the biodegradable mechanism of the calcium pyrophosphate with sodium phosphate ceramic and speculate why the ceramic can be dissolved or biodegraded in the in vivo
environment.
MATERIALS AND EXPERIMENTS
Materials preparation
Four types of sintered calcium phosphate ceramics were tested in the experiment: CazPz07 with 5wt% Na4Pz07.10Hz0 (SDCP), pure CazPz07 (DCP), HA and /?-TCP.
DCP powder with mean grain size about O.l,um was used in the experiment. The specific surface area determined by Brunauer-Emmett-Teller (BET) analysis was 51 f 0.2 cm2 gg’ and /?-TCP powders were spherical in shape and about 0.06pm in grain size according to scanning electron microscopic observation, and their BET specific surface areas were approximately 70 and
59 cm2 g-’ respectively. Trace elements that might be connected with biocompatibility were detected by atomic absorption analysis. The concentrations of the trace elements in the four ceramics were much lower than the maximum tolerable level.
The DCP powder was mixed with 5wt%
Na4Pz07.10Hz0 in water and dried at 70°C for 3 days. The well mixed and dried cake was ground and sieved into 40-60 meshed particles. The sieved particles were then compacted in a stainless-steel die under a hydrostatic pressure of 270MPa, and a green density of about 60% T.D. was obtained. The prepared green body was placed on a platinum sheet and heated up to 930°C at a heating rate of 3”Cmin-l in a conventional Ni-Cr coiled furnace and then maintained in air for 1 h after the sintering temperature of 930°C was reached’0,12. HA, TCP and calcium pyrophosphate discs were also prepared using the same procedures, but no Na,Pz07.10HZ0 was added. The sintering temperatures of the three ceramics were 1250, 1200 and 930°C respectively, with the same heating rate of 3”Cmin-*. Dense ceramics with specific density of over 98% were prepared for the following experiment.
Soaking process
The four ceramics were soaked in distilled water. For each material, 1 g was immersed in 50ml distilled water at 37°C for l-30 days. After soaking, the ceramics were taken out of the solution, gently washed in
distilled water and subsequently in acetone, and dried at room temperature13’16.
Evaluation and measurements
After the specimens were taken out of the immersion solution, the microstructure and morphology of the crystal deposited on the surface were observed under the scanning electron microscope (SEM). The surfaces were coated with a thin layer of evaporated carbon. They were then observed by SEM and analysed using an energy-dispersive electron probe X-ray microana- lyser. Phosphorus, calcium and sodium were analysed across the grains and grain boundaries. An electron beam maintained at 2 x lo-” A was used and X-ray intensities in counts per second (cps) were recorded”. The accelerating voltage was 12 kV. Ultra-thin sections for transmission electron microscopy (TEM) were obtained from the sintered specimen by slicing with a diamond blade saw and ultrasonic cutter. The slices were polished with diamond abrasive to a thickness of 30pm on a dimple grinder and then mounted on a copper ring. The specimens were finally thinned by ion-beam milling. The crystal structure of the intergrain material was investigated using a Hitachi-700 STEM operating at 175 kV”,17. Selected area diffraction patterns were recorded with photographic plates. Tilting crystals from one orientation to another was carried out in the selected area diffraction mode using a double tilt holder. Calcium, sodium and phosphorus ion contents in the supernatant solution were detected by atomic absorption analysis and ion-coupled plasma.
Leucocyte isolation and cell culture
Leucocytes were isolated from the mature male New Zealand rabbit. Blood (8ml) was extracted from the rabbit and mixed with z ml EDTA solution. The well- mixed solution was then poured into a test tube along the tube sidewall, where 1 ml HESPN solution had been prepared previously. After 20min or after a translucent solution appeared, the upper part of the solution contained the isolated leucocytes’a*lg.
The block ceramic (0.2g) was co-cultured with the leucocytes for about 2 days. The cell density was about 1 x lo4 cells per 940mm’ and 3 ml medium was seeded for each Petri dish. The culture medium used in the experiment was Dulbecco’s modified supplemented with 10% fetal calf serum (Gibco, UK), penicillin G solution (100 units ml-‘) and streptomycin (lOOmgml-l; Gibco, UK). The dishes were incubated at 37°C in an atmosphere supplemented with 5% CO2 for 48 h observation. We were able to observe how the ceramic grains released from the materials were digested by the leucocytes. The dynamic observation of the digested process of the material was recorded using an optical microscope with an immersion-oiled objective lens (x100). The experiment was repeated six times for each ceramic.
RESULTS AND DISCUSSION
Ion concentration analysis
Figure 1 shows the calcium ion concentration after the four tested materials were immersed in distilled water Biomaterials 1997, Vol. 18 No. 13
Degradation behaviour of bioceramics: Feng-Huei Lin et a/.
‘“II
0
i ’ D. I I I I ‘I
0 5 10 15 a0 25 33
Immersion time (days)
0 5 10 15 23 25
Immersion time (days)
Figure 1 The calcium ion concentration in distilled water Figure 2
after the four tested materials were immersed for a time
PO:- ion concentration in distilled water after the four tested materials were immersed for a time period period ranging from 1 to 30 days. ranging from 1 to 30 days.
for l-30 days. The calcium ion concentration of TCP ceramic increased rapidly with immersion time during the initial 7 days and then reached a plateau at a constant value of about 1 mM. For the HA and DCP groups, the calcium ion concentration was virtually zero throughout. There was no significant increase and not much change for the calcium ion concentration of the HA and DCP g.roups during the experimental period. On the contrary, the calcium ion concentration of SDCP was increaseId with immersion time.
PO:- concentration curves with the four tested materials immersed in distilled water for 1-30 days are shown in Figure 2. The four curves showed the same tendency as for calcium ion concentration. The PO:- concentration of SDCP increased with immersion time, while the HA and DCP were not much changed and maintained a value of 0.1-0.2 mM. During the initial 7 days, the PO:- concentration of TCP increased with immersion time, thereafter maintaining a constant value of about 28 mM.
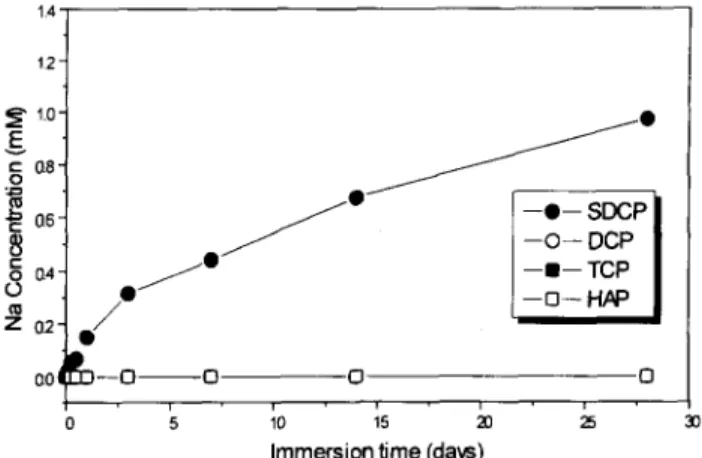
Figure 3 shows the relationship between sodium ion concentration and immersion time after the four tested materials were imme:rsed in distilled water for 1-30 days. There was no sodium ion release from the TCP, HA and DCP ceramics, as shown in Figure 3, because there is no sodium in these ceramics. However, the sodium concentration constantly increased with immersion time for the SDCP ceramic.
From the results of the ion concentrations in distilled water, we speculate that:
(1)
(2)
(3)
(4)
HA and DCP ceramics could not be dissolved in distilled water and thus were stable and inert in this solution;
SDCP ceramic could be dissolved in distilled water and released Ca’+, Naf and PO:- constantly from the ceramic without saturation before immersion for 30 days;
TCP ceramic dissolved substantially during the initial 7 days, and then the Cazt and PO:- concen- trations in the solution reached saturation, result- ing in a constant value for the two ions’ concentration;
the dissolution rate of TCP was much higher than that of SDCP ceramic during the initial 7 days. According to the solubility diagram for the system CaO-P205-H20, at a pH range of 4.2-8.0, HA is less
0 0
14/
‘21
/* -o- SDCP -0- DCP -m- TCP -o-HA= 00 -0-o 0 0 0 5 10 15 al 25 3Immersion time (days)
3
Figure 3 The sodium ion concentration in distilled water after the four tested materials were immersed for a time period ranging from 1 to 30 days.
soluble than TCP. In addition, it is known that HA dissolves less rapidly than TCP. In particular, the TCP dissolves 12.3 times faster than HA in acidic medium and 22.3 times faster than HA in basic medium”. Another in vitro investigation revealed that the dissolution rate of TCP was three times greater than that of HA in lactate buffer solution. There has been no report on the biodegradability of calcium pyrophosphate (DCP)4321. However, it has been reported that DCP of bulk type did not produce the rapid sequence of membrane lysis or cell death, and was very stable in viva”.
Based on the previous literature, HA and DCP can be considered as stable in distilled water, and TCP ceramic was degradable in the same solution. In this study, the results of ion concentration analysis agreed with the previous reports. SDCP consisted of 85% calcium pyrophosphate and 5% sodium phosphate in chemical composition. However, it revealed a great tendency to degrade in distilled water and it has proven to be biodegradable in the physiological environment.
Microstructure and X-ray difiaction analysis
An understanding of the bioresorbability behaviour can be gained by considering the bioceramics from two points of view-their crystal/chemical composition and their structure as materials8V’4. Figure 4 shows the
918 Degradation behaviour of bioceramics: Feng-Huei Lin et al.
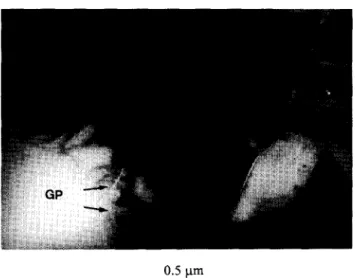
Figure 4 Transmission electron micrograph showing the microstructure of sintered SDCP. GP, glass phase on the grain boundary.
Figure 5 X-ray diffraction patterns of SDCP after immersion in distilled water for 130 days. The characteris- tic peaks of SDCP are indicated as solid lines connected with the horizontal ground. 0, Characteristic peaks of the second phase (Ca,Na)(PO&
TEM microstructure of sintered SDCP. The glass phase, with a chemical composition of 40% NaP03 and 60% CaP206 by weight, can be seen along the grain boundary. The glass was easily dissolved in distilled water, which caused the grain to be slashed from the ceramic. We could observe X-ray diffraction (XRD) patterns of SDCP ceramic immersed in distilled water for l-30 days. A characteristic peak of the second phase with chemical formula (Na,Ca)(PO& gradually disappeared with immersion time, as shown in Figure 5. We suppose that the DCP added with 5wt% sodium phosphate could form a glass phase and a compound that could be dissolved in distilled water, leading to SDCP disintegration. The sodium ion was constantly released from the SDCP ceramic, which possibly stemmed from the glass and second phase dissolving in the solution.
Figures 6 and 7 show the XRD patterns of HA and DCP respectively after the two ceramics were immersed in distilled water for l-30 days. All characteristic peaks of the two patterns are in agreement with the HA and DCP X-ray diffracted JCPD data file. The two ceramics were very stable, phase transformation did not occur
and no other phases appeared during the immersion period. Figure 8 shows the XRD patterns after TCP was immersed in distilled water for l-30 days. A known precipitate was formed on the surface of the TCP ceramic. Needle-like crystals 0.5pm in diameter were observed on the surface of the TCP after immersion in distilled water for about 1 day. The crystals then became thicker, with an average grain size of about 3- 5pm, and plate-like when immersed for 7 days. After
Figure 6 X-ray diffraction of HA after immersion in distilled water for l-30 days.
Figure 7 X-ray diffraction patterns of DCP after immersion in distilled water for l-30 days,
Figure 6 X-ray diffraction patterns of TCP after immersion in distilled water for 130 days. The characteristic peaks of TCP are indicated as solid lines connected with the horizon- tal ground. H, characteristic peaks of HA.
- Biomaterials 1997, Vol. 18 No. 13
Degradation behaviour of bioceramics: Feng-Huei Lin et al. 919
immersion for 30 days, the crystals covered the surface of the TCP like a blanket. The precipitate on the TCP surface could be identified by its crystal structure and chemical composit:ion by XRD electron probe microanalysis (EPMA) respectively. It was shown that the precipitate was in agreement with that for HA. The characteristic peaks of HA gradually appeared in the XRD patterns after TCP immersion for 7 days. The TCP was supposed to be dissolved in solution during the initial 7 days and then reprecipitated the HA crystals onto its surface. The HA crystals precipitated on the TCP ceramic could be observed with SEM after immersion for 7 days. as shown in Figure 9. HA crystals
could be seen elsewhere on the TCP surface after SO- day immersion (Figure 10). Analysis of the blanket formed on TCP demonstrated the appearance of low but characteristic peaks of HA.
Figure 11 shows the weight loss curves of the four tested materials immersed in solution for l-30 days. HA and DCP showed no significant change in weight loss, because they were stable and inert in distilled
Figure 9 HA crystals could be observed on the TCP
surface under scanning electron microscopy examination after TCP immersion for 7 days. Tc, TCP matrix; Ha, repreci- pitated HA crystals.
Figure 10 HA blanket coverage on the surface of TCP after
immersion for 30 days. Tc, TCP grains; Ha, reprecipitated HA blanket.
SIO I, I v I ' I
0 5 10 15 22 a
Immersion time (days)
Figure 11 Weight loss curves of the four tested materials
immersed in solution for l-30 days.
water. SDCP constantly lost weight during the experimental period. The weight loss curve of TCP immersed in distilled water shows that the weight decreased in the first 7 days and then increased.
Jarchog expected that the compositions of any solids deposited on the surface of TCP would largely be determined by the surrounding media. Immersion tests of TCP in distilled water revealed significant amounts of Ca’+ and PO:- in the surrounding environment. The PO:- in the solution may adsorb onto the TCP ceramic and form an HA layer in combination with dissolved Cazt from the material. The precipitation of HA crystals has been known to be principally determined by [Cal x [PI and condition of the nucleation siteI “I 23. Although the nucleation sites of these materials were not clear, the lower solubility of HA and DCP might be the main reason they failed to form a surface film in distilled water. This assumption is supported by the fact that TCP immersion in distilled water initially saturated with Ca*+ and PO:-, and TCP produced HA crystals on its surface. We speculate that the initial decrease in weight of TCP results from ceramic dissolution in distilled water, which leads to Cazt and PO:- ion concentration increases in solution. After the two ions reached saturation conditions in the solution, they then reprecipitated as a crystal form of HA on the surface of the TCP ceramic and the weight increased again.
Phagocytosis of SDCP particles by leucocytes
Microporosity, however, played a more dominant role in the resorption than macroporosity*4324. The microporosity determined the geometry of ‘necks’ between sintered particles, while macroporosity determined the member of necks in contact with the environment. The ‘neck’ formation was dependent on the preparation technique, i.e. sintering temperature and the pressure applied to compress the powder into a tablet before sintering”“‘. Additionally, the assumption was made that two different biological resorption pathways existed, solution-mediated processes (the implants dissolve in physiological
solution) and cell-mediated processes
(phagocytosis)‘3p lg. “, *‘.
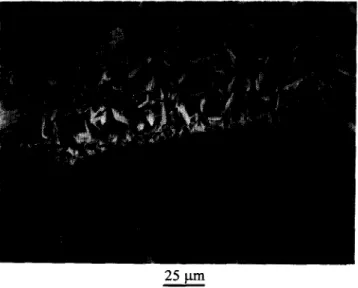
It is quite possible that this microporosity could aid in bioresorption by causing microscopic ‘break-up’ secondary to solution-mediated resorption. Thus, partially dissolved materials could slough off
920 Degradation behaviour of bioceramics: Feng-Huei Lin et al.
2.6 urn
Figure 12 Partially dissolved materials could slough off
individual crystals or fragments from the SDCP. Sd, SDCP matrix; Pf, slashed particles or fragments; DW, distilled water.
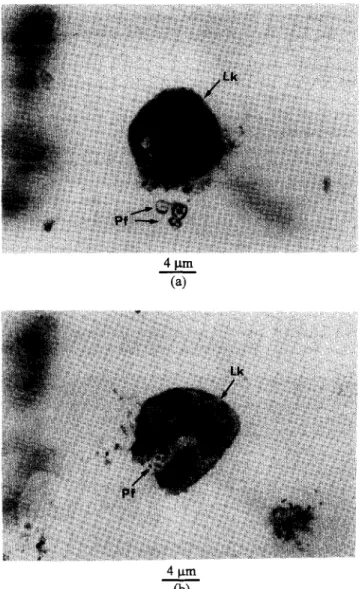
individual crystals or fragments, as shown in Figure 12, which are sufficiently small to allow for aggressive cell- mediated removal. Figure 13 shows a series process of SDCP particles dissolved from the material and digested by leucocytes. Moreover, much of the sodium-calcium-phosphate glassy phase was detected on the grain boundary and intergranular area in the SDCP bioceramic, which was described in the previous section. This would enhance the material’s dissolution process, because the glassy phase was easily attacked by the in vivo fluid. Evidence in support of the above hypothesis has been the observation of multinuclear- type cells in close proximity to the developed calcium phosphate ceramic. In some cases, ceramic fragments were indeed found to be present in vesicles of these cells. From the mentioned biodegradable behaviour of
the sintered DCP with 5 wt% addition of
Na4P207.10Hz0, the conclusion can be drawn that biodegradation occurred in two steps. The first and most important step is the extracellular disintegration of the implant. The second step is the digestion and migration of the particles.
CONCLUSIONS
From the results of the ion concentration, weight loss and XRD analysis of the four ceramics immersed in distilled water, our findings were as follows:
(1)
(2)
(3)
HA and DCP ceramics could not be dissolved in distilled water and were thought to be stable and inert in solution.
SDCP ceramic could be dissolved in distilled water, and Ca’+, Na+ and PO:- were constantly released from the ceramic during the experiment period.
TCP ceramic was readily dissolved during the initial 7 days, and the Ca2+ and PO:- concentra- tions in the solution reached saturation, which resulted in a constant value for the two ion concentrations. The weight loss curve of TCP immersed in distilled water had a negative tendency with immersion time during the first 7
4 cLm cb)
Figure 13 Optical microscopic observation of small SDCP
grains digested by leucocytes. a, Leucocytes close to the
SDCP particles; b, SDCP particles were engulfed by leucocytes. Lk, leucocyte; Pf, SDCP fragments.
days and then increased, due to HA crystals precipitating onto the TCP surface after Ca’+ and PO:- reached saturated conditions.
SDCP contained 95% calcium pyrophosphate and 5% sodium phosphate in chemical composition. However, it revealed a great tendency to dissolve in distilled water and it was also shown to be biodegrad- able in the physiological environment. Based on this study, the degradable behaviour (in vivo and in vitro) of the sintered DCP bioceramic doped with 5wt% Na4Pz07.10Hz0 could be considered as occurring in two steps. The first step is the extracellular disintegra- tion of the implant. The second step is the digestion and migration of the particles. The developed material will be phased out in the physio-chemical environment and subsequently replaced by regenerated bone tissue. It is thought to have great potential in the field of orthopaedics in the near future.
ACKNOWLEDGEMENTS
We would like to thank the National Science Council of the ROC for their financial support of this research. Biomaterials 1997. Vol. 18 No. 13
Degradation behaviour of bioceramics: Feng-Huei Lin et al. 921
We also thank K. Y. Tan of the Laboratory for Electron Microscopy, Department of Gastroendoscopy, College of Medicine, National Taiwan University.
REFERENCES
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13.Daculsi, G., Leberos, R.Z., Nery, E., Lynch, K. and Kerebel, B., Transformation of biphasic calcium phosphate ceramics in viva ultrastructural and physico-chemical characterization. I. Biomed. Mater. Bes., 1989, 23, 883-894.
Hench, L. L., Bioactive ceramics: from concept to clinic. J. Am. Ceram. Sot., 1992, 74,1487-1510.
Radin, S. and Ducheyne, P., The effect of calcium phosphate composition and structure on in vitro behaviour. II. Precipitation. J. Biomed. Mater. Res., 1993,27, 35-44.
Kohri, M., Miki, K., Waite, H., Nakajima, H. and Okabe, T., In vitro stability of biphasic calcium phosphate ceramics. Biomatericzls, 1993, 14(4), 299-304.
Hupp, J. R. and McKenna, S. J., Use of porous hydroxy- apatite blocks for augmentation of atrophic mandibles. J. Oral Maxillofac. Surg., 1988, 46, 533-545.
El Deeb, M. and Roszkowski, M., Hydroxyapatite blocks as an extracranial augmenting material in rhesus monkeys. J. Oral Maxillofac. Surg., 1988, 46, 33-40. deGroot, K., Bioceramics consisting of calcium phosphate salts. Biomaferials, 1980, 1, 47-55.
Driessens, F. C. M., Formation and stability of calcium phosphates in relation to the phase composition of the mineral in calcified tissues. In Bioceramic of Calcium Phosphates, ed. K. deGroot. CRC Press, Boca Raton, FL, 1983, pp. l-32.
Jarcho, M., Calcium phosphate ceramics as hard tissue prosthetics. Clin. C&hop. Rel. Res., 1981, 157, 259-278. Lin, F. H., Lin, C. C., Liu, H. C., Huang, Y. Y. and Wang, C. Y., Sintered porous DP-bioactive glass and hydroxy- apatite as bone substitute. Biomaterials, 1994, X1(13),
1087-1096.
Klein, C.A.P. T. and deGroot, K., Interaction of biodegradable /?-TCP ceramics with bone tissue. Biomateriak, 1983, 6, 189-195.
Lin, F. H., Liau, C. J., Chen, K. S. and Wang, C. Y., The preparation and ientative in-t&o evaluation of the sintered Ca,PZO, bioceramic with NqPzO,lOHzO addition. J. Biomed. Eng. Applic. Bas. Commun., 1995, 7(5), 515-518.
Radin, S.R. and Ducheyne, P., Effect of bioactive ceramic composition and structure on in vitro
14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25.
behavior. III. Porous versus dense ceramics. J. Biomed. Mater. Res., 1994, 26, 1303-1309.
deGroot, K., Effect of porosity and physiochemical properties on the stability, resorption and strength of calcium phosphate ceramics. In Bioceramics: Material Characteristics Versus In Vivo Behatiour, ed. P. Ducheyne and J. Lemmons. New York Academy of Sciences, New York, 1988, pp. 227-233.
Li, R., Clark, A. E. and Hench, L. L., An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater., 1991, 2, 231-239.
Asada, M., Miura, Y. and Osaka, A., Hydroxyapatite crystal growth on calcium hydroxyapatite ceramics. J. Mater. Sci., 1988, 23, 3202-3205.
Lin, F.H., Lu, C.M., Chen, R. S. and Tsai, Y.H., Mechanical properties of composite resin. J. Biomed. Eng. Applic. Bas. Commun., 1993, 5, 6784-795.
Kawaguchi, H., Koiwai, N., Ohtsuka, Y., Miyamoto, M. and Sasakawa, S., Phagocytosis of latex particles by leukocytes. I. Dependence of phagocytosis on the size and surface potential of particles. Biomaterials, 1986, 7, 61-66.
Gomi, K., Lowenberg, B., Shapiro, G. and Davies, J. E., Resorption of sintered synthetic hydroxyapatite by osteoclasts in vitro. Biomaterials, 1993, 14(Z), 91- 96.
Hyakuna, K., Yamamuro, T. Kotoura, Y. et al., Surface reactions of calcium phosphate ceramics to various solutions. J. Biomed. Mater. Res., 1990, 24, 471-488. Komatsu, T., Ohira, N., Oshida, M. and Sasaki, K., Massive deposition of calcium pyrophosphate dihydrate crystals in the knee. J. Bone Joint Surg., 1990, 72A, 931-935.
Burt, H. M., Jackson, J. K. and Rowell, J., Calcium pyrophosphate and monosodium urate crystal interac- tions with neutrophils: effect of crystal size and lipopro- tein binding to crystals. J. Rheumatol., 1989, 16, 809- 817.
Li, P., Nakanishi, K., Kokubo, T. and deGroot, K., Induction and morphology of hydroxyapatite, precipi- tated from metastable simulated bofy fluids on sol-gel prepared silica. Biomaterials, 1993, 14(13), 963-968. Kitsugi, T., Yamamuro, T., Nakamura, T., Kotani, S., Kokubo, T. and Takeuchi, H., Four calcium phosphate ceramics as bone substitute for non-weight-bearing. Biomaterials, 1993, 14(3), 216-224.
Yamada, S., Nakamura, T., Kokubo, M., Oka, M. and Yamamuro, T., Osteoclastic resorption of apatite formed on apatite-wollastonite-containing glass- ceramic by a simulated body fluid. J. Biomed. Mater. Res., 1994, 16(11), 1357-1363.