E L S E V I E R Journal of Chromatography A, 745 (1996) 217-223
JOURNAL OF
CHROMATOGRAPHY A
Analysis of chlorophenoxy acid herbicides by cyclodextrin-modified
capillary electrophoresis
Y o u - Z u n g H s i e h * , H s i - Y a H u a n g
Department (?f'Applied Chemisto', National Chiao Tung UniversiO'. Hsinchu. Taiwan, ROC
Abstract
In this study, we developed a capillary electrophoresis method involving cyclodextrins to separate seven chlorophenoxy acid herbicides and their enantiomers. The cavity size and the concentration of cyclodextrins heavily influenced the migration time of individual herbicides. The peak resolution also varied with different concentration ratios of cyclodextrins. Adding 4 mM c~-cyclodextrin and l mM /3-cyclodextrin in the buffer was found to produce the optimum experimental condition. The herbicides examined in this study could be successflflly separated within 7 min; meanwhile, the two pairs of enantiomers could also be completely resolved. The calibration lines" correlation coefficients for the separation conditions were all above 0,996. The dynamic range of the analysis of seven herbicides was at least two orders, from 1 ppm to 100 ppm. Analyzing the seven herbicides by capillary electrophoresis posed the advantages of a high resolution, high separation efficiency and good reproducibility. By combining with a C ,, disk precuncentration method, the herbicides' detection limits in the environmental water were below 1 ppb.
Kevuordx: Environmental analysis: Pesticides: Chlorophenoxy acids
1. Introduction
Chlorophenoxy acids are used as selective growth herbicides in agriculture [1-3]. The widespread use o f these compounds has led to increased water and soil pollution. Their toxicity to humans and living organisms necessitates development of sensitive methods to analyze them 14,5]. Gas chromatography ( G C I is a conventional analysis method for these herbicides [6,7]. However, derivatization prior to analysis is necessary for the carboxyl group to increase its stability during GC separation. Previous studies combined high-performance liquid chroma- tography ( H P L C ) with GC to simultaneously analyze these compounds [8,9]. Satisfactory results were obtained by reversed-phase with acidified eluents and
*Corresponding author.
UV or MS detection. The acid condition can suppress the ionization of the acids. Some of the chloro- phenoxy acids are racemic mixtures and only the d-isomers are the active ingredients. LC using a chiral stationary phase can be applied for these separations [10]. However, the disadvantages of these approaches are that by using chiral LC, the columns are expensive and that different types of columns may be needed to analyze relatively similar racemic compounds.
In the last decade, capillary electrophoresis (CE) has become a highly effective tool in separating ionic and neutral compounds [11-15]. It has been applied to analyze many different compounds: from small ions to large molecules such as proteins and DNA. CE offers the advantages of high resolution, high separation efficiency, and good reproducibility. Sev- eral investigators have analyzed pesticides by CE
0021-9673/96/$15.00 © 1996 Elsevier Science B.V. All rights reserved
218 Y. Hsieh, H. Huang / J. Chromatogr. A 745 (1996) 2 1 7 - 2 2 3
[ 16-19]. Wu et al. [ 16] separated different herbicides by micellar electrokinetic chromatography (MEKC). Their results indicated that free solution electro- phoresis did not have a sufficient amount of res- olution to separate 2-(2,4-dichlorophenoxy)-propion- ic acid (2,4-DP) and 2-(4-chloro-2-methylphenoxy) propionic acid. Adding SDS and Brij-35 was deemed necessary to achieve the baseline resolution. Yeo et al. suggested using different cyclodextrins to in- crease the necessary resolution [17]. Also, Nielen used modified cyclodextrin added to the separation buffer to confirm an actual product's impurities [18,19].
This study examines the feasibility of using capil- lary electrophoresis with different cyclodextrin addi- tives (ce-cyclodextrin and/3-cyclodextrin) to separate and quantify seven chlorophenoxy acid herbicides in water samples. The effects of various concentrations of cyclodextrin on the separation are also investi- gated. In addition, the baseline separation of these herbicides and of the two pairs of enantiomers is further studied by mixing a-cyclodextrin and /3- cyclodextrins in the running buffer.
2. Experimental
2.1. Apparatus
Beckman P/ACE 2000 and 5500 capillary electro- phoresis systems (Beckman Instruments, Palo Alto, CA, USA) were used. The UV absorbance detector operated at a wavelength of 200 nm. CE was performed in a 47 cm (40 cm to detector)×50 # m I.D. fused-silica capillary tube (Polymicro Tech- nologies, Phoenix, AZ, USA). The capillary column was assembled in the cartridge format of Beckman Instruments. The voltage of the electrophoresis sepa- ration is 25 kV, and the temperature of capillary column was set at 22 °C. Pressure injection was the sample injection method. Data analysis was per- formed on System Gold software.
2.2. Chemicals
Disodium hydrogenphosphate and sodium di-
hydrogenphosphate were obtained from Fluka
(Buchs, Switzerland), c~- and /3-cyclodextrins from
Sigma (St. Louis, MO, USA). 2,4-dichlorophenoxy- acetic acid (2,4-D), 2-(2,4-dichlorophenoxy) propi- onic acid (2,4-DP), 4-(2,4-dichlorophenoxy) butyric acid (2,4-DB), 4-chloro-2-methylphenoxyacetic acid (MCPA), were obtained from Sigma (St. Louis, MO, USA). 4-(4-chloro-2-methylphenoxy) butyric acid (MCPB), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) and 2-(2,4,5-trichlorophenoxy) propionic acid (2,4,5- TP) were obtained from Aldrich (Milwaukee, WI, USA). All other chemicals were of analytical grade and purchased from Merck (Darmstadt, Germany). Water was purified in a Milli-Q water system (Millipore, Bedford, MA, USA). 47 mm diameter C~8 disk was purchased from J.T. Baker (Phillips- burg, N J, USA)
2.3. Procedure
2 m g / m l stock solutions of the herbicides were prepared in isopropanol. The various concentration sample solutions were prepared by dissolving the stock solution in isopropanol and buffer (1:1, v/v). Electrophoresis buffers were prepared by dissolving 0.1 M disodium hydrogenphosphate and sodium dihydrogenphosphate in water. The C~8 disk was first cleaned with 5 ml methanol and 5 ml water. A 500 ml sample solution was next eluted with 25 ml/min speed through the disk. The herbicides in the C~s disk were extracted twice by 4 ml of methanol. The methanol solution was then evaporated to a final volume of 2.5 ml.
3. Results and discussion
The chlorophenoxy acid herbicides under study could be divided into two groups by different chemical structures. One group (2,4-DB, MCPB, 2,4-DP, 2,4-D, and MCPA) had three substitutes in the benzene ring, and the other (2,4,5-TP and 2,4,5- T) had four substitutes. At the pH of the phosphate buffer used in this study, all of the herbicides were partially or totally dissociated in the buffer. This finding suggests that these herbicides carry a nega- tive charge throughout the separation.
Fig. 1 reveals that analyzing the structurally related herbicides by capillary electrophoresis with- out any additives could not yield a complete sepa-
Y. Hsieh, H. Huang / J. Chromatogr. A 745 (1996) 217-223 219 0.012 < ' 0.008 8 2 0 . 0 0 4
rl
!i
I 1,2 3 7 5,4 0 0 2 4 Time (rain)F~g. 1. Electropherogram of the chlorophenoxy acid herbicides, Voltage, 25 kV; separation tube, 47 cm long (40 cm to detector)× 50 /zm I.D. ; buffer, phosphate buffer pH 5.6; conductivity, 2.05 mS/cm; pressure injection, 4 s; detector wavelength 200 nm. Peaks: I - 2,4-DB; 2= MCPB; 3= 2,4-DP; 4= 2,4-D; 5= MCPA; 6= 2,4,5-TP; 7= 2,4,5-T.
ration. More specifically, two pairs of the analytes, 2,4-DB/MCPB and 2,4-D/MCPA, could not be resolved, respectively. The poor resolutions are owed to the components in each of these two pairs having differed only by a substituent, changing from a chlorine to a methyl group in the benzene ring, thereby making it difficult for them to be separated. Therefore, to obtain an enhanced resolution for these two pairs and isomers of 2,4-DP and 2,4,5-TP, various cyclodextrins or surfactants were added in the running buffer. Results obtained from adding surfactants were unsatisfactory. However, adding cyclodextrins significantly improved separation. Thus, a more detailed discussion follows regarding the influence of adding different cyclodextrins in capillary electrophoresis for chlorophenoxy acid analysis.
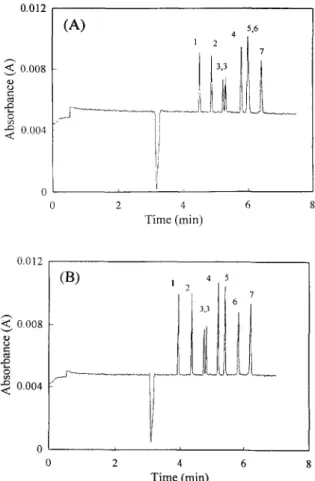
3.1. The effects of a-cyclodextrin on separation Fig. 2 summarizes the results of adding 1 mM and 2 mM c~-cyclodextrin in a phosphate buffer. The baseline resolution was obtained with 1 mM cr- cyclodextrin, except for MCPA and 2,4,5-TP. The two enantiomers of 2,4-DP could also be separated completely. By adding 2 mM of a-cyclodextrin, the baseline resolution could be obtained for seven herbicides including the enantiomers of 2,4-DP.
0.012
g
0.008 e" 0.004 0.012 (A) s.6 4 [ i 2 4 6 Time (rain) "< 0.008 8 e- ..Q 0.004 ( B ) , 4 5 2 7 I I 1 6 , 0 J i i 0 2 4 6 T i m e (min)Fig. 2. Electropherogram of the chlorophenoxy acid herbicides in buffer with different a-cyclodextrin concentrations, (A) 1 mM a-cyclodextrin; (B) 2 mM a-cyclodextrin. Others are the same as in Fig. 1.
Comparing Fig. 1 and Fig, 2 obviously indicates that most migration times decreased with a - c y c l o - dextrin. The change in migration time therefore suggests a certain interaction between the herbicides and c~-cyclodextrin. If the herbicide molecules enter the cyclodextrin's interior to form a stable inclusion complex, the migration time of the herbicide should decrease. As to the stability of the cyclodextrin inclusion complex, it is governed by several factors, e.g., hydrogen bonding, hydrophobic interactions, and the molecules' space-filling ability [20]. The current results seem to suggest that the molecular size of the herbicides approach the interior size of cyclodextrin, thus a relatively stable inclusion com- plex could be formed.
220 Y. Hsieh, H. Huang / J. Chromatogr. A 745 (1996) 217-223 6 .=.
g
4 l - a - - ~ - : ~ 3 , 3 l - ~ 1 i , , , i i 0 1 2 3 4 6 8 10 Concentration (mM)Fig. 3. Relationship between migration time and ce-cyclodextrin concentration of buffer, a-cyclodextrin concentration is from 1 mM to 8 mM. Voltage, 25 kV; separation tube, 47 cm long (40 cm to detector)×50 #m I.D. ; buffer, phosphate buffer pH 5.6; conductivity, 2.05 mS/cm; pressure injection, 4 s; detector wavelength 200 nm.
buffer markedly affected the migration times of most chlorophenoxy acid herbicides, as indicated in Fig. 3. The five herbicides, i.e., 2,4-DB, MCPB, 2,4-DP, 2,4-D, and MCPA, eluted first and migrated faster as the concentration of o~-cyclodextrin increased. How- ever, the migration velocity o f the other two her- bicides, 2,4,5-TP and 2,4,5-T, did not change with the concentration o f ce-cyclodextrin. The effects of different concentrations of o~-cyclodextrin on these herbicides depend primarily on the herbicides' mo- lecular size. As previously mentioned 2,4,5-TP and 2,4,5-T have four substitutes in the benzene ring, whereas the other five herbicides have three. Since each of the five herbicides has a relatively small molecule suitable for forming an inclusion complex with ce-cyclodextrin, the ratio o f the inclusion com- plex to free herbicides increased when more o~- cyclodextrins were added to the buffer. Nevertheless, 2,4,5-T and 2,4,5-TP are too large to enter the interior of a - c y c l o d e x t r i n , subsequently leading to insensitivity to the concentration of o~-cyclodextrin. As indicated in Fig. 3, after adding 8 m M a - cyclodextrin in the buffer, the two enantiomers of 2,4-DP could not be resolved well. From the peak resolution calculated on different concentrations of c~-cyclodextrin, the optimum approach to separate the seven herbicides and the two enantiomers o f 2 A - D P was adding 2 m M ce-cyclodextrin.
3.2. The e f f e c t s o f f l - c y c l o d e x t r i n on s e p a r a t i o n
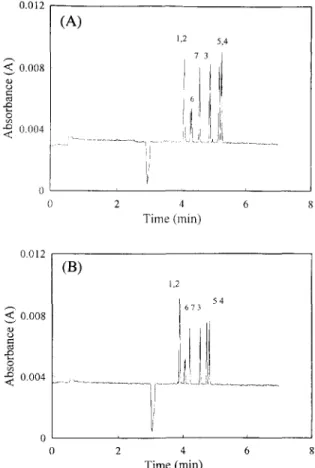
As discussed in the previous section, the mole- cules of 2,4,5-TP and 2,4,5-T are too large to form an inclusion complex with o~-cyclodextrin. The interior cavity of fl-cyclodextrin has a larger volume than a - c y c l o d e x t r i n . Therefore, fl-cyclodextrin was chosen to replace o~-cyclodextrin for resolving the structurally related herbicides, particularly for those compounds with four substitutes in the benzene ring. Fig. 4 shows the results of adding 2 mM and 7 m M /3-cyclodextrin in the buffer. The baseline resolution was obtained with 2 m M / 3 - c y c l o d e x t r i n , except for 2,4-DB and MCPB. The two enantiomers
0.012 0.008 0.004 (A) 1,2 5,4
! J 73
,i,i i I i jIi
0.012 2 4 6 Time (rain)(B)
0.008 ,.o 0.004 . . . 1 i] 0 0 2 1,2 673 54L J;
V
i i 4 6 8 Time (min)Fig. 4. Electropherogram of the chlorophenoxy acid herbicides in buffer with different fl-cyclodextrin concentrations, (A) 2 mM ,B-cyclodextrin: (B) 7 mM ,B-cyclodextrin. Voltage, 25 kV: sepa- ration tube, 47 cm long (40 cm to detector)×50/xm I.D. : buffer, phosphate buffer pH 5.6: conductivity. 1.74 mS/cm: pressure injection, 4 s: detector wavelength 200 nm.
Y. Hsieh, H. Huang / J. Chromatogr. A 745 (1996) 2 1 7 - 2 2 3 221
of 2,4,5-TP can be separated partially. Results ob- tained after pouring 7 mM /3-cyclodextrin in the buffer were similar to those of 2 mM/3-cyclodextrin except for the more rapid migration time.
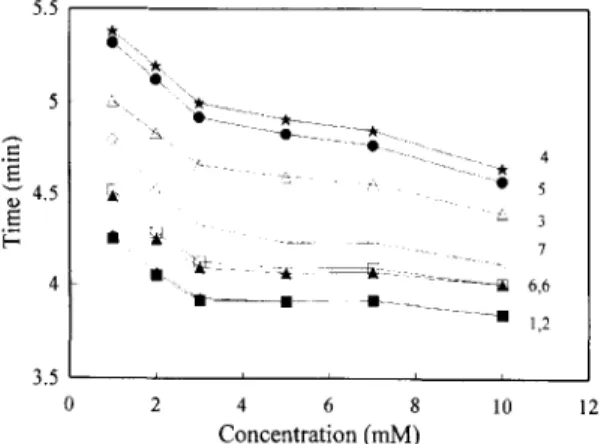
The migration times of the herbicides are shown in Fig. 5 as a function of the concentration of /3- cyclodextrin in the buffer. Fig. 5 reveals that all of the seven herbicides increased their migration ve- locity with an increasing concentration of /3-cyclo- dextrin. This phenomenon indicates that all of the herbicides could form an inclusion complex with /3-cyclodextrin. In addition, more inclusion complex- es were formed as more/3-cyclodextrins were added. The migration order of these seven herbicides in capillary electrophoresis with o~-cyclodextrin and/3- cyclodextrin in the buffer were further compared (Fig. 3 and Fig. 5). Marked differences for 2,4,5-TP and 2,4,5-T were particularly observed between these two graphs, which could be accounted for by the difference of the stability constants of the inclusion complex formed. As previously mentioned, 2,4,5-TP and 2,4,5-T have larger molecules than the other five herbicides. Therefore, these two compounds form a more stable inclusion complex with /3-cyclodextrin which has a two-fold volume interior cavity than a-cyclodextrin. Thus, in the buffer with /3-cyclo- dextrin, 2,4,5-TP and 2,4,5-T migrated faster than 2,4-DE 2,4-D and MCPA.
The molecular sizes of 2,4,5-TP and 2,4,5-T are better fitted in /3-cyclodextrin than the other five
5.5 E ~ 4 . 5 L 3 - 7 - - ~ l l 1 , 2 3 . 5 ~ , i , i 0 2 4 6 8 10 12 Concentration (mM)
Fig. 5. Relationship between migration time and /3-cyclodextrin concentration of buffer. /3-cyclodextrin concentration is from I mM to 10 raM. Others are the same as in Fig. 4.
herbicides. However, 2,4-DB and MCPB migrated first in /3-cyclodextrin modified buffer. The more rapid migrations of 2,4-DB and MCPB at pH 5.6 buffer can be accounted for by their lower dissocia- tion degrees (the pK a values, 4.58 and 4.86, respec- tively) than those of other herbicides (the pK a values, all around 3.00) [2]. The lower dissociation degrees suggest that 2,4-DB and MCPB form less negative ions, thereby leading to the most rapid elution. Thus, the migration velocity is affected by both the inclusion complex's stability and the pH value of the running buffer. Such an explanation is compatible with the theoretical model proposed by Rawjee et al. [21,22].
3.3. The effects of ~- and/3-cyclodextrin on separation
As previously mentioned, the two enantiomers of 2,4,5-TP could not be separated completely as o~- cyclodextrin inclusion complex. Similarly, the en- antiomers of 2,4-DP could not be separated in /3- cyclodextrin. To separate these two pairs of enantio- mers in one single run, the electrophoretic buffers were prepared by adding o~- and/3-cyclodextrin with different ratios. Such a method was used in MEKC to separate some chiral/3-blockers successfully [23]. According to Fig. 5, altering the concentration of /3-cyclodextrin would not produce an enhanced resolution for the enantiomers of 2,4,5-TP. Hence, the concentration of /3-cyclodextrin was fixed at 1 mM. Fig. 6 shows the migration times which varied with the concentration of a-cyclodextrin in the buffer ranging from 1 mM to 8 mM. Thus, each herbicide had the opportunity to interact with c~- cyclodextrin or /3-cyclodextrin. The subsequent mi- gration order was quite similar to that with only a-cyclodextrin in the buffer.
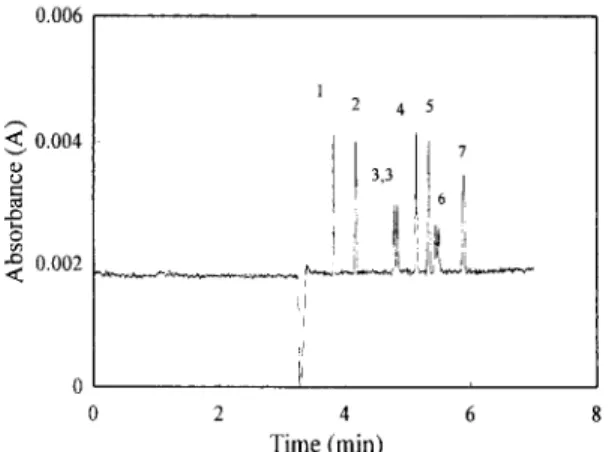
Calculating the peak resolution with various con- centrations of a-cyclodextrin at the fixed concen- tration of/3-cyclodextrin at 1 mM revealed that the optimum choice was 4 mM o~-cyclodextrin. Fig. 7 presents separation results with 4 mM c~-cyclodextrin and 1 mM /3-cyclodextrin. The baseline resolution was obtained for all seven herbicides; in addition, the two ;airs of enantiomers of 2,4-DP and 2,4,5-TP could be separated.
222 Y. Hsieh, H. Huang / J. Chromatogr. A 745 (1996) 217-223 5 bZ i..-*-_ 4 3,3 2 1 3 ~ r 0 2 4 6 8 10 Concentration (mM)
Fig. 6. Relationship between migration time and o~-cyclodextrin concentration of buffer, a - c y c l o d e x t r i n concentration is from 1 mM to 8 mM. Voltage, 25 kV; separation tube, 47 cm long (40 cm to d e t e c t o r ) × 5 0 # m I.D.; buffer, phosphate buffer pH 5.6; /3- cyclodextrin 1 raM; conductivity, 2.65 m S / c m ; pressure injection, 4 s; detector wavelength 200 nrn.
3.4. Optimum separation condition
Based on the results in this study, two separation conditions are recommended to analyze these seven herbicides. One condition employs 2 mM ~-cyclo- dextrin to modify pH 5.6 phosphate buffer. This condition can be used to separate the seven analytes
and the enantiomers of 2,4-DP. The other condition uses 4 mM a-cyclodextrin and 1 mM/3-cyclodextrin to modify pH 5.6 phosphate buffer. The latter condition can be used to separate the seven analytes and the enantiomers of 2,4-DP and 2,4,5-TE The correlation coefficients of the calibration lines for these two separation conditions were all above 0.996. The dynamic range of analyzing the seven herbicides was at least two orders, from 1 ppm to 100 ppm.
3.5. Analysis of herbicides spiked on lake water Fig. 8 summarizes the analysis results of l0/~g of each herbicides spiked on 500 ml of lake water. The herbicides in lake water were first extracted by a C j8 membrane disk before injected to the capillary electrophoresis. The recovery of herbicides in lake water by C~8 disk ranged between 73% and 90%. The herbicides' concentrations were increased by approximately 150 fold. Therefore, the detection limits of herbicides in lake water are below 1 ppb. Other environmental solution were also examined by the C is disk extraction method. The recoveries are all above 70%, which is unaffected by different matrix backgrounds. This could possibly be owing to the solution being adjusted to pH 2.0 before ex- traction by a C~8 disk. This procedure could decrease the background matrix adsorbed by the C~s disk.
0.006 "< 0.004 0.002 1 2 4 5 7
, t! i
2 4 Time (rain) 6 8Fig. 7. Elecm)phemgram of the chlorophenoxy acid herbicides. e~-cyclodextrin 4 mM: /3-cyclodextrin I raM. Others are the same as in Fig. 6. 0.003 < 0.0015 e~ < 1 4 5 2
t
I
3 , 3 1 t ~ 6 2 4 6 Time (rain)Fig. 8. Electropherogram of the 20 # g / I chlorophem~xy acid herbicides spiked on lake water. Others are the same as in Fig. 7.
Y. Hsieh, H. Huang / J. Chromatogr. A 745 (1996) 2 1 7 - 2 2 3 223
4. Conclusion
In this study, w e h a v e s i m u l t a n e o u s l y separated the s e v e n h e r b i c i d e s w i t h i n 7 m i n and c o m p l e t e l y r e s o l v e d the t w o pairs o f e n a n t i o m e r s . T h e resolution was h e a v i l y i n f l u e n c e d by the c a v i t y size and c o n c e n t r a t i o n o f c y c l o d e x t r i n s . A n a l y z i n g the s e v e n h e r b i c i d e s by c a p i l l a r y e l e c t r o p h o r e s i s poses advan- tages o f a h i g h resolution, h i g h separation e f f i c i e n c y and g o o d r e p r o d u c i b i l i t y . M o r e o v e r , capillary elec- trophoresis can g i v e d e t e c t i o n limits o f herbicides in the sub-ppb range w i t h a p r e c o n c e n t r a t i o n o f her- bicides in the e n v i r o n m e n t a l solution by a C J8 disk.
Acknowledgments
T h e authors w o u l d like to thank the N a t i o n a l S c i e n c e C o u n c i l o f T a i w a n for the financial support o f this w o r k ( N S C 8 3 - 0 2 0 8 - M - 0 0 9 - 0 2 3 ) .
References
[1] W.T. Thomson, Agricultural Chemicals book (II): Her- bicides, Thomson, Fresno, CA, ca. 1989.
[2] RC. Kearney and D.D. Kaufman, Herbicides, Marcel Dekker, New York, 2rid ed., 1975.
[3] S.K. Dedatta and R.Q. Lacsina, Weed Sci., 19 (1971) 203. [4] D.J. Munch, R.L. Graves, R.A. Maxey and T.M. Engel,
Environ. Sci. Technol., 24 (1990) 1446.
[5] W.C. Brumley and C.M. Brownrigg, J. Chromatogr., 646 (1993) 377.
[6] M.J.M. Wells and J.L. Michael, Anal. Chem., 59 (1987) 1739.
[7] J. Hajslova and F, Pudil, J. Chromatogr., 438 (1988) 55. [8] S. Butz, Th. Heberer and H.-J. Stan, J. Chromatogr. A, 677
(1994) 63.
[9l R.O. Mumma and J.D. Spittler, J. Agric. Food Chem., 36 (1988) 492.
[10] B. Blessington and N. Crabb, J. Chromatogr., 454 (1988) 450.
[11] J.W. Jorgenson and K.D. Lukacs, Science, 222 (1983) 266. [12] T. Tsuda, JJv'. Sweedler and R.N. Zare, Anal. Chem., 62
(1990) 2149.
[13] F. Foret, L. Krivfinkov~ and R Bocek, in B.J. Radola (Editor), Capillary Zone Electrophoresis, Cambridge Univ. Press, New York, 1993.
[14] M. Novotny, H. Soini and M. Stefansson, Anal. Chem., 66 (1994) 646A.
[15] S. Terabe, K. Otsuka and T, Ando, Anal. Chem., 57 (1985) 834.
[16] Q. Wu, H.A. Claessens and C.A. Cramers, Chromatographia, 34 (1992) 25.
[17] S.K. Yeo, H.K. Lee and S.F.Y. Li, J. Chromatogr., 594 (1992) 335.
[18] M.W.F. Nielen, J. Chromatogr., 637 (1993) 81. [19] M.W.F. Nielen, Trends Anal. Chem., 12 (1993) 345. [20] J. Snopek, I. Jelfnek and E. SmolkovA-Keulemansov~i, J.
Chromatogr., 452 (1988) 571.
[21] Y.Y. Rawjee, D.U. Staerk and Gy. Vigh, J. Chromatogr., 635 (1993) 291.
[22] YN. Rawjee and Gy. Vigh, Anal. Chem., 66 (1994) 619. [23] H. Siren, J.H. Jumppanen, K. Manninen and M.L. Riekkola,