j. CHEM. SOC. PERKIN TRANS. 2 1995 58 I
Face Selectivity in the Paterno-Buchi Reactions of Methacrylonitrile t o
5-Substituted Adamantan-2-ones
Wen-Sheng Chung," Yei-De Liu and Nae-Jean Wang
Department of Applied Chemistry, National Chiao Tung University, Hsinchu, Taiwan, 30050, R. 0. C.
The photocycloaddition of methacrylonitrile to 5-substituted adamantan-2-ones (1 - X ) produces two geometrically isomeric oxetanes in which the oxygen atom and the 5-substituent are in anti or syn positions. The substituent was varied from fluoro, chloro, bromo, phenyl to SiMe, and the product ratios, except for SiMe,, were similar (ca. 56:44) in all instances. Structure determination of the
anti and syn oxetanes were carried out by ' H and 13C NMR and mass spectroscopy. The structure
of syn-5- bromospiro[adamantane-2,2'-oxetane] (syn-5- Br) was further supported by single-crystal X-ray crystallography. The product formation bias resulting from the attack on the zu-face is discussed in terms of transition-state hyperconjugation, but new results for photocycloaddition reactions of 5- trimet hylsilyl -su bstituted adamantan - 2-one (1 - Si Me,), with both fumaronitrile and met hacrylonitrile, show an unprecedented o-electron-withdrawing characteristic with this substituent.
n-Facial stereoselectivity by various reagents in nucleophilic, electrophilic, radical and cycloaddition reactions to trigonal carbon centres continues to attract considerable theoretical and experimental attention. Although several model studies have clearly demonstrated the importance of long-range electronic effects to be a determining factor in diastereofacial selectivity, the precise nature of the electronic interaction remains controversial.'-6 Transition-state hyperconjugation'*3-5 and electrostatic field i n t e r a c t i ~ n * * ~ ~ ~ are the two most popular explanations of the face-selectivity results to be found in the literature.
5-Substituted adamantan-2-ones 1-X and their derivatives have proved to be useful probes in research aimed at understanding the electronic factors in face selection. The advantages of their use include the presence of two virtually isosteric faces, the absence of conformational uncertainty and the possibility of forming only two geometrically isomeric products. Studies by le Noble et aL3 of a variety of reactions indicate that the reagent prefers to attack the face that is antiperiplanar to the more electron-rich vicinal bonds (zu and en face preference in 1 when X equals an electron-withdrawing and electron-donating group, respectively). These results have been reconciled with Cieplak's transition-state hyper- conjugation model.'
The photocycloaddition of ketones to olefins (also known as the Paterno-Buchi r e a ~ t i o n ) ~ was one of the earliest examples to demonstrate the usefulness of photochemistry to the synthetic chemist. Mechanistic studies' have been done, primarily with acetone, but very little is known about the stereochemistry of this reaction when the two faces of the carbonyl group are different. Variously 7,7-disubstituted 2- norbornanones have been shown to undergo cycloaddition on the e m side, l o but there appears to be no guidance for instances in which the face differential results from an electronic perturbation. Recently, we have reported' l a that photo-
cycloaddition of the electron-poor olefin ( E ) - 1 ,Zdicyano- ethylene 2 to 5-substituted adamantanones occurs with the formation of products in accord with this concept. It was later found' I b that the use of (2)-1,2-diethoxyethylene 3 gave the
same stereochemical result even though this electron-rich olefin is known to undergo the reaction uia a very different pathway. Besides using electron-withdrawing 5-substituents (e.g. halogens and the phenyl group) of 1-X, we also used an electron- donating/neutral substituent (such as 1-SiMe,) to study face selectivity. We report here our studies on the photocycloaddition of 5-substituted adamantan-2-ones (1-X) to methacrylonitrile (4).
Results and Discussion
The singlet n,n* state of 1-X is trapped by an a,P-unsaturated nitrile such as 2 and 4 via a concerted [2
+
23 cycloaddition involving the now occupied n* orbital (face attack) which leads to a regiospecific reaction. The triplet state does not directly lead to an oxetane but results in olefin dimerization (Scheme 1).13 14 hv 1-x + 4
-
CH&N+ 8
10 4 s x O 6 6 (al7ti)b-X (syn )-5-x Scheme 1 6Exciplex formation has been proposed to be the initial step of a quenching interaction between ketone n,n* singlet or triplet states and olefins.8u*b The exciplex appears to have a charge transfer characteristic, with the n,n* state acting as an electron donor to electron-poor olefin (4)."vb Once formed, the exciplex is subject to dissociation, or to bond formation leading directly to oxetanes.
Thus, when a Xe/Hg lamp was used in conjunction with a potassium chromate filter to irradiate an acetonitrile solution of
1-F and excess 4, the product of brief exposure was shown by means of VPC to be a mixture of two components in the ratio of
582
J. CHEM. SOC. PERKIN TRANS. 2 1995 54: 46 (see Scheme 1).-/-~' Integration of the oxetane-ring proton(CH,CNCCH,) NMR signals confirmed the ratio. Yields of oxetanes were 70-95%, based on converted l-X; dimers (6) and polymers of 4 were also produced. The proton NMR spectra of the oxetanes confirmed structures 5, rather than those, 7, from
5 7
the alternative orientation of cycloaddition. Arnold
'
has suggested that hydrogen on the carbon cc to the oxygen of an oxetane ring has a chemical shift 4-5 ppm, whereas hydrogen on the P-carbon atom has a chemical shift of 2.5-3.6 ppm. In the eleven oxetanes we have studied, the oxetane ring proton absorptions fell in the region of 2.4-2.9 ppm (see the Experi- mental), which is in good agreement with the above description. The magnitude of the geminal coupling (ca. 11.7 Hz) of the oxetane ring protons provides further evidence for the oxetane structures 5. Barltrop and Carless,13 in their study of photocycloadditions of aliphatic ketones to a series of a,P-unsaturated nitriles, obtained a value for the geminal coupling constant of the oxetane ring C, protons of ca. 11.5 Hz (structures 8-10) contrasting with the geminal coupling J,,, of
6.0 Hz obtained by Lustig et ~ 1 . lfor the oxetane ring C, ~ ~
protons. This, along with other examples of oxetane ring coupling constants taken from the l i t e r a t ~ r e , ' ~ shows that J , , , is of the order of 11-12 Hz, whereas J,,, is 5-7 Hz. Another indication of structure 5 instead of 7 comes from 13C NMR study, where the chemical shift difference (Ad) between C-8 and C-10 and between C-4 and C-9 is similar and falls into the range of0.2-0.3 ppm (see Table 1). However, previous studies"* show that a large difference in chemical shifts for carbons close to one of the cyanomethylene group is expected; i.e. if 7 were formed, the chemical shift difference (Ad) should have been larger than 0.5 ppm.
8 9 10
The configuration assignment of the spiro skeleton was found to be consistent with a 13C NMR study of a type described by le Noble et In essence, this is an additivity scheme in which the chemical shifts are calculated from those of the corresponding carbons in adamantane, 1 -fluoroadamantane For the sake of convenience and continuity, we refer to all of our adamantanes as 2,5-substituted even though this is sometimes technically incorrect (e.g. 2,s-dimethyladamantane is really 1,4-, etc.). We also wish to point out that the E,Zdescriptors now in general use for olefins (J. E. Blackwood, C. L. Gladys, K. L. Loening, A. E. Petrarca and
J. E. Rush, J. Am. Chem. Soc., 1968,90,509; footnote 2) are generally not adequate for disubstituted rings since these will often be chiral; however, even-membered carbocyclic rings with two-four substituents at two opposite corners have C, symmetry, and the E/Z symbols can then be used without ambiguity (olefins may be regarded as a special case, with two-membered rings). 2,5-Disubstituted adamantanes fit this criterion, and hence we have used these descriptors for them.
and the parent spirooxetane. The C-4,9 and C-8,lO pairs, by far the most informative beacons in this regard, are readily distinguished from one another by virtue of the 13C-19F splittings, which are roughly 20 and 0 Hz, respectively (Table 1). In the parent compound, they can be recognized by means of their chemical shifts: when one of the two substituents at C-2 is an electronegative atom such as oxygen, the carbon pair 'below' it is always shielded compared to the anti pair.
' '
The chemical shifts thus computed for C-4,9 and C-8,lO agree with the observed values to within a few tenths of 1 ppm; differences of 1 ppm or more result if the opposite configurations are assumed (Table 1).The experiments with 1-Cl, -Br, -Ph and -SiMe, followed a similar course (Table 2); the anti and syn assignments in these instances were also based on the 13C additivity method. As with the fluoro products, the chemical shifts calculated for C-4,9 and C-8,lO agree with the observed values to within a few tenths of 1 ppm (see Table 1). Column chromatography was used to obtain samples of these compounds in pure form. The minor product (syn-5-Br) from 1-Br was separated and crystallized in ethyl acetate-hexane (1 : 7, v/v), whose structure was further supported by way of a single-crystal X-ray diffraction study (Fig. 1).
The mass spectra of the oxetanes are also given in the Experimental section. They generally showed weak molecular ions (0-30% by the EI method); the main fragments arise from fission across the oxetane ring. Such ring fission is a known process;' ,16 thus, the parent oxetane predominantly gives ethylenes via ring cleavage. 16v1' Again, the possible structures 7
were eliminated from consideration by the lack of a large peak at m/z corresponding to the loss of HCOH from the molecular ion. Peaks at m/z corresponding to the loss of O=C(CN)CH, are clearly observed, also supporting the formation of oxetane structure 5 . The use of chemical ionization techniques'
*
greatly improved the detection of molecular ions of all oxetanes 5.The electron-donating (neutral) character of 5-trimethylsil-
Fig. 1 ORTEP drawing for structure syn-5-Br
4 v rn ier
z
4z
trans-oxet anes r/l Er
Table 1 Calculated' and observed' chemical shifts in 5-substituted-4'-cyano-4'-methylspiro[adamantane-2,2'-oxetanes] N c-1, c-3 39.91 37.84 c-2 86.35 C-4, C-9 33.27 32.89 c-5 26.07c-7
26.0 1 C-6 36.21 C-8, C-10 31.55 31.31 c-3' 43.73 c-4' 68.20 CN 121.90 CH3 28.40 C-ic-0
C-m C-P 42.28 (42.78) J = 11.0 40.13 (40.68) J = 10.1 84.58 (84.32) 37.98 (38.01) J = 19.8 37.65 (37.66) J = 19.8 90.69 (89.94) J = 184.6 29.25 (28.88) J = 9.8 41.67 (40.95) J = 15.4 30.15 (29.52) 29.94 (29.29) 43.64 68.49 121.61 28.40 43.01 (42.78) J = 11.0 40.90 (40.68) J = 10.2 84.08 (84.32) 36.66 (36.52) J = 19.8 36.46 (36.09) J = 19.8 90.55 (89.88) J = 183.5 29.33 (28.94) J = 9.9 41.54 (40.95) J = 17.5 3 1.49 (30.86) 42.59 68.43 121.70 28.43 31.84 (31.21) 42.62 (43.08) 40.49 (40.98) 84.28 (84.12) 43.06 (43.1 1) 42.71 (42.76) 65.43 (65.74) 29.39 (29.18) 46.44 (46.05) 29.83 (29.32) 29.60 (29.09) 43.61 68.46 121.58 28.40 CL Carbon 5-H anti-5-F syn-5-F unti-5-Cl syn-9C1 anti-5-Br syn-5-Br anti-5-Ph syn-5-Ph anti-5-SiMe, syn-5-SiMe, \o \o Jl 43.06 (43.08) 40.99 (40.98) 83.88 (84.12) 41.42 (41.42) 41.19 (41.19) 65.42 (65.68) 29.45 (29.24) 46.38 (46.05) 31.58 (31.01) 31.23 (30.66) 42.76 68.49 121.67 28.46 43.44 41.34 84.14 44.57 44.25 61.73 30.24 47.92 29.80 29.60 43.61 68.49 121.58 28.40 43.73 41.69 83.79 ' 42.88 42.88 61.97 30.18 47.83 3 1.52 31.17 42.68 68.46 121.61 28.43 40.55 38.37 85.89 38.77 38.45 34.90 26.83 42.35 30.85 30.64 43.94 68.46 121.96 28.55 149.17 124.73 128.25 125.98 40.75 38.60 85.51 37.23 37.1 1 34.64 26.91 41.57 32.57 32.25 43.41 68.46 121.99 28.58 149.58 124.8 1 128.14 125.78 39.58 37.41 86.52 33.36 33.01 19.15 25.69 35.86 31.42 31.23 44.01 68.36 122.12 28.61 SiMe, - 5.52 39.68 37.49 86.71 31.60 31.41 19.34 25.63 35.88 33.42 33.08 43.90 68.37 122.09 28.63 SiMe, - 5.43 t ' Calculated values are in parentheses. Measured by VXR-300 NMR operated at 75.4 MHz and reported in 6 scale, CDCI, (6 77.00), J is in Hz. See Scheme 1 for the numbering system. In the parent compound 5-H, the oxygen is understood to be syn to C-8 and C-10. ' Two peaks at 6 42.88 overlap.584 J. CHEM.
soc.
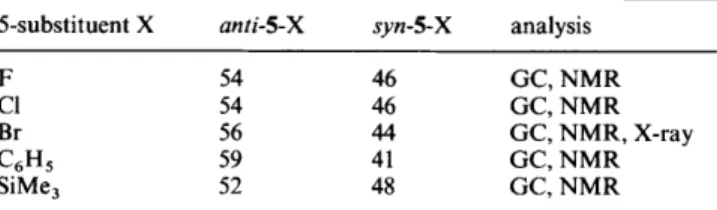
PERKIN TRANS. 2 1995Table 2 anti:syn epimer ratios (%) in the photocycloadditions of 4 to 5-substituted adamantanones (1-X)o in acetonitrile at room temperature
5-substituent X anti-5-X syn-5-X analysis
F 54 46 GC, NMR
c1
54 46 GC, NMRBr 56 4 4 GC, NMR, X-ray
C6H5 59 41 GC, NMR
SiMe, 52 48 GC, NMR
a Analysis by GC and 'H NMR; error limit for GC analysis is k 2% and
for 'H NMR analysis is 4 5%. Yields are 70-90% based on converted
l-x.
yll substituted adamantanone has been independently shown by le Noble and Adcock in reduction,20c,21 and methylation2' reactions. We have confirmed the reduction results to be 50: 50 in face selectivity, i. e. SiMe, fails to exert any influence on facial selectivity. Present results for the photocycloaddition reaction of l-SiMe, with both fumaronitrile 2 (anti-11 :syn-11 = 59 :41, Scheme 2)'" and methacrylonitrile 4 (anti-5:syn-5 = 52: 48)
CN CN
(anti)-l l-SiMe3 (syn )-1 l-SiMe3
(59%) (41%)
Scheme 2
show an unprecedented zu face attack preference, i.e. trimethylsilyl behaves like a o-electron-withdrawing group in excited states. Long-range steric interaction by the relatively bulky substituent used in the explanation of free cation6 results cannot be applied here. The exact nature of SiMe, and its homologue in the excited states is not clear, possible involvement of the electrostatic field effects is not excluded.
The present results add to the already extensive evidence for the proposition that addition to trigonal carbon occurs at the face antiperiplanar to the more electron-rich vicinal bond(s). It would seem difficult to find a reason for this preference in so many reactions as described here and elsewhere without involving transition-state hyperconjugation. We must note that, while all of the 5-substituents except SiMe, used in this study are electron-withdrawing and all induce predominant zu face attack, there is no obvious correlation between the magnitude of the antilsyn ratios and the strength of the induction.$ Such a correlation is clearly present in reactions producing 2- adamantyl cations20*22 and a more modest one has been observed in reductions and methylations of l-X.21*23 It must be realized, however, that our knowledge of inductive power is based almost exclusively on ground-state chemistry, and also that with the antilsyn ratios generally rather close to unity, it will remain difficult satisfactorily to prove or disprove such correlation. We are continuing with research aimed at studying the electronic effects of photochemical reactions in other series.
Experimental
Proton nuclear magnetic resonance spectra were taken on 300 and 400 MHz spectrometers. The data reported were recorded
$ This observation is similar to previous observation in other olefins (see ref. 1 la,b).
at 300 MHz unless otherwise specified. Natural abundance NMR spectra were taken by means of pulsed Fourier- transform, on a Varian Unity-300 MHz, high resolution NMR spectrometer, operating at 75.4 MHz. Broad-band decoupling was used to simplify spectra and aid peak identification. Chemical shifts are reported in parts per million and coupling constants in Hz for both nuclei, with the solvent (usually CDCl,) peak as an internal standard. The reference peak for I3C is 6 77.00, which is set at the centre peak of CDCl,, and for 'H it is 6 7.25 of CHCl,.
Gas chromatographic analyses were carried out on an instrument equipped with a flame ionization detector and a reporting integrator. The capillary column employed included HP- 1 crosslinked methylsilicone (SE-30, 25 m) and carbowax columns (25 m). GC/Mass spectral analyses were carried out by EI and CH, chemical ionization.
'
IR spectra were measured with a Nicolet 520 spectrometer; all samples were studied in the form of KBr disks.Materials.-All commercially obtained chemicals were reagent or spectrophotometric grade and were not purified prior to use unless otherwise specified. The synthesis of 5- phenyladamantan-2-one (l-Ph),24 5-chloro- ( l-Cl),24 5-bromo- (l-Br),25 and 5-fluoro-adamantan-Zones (1-F)22*26 have all been described. l-F was obtained by refluxing a mixture of 20 mol dm-, of the l-Br in 150 cm3 of cyclohexane with 50 mmol dm-, anhydrous silver fluoride,26 which is an adaptation of a procedure given by Schleyer for 1 -fluoroadamantane. The synthesis of l-SiMe, has been described by le Noble;21 following their procedures we could obtain the desired ketone plus a new product 5-pentamethyldisilyladamantan-2-one (1-
X-Ray Structure Analysis of anti-5-Br.--anti-5-Br, a colour- less solid with mp 108-1 12 OC, was crystallized from 15% ethyl acetate in hexanes. Its structure was determined by means of single-crystal X-ray analysis on a Rigaku AFC6S diffractometer with a graphite monochromated Cu-Ka
(A
= 1.541 78A)
radiation at 296 k 1 K, with an omega-28 type scan at 16 degree/min (in m). The crystals are primitive monoclinic, with space group P2,/n (14) and unit cell dimensions a = 10.459(4), b = 11.684(3), c = 11.566(4)A,
/I = 11 1.06(3)", V = 1318.9(9)A3,
2 = 4, pcalc = 1.34 g cm-,, crystal size (mm) 0.72 x 0.48 x 0.32, p(Cu-Ka) = 40.18 cm-', F(OO0) = 544, 2193 reflections, 2070 unique reflections, 1728 with I > 3.00a(I) and with 154 variable parameters. The model, which includes Br, 0, N, C atoms treated anisotropically and H atoms isotropically, was refined by the least-squares methods with weight m = 1/[a2(F,,)] to final R values of 0.040 and Rw = 0.029.8Irradiation of l-X with 4.-A relatively high concentration of 4 (0.75 mol dm-,) was employed to favour the formation of
oxetanes. Thus, a solution of 0.1 g of l-X and 1 g of 4 in 20 cm3 of spectrograde acetonitrile was placed in a Pyrex tube stoppered with a rubber septum. The solution was irradiated (Xe/Hg 500W lamp) for 48 h (ca. 6&90% conversion), through a 3 13 nm filter solution (0.002 mol dm-, K2Cr0, in 1% aqueous K2C0,). The solvent was removed from the dark red solution on a rotary evaporator and the residue was redissolved in ethyl acetate with sonication. The solution was filtered to remove undissolved solids, then concentrated on a rotary evaporator. The residues of all 5-substituted oxetanes (except 5-phenyl-) 5 Tables of atomic co-ordinates, bond lengths and angles and thermal parameters have been deposited at the Cambridge Crystallographic Data Centre. For details of the deposition scheme see 'Instructions for Authors (1995)', J. Chem. SOC., Perkin Trans. 2, 1995, issue 1.
J . CHEM. SOC. PERKIN TRANS. 2 1995 585 were chromatographed over silica gel with ethyl acetate in
hexanes (10 to 20% by volume) as eluent. The use of 40-63 pm silica gel 60 (E. Merck No. 9385) and a pressure-driven rate of 1 .O in min
'
leads to a successful ~ e p a r a t i o n . , ~ In every instance, anti-5-X eluted first, followed by syn-5-X. anti- and syn-5-Ph were separated by crystallization from 20% ethyl acetate in hexanes.The peak patterns in the 'H (CDCl,; 300 MHz) and I3C NMR spectra for all the oxetanes are very similar (for complete assignments of the "C peaks see Table 1).
4'-Cyano-4'-methylspiro[adamantane-2,2'-oxetane] (5-H). A
colourless liquid, 6, 2.84 (1 H, d, J 1 1.7), 2.42 (1 H, d, J 1 1.7), 2.38 (1 H, br s), 2.07-1.95 (4 H, m) and 1.77-1.58 (12 H, m); MS(E1) m/z 217 (M', 0.7%), 150 (M+ - 4, loo), 148 [M+ -
O=C(CN)CH,, 81, 80 (31), 79 (37).
oxetane] (anti-5-F). A colourless liquid, 6,2.88 (1 H, d, J 1 1.7), 2.59(1H,brs),2.48(1 H,d,J11.7),2.17(2H,brs),2.04-1.78 (1 1 H, m) and 1.53-1.42 (2 H, m); MS(E1) m/z 235 (M +, 0.4%),
an ti -4'
-
Cyano - 5 -flu0 ro-4'-me thy lsp ir o [adaman tane- 2,2'-168 (M' - 4, loo), 166 [M' - O=C(CN)CH,, 9],97 (36). syn-4'- Cyano-5-Jluoro-4'-methylspiro[adamantane-2,2'- oxetane] (syn-5-F). A colourless liquid, d,2.85 (1 H, d, J 11.7), 2.65 (1 H, br s), 2.46 (1 H, d, J 11.7), 2.24-1.87 (7 H, m) and 1.76-1.51 (8 H, m); MS(E1) m/z 235 (M', 473, 169 (32), 168 (M' - 4, loo), 166 [M' - O=C(CN)CH,, 621, 97 (86), 91
(34).
oxetane] (anti-5-Cl). A colourless solid (mp 110-1 14 "C),
BH
2.88(1H,d,J11.7),2.53(1H,brs),2.48(1 H , d , J l l . 7 ) , 2 . 1 1 - 1.91 (10H,m), 1.75(3H,s)and 1.66-1.48(2H,m).MS(ET)m/z anti-5- Chloro-4'-cyano-4'-methylspiro[adamantane-2,2'- 251 ( M + , 0.9%), 216 (M' - C1, 2) 186 ( 5 5 ) , 184 (M+ - 4, loo), 182 [M' -O=C(CN)CH,, 6],91 (45), 79 (41). syn-5- Chloro-4'-cyano-4'-methylspiro[adamantane-2,2'- oxetane] (syn-5-Cl). A colourless solid (mp 86-89 "C), 6, 2.84 (1 H, d, J 11.7), 2.57 (1 H, br s), 2.46 (1 H, d, J 11.7) in 2.47-2.43 (3 H, m), 2.161.93 (6 H, m) and 1.93-1.75 (7 H, m). MS(E1) m/z 251 ( M + , 0.6%), 216 (M+ - C1,6) 186 (36), 184 (M+ - 4,
loo), 182 [M' - O=C(CN)CH,, 71.
anti-5- Bromo-4'-cyano-4'-methylspiro[adamantane-2,2'- oxetane] (anti-5-Br). A colourless solid (mp 129-131 "C), SH 2.89(1 H , d , J11.7),2.48(1 H , d , J11.7)in2.50-2.46(2H,m),
2.33-2.21 (6 H, m), 2.09-1.96 (4 H, m), 1.75 (3 H, s) and 1.65- 1.53 (2 H, m); MS(E1) m/z 295 (M', 0.1%), 216 (M' - Br, 161, 91 (47), 79 (Br', 46). HRMS (m/z). Calc. for CI4- H,879BrNO: 295.0572. Found: 295.0570.
oxetane] (syn-5-Br). A colourless solid (mp 108-1 12 "C), B, 2.82 (1 H, d, J 11.7), 2.69-2.54 (3 H, m), 2.43 (1 H, d, J 11.7), 2.31 (2 H, br s), 2.21-2.09 (4 H, m) and 1.83-1.55 (7 H, m); MS(E1) m/z 295 ( M + , 0.1%), 217 (51), 216 (M' - Br, 100) 160 (43), 91 ( 5 9 , 79 (Br', 55), 41 (38). HRMS (m/z). Calc. for
c
4H 879BrNO: 295.0572. Found: 295.0565.oxetane] (anti-5-Ph). Colourless solid (mp 158-161 "C), d, 7.33-7.26 ( 5 H, m), 2.89 (1 H, d, J 11.7), 2.55 (1 H, br s), 2.47 (1 H, d, J 11.7), 2.13-1.77 (12 H, m) and 1.67-1.56 (3 H, m); MS(E1) m / z 293 (M', 38%), 226 (Mf - 4, loo), 224 [Mf -
(m/z). Calc. for C,,H,,NO: 293.1780. Found: 293.1777. oxetane] (syn-5-Ph). Colourless liquid, 6, 7.35-7.18 ( 5 H, m), 2.91 (1 H, d, J 11.7), 2.55 (1 H, br s), 2.50 (1 H, d, J 11.7) and 2.32-1.61 (15 H, m); MS(EI)m/z293 (M', 64%), 226 (M' - 4, loo), 224 [M' - O=C(CN)CH,, 81, 168 (38), 155 (75), 91 (61),
41 (38). HRMS (m/z). Calc. for C,,H,,NO: 293.1780. Found: 293.1778.
100) 160 (59), 149 (216 - 4, 34), 147 [216 - O=C(CN)CH,,
syn-5- Bromo-4'-cyano-4'-methylspiro [adaman tane-2,2'-
(59), 149 (216 - 4, 25), 147 [216 - O=C(CN)CH,, 341, 121
anti -4'- Cyan o -4'-m e t h yl- 5 -pheny lsp ir o [adaman tane-2,2 I -
O=C(CN)CH,, 81, 168 (M), 155 (60), 91 ( 5 3 , 41 (50). HRMS
syn-4'- Cyano-4'-methyl-5-phenylspiro[adamantane-2,2'-
an ti-4'- Cyano-4'-meth yl-5- trimethy lsiIylspiro [udamantane-
2,2'-oxetane] (anti-5-SiMe3). A colourless solid (mp 151- br s), 1.92-1.55 (15 H, m) and -0.12 (9 H, s); MS(E1) m/z 289 ( M + , 76%), 246 (33), 220 (6), 147 (loo), 105 (61), 73 (32). HRMS (m/z). Calc. for C,,H,,NOSi: 289.1863. Found: 289.1859.
syn-4'- Cyano-4'-methy1-5-trimethylsilylspiro[adamantane- 2,2'-oxetane] (syn-5-SiMe3). A colourless solid (mp 99-1 00 "C), dH 2.81 (1 H, d, J 11.7), 2.39 (1 H, d, J 11.7), 2.36 (1 H, br s),
1.94-1.45 (15 H, m) and -0.12 (9 H, s); MS(E1) mjz: 289 (M', loo), 246 (1 8), 220 (9), 147 (1 5 ) , 73 (58). HRMS (m/z). Calc. for C,,H,,NOSi: 289.1863. Found: 289.1850.
152 "C),6, 2.83 (1 H, d, J 11.2), 2.40 (1 H, d, J 11.7), 2.34 (1 H,
Acknowledgements
We thank Dr. Michael Y. Chiang for the X-ray crystal structure determination of syn-5-Br. This work was supported at NCTU by the National Science Council of the R.O.C.
References
1 ( a ) A. S. Cieplak, B. Tait and C. R. Johnson, J. Am. Chem. Soc.,
1989, 111, 8447; (b) A. S. Cieplak, J. Am. Chem. Soc., 1981, 103, 4540.
2 M. N. Paddon-Row, Y.-D. Wu and K. N. Houk, J. Am. Chem. Soc., 1992, 114, 10638 and references cited therein.
3 V. R. Bodepudi and W. J. le Noble, J. Org. Chem., 1994,59, 3265; 1991,56, 2001 and references cited therein.
4 B. Ganguly, J. Chandrasekhar, F. A. Khan and G. Mehta, J. Org.
Chem., 1993,58, 1734 and references cited therein.
5 R. L. Halterman, B. A. McCarthy and M. A. McEvoy, J . Org. Chem., 1992,57, 5585 and references cited therein.
6 W. Adcock, J. Cotton and N. A. Trout, J. Org. Chem., 1994,59, I867 and references cited therein.
7 (a) L. Paterno and G. Chieffi, Gazz. Chim. Ztal., 1909, 39, 341;
(6) G. Biichi, C. G. Inman and E. S . Lipinsky, J. Am. Chem. Soc.,
1954,76,4327.
8 For reviews, see ( a ) N. J . Turro, Pure Appl. Chem., 1971,27,679; (b) N. J. Turro, J. C. Dalton, K. Dawes, G. Farrington, R. Hautala, D. Morton, M. Niemczyk and N. Schore, Acc. Chem. Res., 1972,
5 , 92; (c) S. W. Schreiber, Science, 1985, 227, 858; (d) H. A. J.
Carless, in Synthetic Organic Photochemistry, ed. W. M. Horspool, Plenum Press, New York, 1984, p. 425; (e) G. Jones 11, in Organic
Photochemistry, ed. A. Padwa, Wiley, New York, 1981, Vol. 5. pp. 1-122.
9 N. J. Turro and P. A. Wriede, J. Am. Chem. Soc., 1970, 92, 320.
10 N. J. Turro and G. L. Farrington, J. Am. Chem. Soc., 1980,102,6056. I I (a) W.-S. Chung, N. J. Turro, S. Srivastava, H. Li and W. J. le Noble,
J. Am. Chem. Soc., 1988, 110, 7882; (b) W . 4 . Chung, N. J. Turro,
S. Srivastava and W. J. le Noble, J. Org. Chem., 1991, 56, 5020;
(c) W.-S. Chung,N. J. Wang, Y.-D. Liu,Y.-J. Leuand M. Y. Chiang,
J. Chem. Soc., Perkin Trans. 2, 1995, 307.
12 D. R. Arnold, R. L. Hinman and A. H. Glick, Tetrahedron Lett., 1964, 1425.
13 J. A. Barltropand H. A. J. Carless, J. Am. Chem. SOC., 1972,94,1951. 14 (a) E. Lustig, E. Ragelis and N. Duy, Spectrochim. Acta, Part A , 1967, 23, 133; (6) D. J. Pate1 and D. I. Schuster, J. Am. Chem. Soc., 1967,89, 184; (c) N. J. Turro and J. R. Williams, Tetrahedron Lett., 1969,321.
15 S. Srivastava, C. K. Cheung and W. J. le Noble, Magn. Reson. Chem., 1985, 23, 232.
16 (a) J. S. Bradshaw, J. Org. Chem., 1966,31,237; (b) J. J. Beereboom and M. S. von Writtenau, J. Org. Chem., 1965,30, 1231.
17 E. J. Gallegos and R. W. Kiser, J. Phys. Chem., 1962,66, 136. 18 For reviews of CI methods in mass spectrometry see ( a ) M. S . B.
Munson and F. H. Field, J. Am. Chem. Soc., 1966, 88, 2621;
(b) R. J. Anderegg, in Biomedical Applications of Mass Spectrometry, ed. C. H. Suelter and J. T. Watson, Wiley, New York, 1990, pp. 1-89; (c) D. Termont, D. D. Keukeleire and M. Vandewalle, J. Chem. Soc.,
Perkin Trans. I , 1977,2349.
19 For trimethylsilyl as a Ssubstituent, see refs. 6 and 21.
20 (a) W. Adcock, J. Couple, V. J. Shiner and N. A. Trout, J. Org. Chem.,
586 J. CHEM. SOC. PERKIN TRANS. 2 1995
1990,55,1411;(6) W. Adcock, A. R. Krstic, P. J. Duggan,V. J. Skiner,
Jr., J. C o o p and M. W, Ensinger, J. Am. Chem. SOC., 1990, 112, 3 140; (c) W. Adcock and N. A. Trout, J. Org. Chem., 1991,56,3229. 21 M. Xie and W. J. le Noble, J. Org. Chem., 1989,54, 3836.
22 M. Xie and W. J. le Noble, J. Org. Chem., 1989,54, 3839.
23 (a) C. K. Cheung, L. T. Tseng, M.-H. Lin, S. Srivastava and W. J. le Noble, J. Am. Chem. Soc., 1986, 108, 1598; (b) H. Li and W. J. le Noble, Tetrahedron Lett., 1990,31,4391.
24 H. W. Geluk, Synthesis, 1972, 374.
25 H. Klein and R. Wiartalla, Synth. Commun., 1979,9,825.
26 I. Tabushi and Y. Aoyama, J. Org. Chem., 1973,38,3447.
27 R. C. Fort and P. v. R. Schleyer, J. Org. Chem., 1965,30,789. 28 W. S. Chung and N. J. Wang, unpublished work. For a similar
reaction outcome using the reagents see K. Krohn and K . Khanbabaee, Angew. Chem., Int. Ed. Engl., 1994,33,99.
29 W. C. Still, M. Kahn and A. Mitra, J. Org. Chem., 1978,43,2923.
Paper 4/05742B Received 20th September 1994 Accepted 22nd November 1994