Pergamon
PII: S0031-9422(96)00375-5
Phytochemistry, Vol. 43, No. 4, pp. 839-842, 1996 Copyright © 1996 Elsevier Science Ltd Printed in Great Britain. All rights reserved 0031-9422/96 $15.00 + 0.0O
TAXANES FROM TAXUS MAIREI
SHUNG-JIM YANG, JIM-MIN FANG and Yu-SHIA CHENG*Department of Chemistry, National Taiwan University, Taipei, Taiwan 106, Republic of China
(Received in revised form 29 April 1996) Key W o r d lndex--Taxus maire; Taxaceae; taxane; twigs; diterpenoids.
A b s t r a c t - - F o u r new taxane diterpenes, 9a-hydroxy-14fl-(2-methylbutyryl)oxy-2a,5a, lOfl-triacetoxytaxa-4(20),
11-diene, 2a,5t~,9ot, 1 Off, 14fl-pentaacetoxytaxa-4(20), 11-diene, 5a-(cinnamoyl)oxy-7fl-hydroxy-9a, 1 Off- 13 a-tri- acetoxytaxa-4(20),ll-diene and 5ct-hydroxy-9a, lOfl,13ct-triacetoxytaxa-4(20),ll-diene, along with 12 known taxa-4(20),ll-dienes, have been isolated from twigs of Taxus mairei and their structures determined by spectral methods. Copyright © 1996 Elsevier Science Ltd
INTRODUCTION
The chemical constituents of the plants of the Taxus
genus have been extensively investigated [1-3], partly due to the finding of an antitumour agent, taxoi [4].
Taxus mairei is the only species belonging to the genus
Taxus found in Taiwan. A few taxane derivatives have been isolated from the heartwood of this plant [5, 6]. We report herein 16 taxane diterpenes having C4(20)- exocyclic double bonds isolated from the twigs of T.
mairei.
RESULTS AND DISCUSSION
A concentrated acetone extract of the twigs of T.
mairei was diluted with water and extracted with ethyl acetate. The ethyl acetate soluble material was chro- matographed to give 16 taxanes (1-16), 12 of which were known: 2a,5a,10fl,14fl- tetraacetoxytaxa-4(20), 11-diene (1) [7], 14fl-(isobutyryl)oxy-2a,5a, lOfl-tri-
acetoxytaxa-4(20),ll-diene (2) [7], 14fl-(2-methyl- butyryl)oxo- 2a,5cr, 10fl- triacetoxytaxa- 4(20), 11 - diene (3) [7], yunnanxane (4) [8], taxuyunnanine B (7) [9], 5 a - (cinnamoyl)oxy - 9tr,10fl,13a - triacetoxytaxa - 4 ( 2 0 ) , l l - d i e n e (8) [10, 11], 2tr-deacetoxytaxinine J (10) [12], taxinine J (11) [6], taxacin (12) [13], taxusin (13) [14, 15], 5a,7fl,9c~,lOfl,13ct-pentaacetoxytaxa-
4(20),1 l-diene (15) [16] and 5or - hydroxy - 2c~,7fl,9a, 10/~, 13 tz - pentaacetoxytaxa - 4(20), 11 - diene (16) [17]. Compounds 1 - 3 have been found in cell cultures of T. chinensis var. mairei [7].
Compound 5 gave rise to a molecular ion [M] ÷ at
mlz 562.312 consistent with a molecular formula C 3 1 H 4 6 0 9 . The ' H NMR spectrum (Table 1) showed
*Author to whom correspondence should be addressed.
resonances of four methyl groups at 8 0.87 (s), 1.12 (s), 1.58 (s) and 2.12 (s), which are characteristic of taxane diterpenes. In addition to the resonances for three acetyl groups at 8 169.8 (s), 169.9 (s), 170.5 (s), 21.3 (q), 21.3 (q) and 21.9 (q), the ~3C NMR spectrum (Table 2) displayed a signal at 8 175.6 (s) attributable to an ester group. The a-methylbutyrate moiety was indicated by the proton resonances at 8 0.85 (t, J = 7.2 Hz,
CH3),
1.11 (d, J = 7.2Hz,
CH3),
1.46 (m, CH2) and 2.34 (m, CH). The proton resonances at 8 4.84 (br s) and 5.28(br s) as well as the carbon signals at 8 141.8 (s) and 117.5 (t) suggested the presence of a methylene group. The structure of 5 was assigned as 9a-hydroxy-14fl-(2- methylbutyryl)oxy - 2a,5ct, 10ft - triacetoxytaxa - 4(20), l l-diene. The methylbutyrate group was located at C-14 by IH-1H COSY, HMBC, and HMQC. The corresponding H-14 signal occurred at 8 4.93 (dd, J =
4.4 and 8.8 Hz). The absolute configuration of the side chain was tentatively assigned to have the (S)-configu- ration based on the literature [7, 8]. The 2fl-, 5fl- and 10o~-protons geminal to the acetoxy groups had chemi- cal shifts [8 5.23 (dd, J = 2.6 and 6.4 Hz), 5.29 (1H, br
s) and 5.80 (d, J = 10Hz)] close to those values in taxuyunnanine B. Compound 5 had a hydroxyl group at C-9 as its H-9fl signal occurred at 8 4.19 (d, J = l0 Hz) whereas the H-9fl signal in taxuyunnanine B occurred at 8 5.77. The ~H NMR spectrum also exhibited a characteristic signal of H-3a at 8 2.92 (lH, d, J = 6 Hz).
Compound 6 was assigned the molecular formula C3oH42Oto ([M] + = m / z 562.276). The 1H and 13C NMR spectra (Tables 1 and 2) were similar to those of compound 7 except that the signals of the methylbutyrate group in 7 were replaced with the signals of an acetyl group in 6. The structure of 6 was assigned as 2a,5a,9ot,10fl,14fl-pentaacetoxytaxa-4(20), ll-diene. From the ~H-1H COSY spectrum the reso- 839
840 SHUNG-JIM YANG et al.
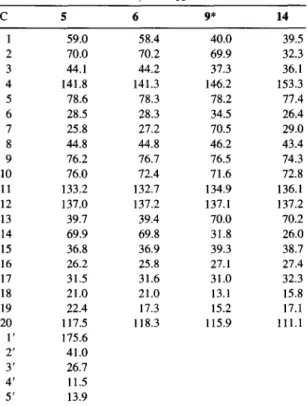
Table 1. 1H NMR spectral data for taxanes 5, 6, 9 and 14 (CDCI3)
H 5 6 9t 14 1 1.82 (d, 2.4)* 190 (d, 2.4) 1.76 (m) 2 5.32 (dd, 5.38 (dd, 2.4, 6.4) 2.4, 6.3) 3 2.92 (d, 6.4) 2.93 (d, 6.3) 2.93 (d, 3.9) 3.26 (d, 4.5) 5 5.29 (br, s) 5.24 (br, s) 5.55 (br s) 4.26 (br s) 7 4.32 (dd, 4.8, 11.1) 9 4.19 (d, 10.0) 5.76 (d, 10.2) 6.05 (d, 11.1) 5.73 (d, 10.2) 10 5.80 (d, 10.0) 6.03 (d, 10.2) 6.25 d, 11.1 ) 6.07 (d, 10.2) 13 2.87 (dd, 2.83 (dd, 5.77 (br t, 5.67 (dd, 9.2, 19.2) 9.0, 19.2) 7.8) 3.3, 7.8) 2.32 (m) 2.40 (m) 14 4.93 (dd, 4.93 dd, 1.12 (dd, 4.4, 8.8) 4.8, 9.0) 4.5, 15.3) 2.78 (m) 16 1.58 (s) 1.68 (s) 1.73 (s) 1.55 (s) 17 1.12 (s) 1.09 (s) 1.01 (s) 0.97 (s) 18 2.12 (s) 2.15 (s) 2.22 (s) 2.08 (s) 19 0.87 (s) 0.82 (s) 0.81 (s) 0.68 (s) 20 4.84 (br s) 4.84 (br s) 4.97 (br s) 4.71 (br s) 5.28 (br s) 5.30 (br s) 5.35 (br s) 5.09 (br s) 2' 2.34 (m) 3' 1.46 (m) 4' 0.85 (t, 7.2) 5' 1.11 (d, 7.2)
*The values in parentheses are coupling constants (Hz).
fCinnamoyl data: 8 7.74 (d, 16.0), 6.52 (d, 16.0) and %37-7.48 (5H).
Table 2. ~3C NMR spectral data for taxanes 5, 6, 9 and 14 (CDC13, 6 in ppm) C 5 6 9* 14 1 59.0 58.4 40.0 2 70.0 70.2 69.9 3 44.1 44.2 37.3 4 141.8 141.3 146.2 5 78.6 78.3 78.2 6 28.5 28.3 34.5 7 25.8 27.2 70.5 8 44.8 44.8 46.2 9 76.2 76.7 76.5 10 76.0 72.4 71.6 11 133.2 132.7 134.9 12 137.0 t37.2 137.1 13 39.7 39.4 70.0 14 69.9 69.8 31.8 15 36.8 36.9 39.3 16 26.2 25.8 27.1 17 31.5 31.6 31.0 18 21.0 21.0 13.1 19 22.4 17.3 15.2 20 117.5 118.3 115.9 1' 175.6 2' 41.0 3' 26.7 4' 11.5 5' 13.9 *Cinnamoyl data: 6 166.0, 118.3, 145.6, 134.0, 128.0, 128.9 and 130.5.
nance at 8 4.93 (dd, J = 4.8 and 9.0 Hz) was assigned to H-14o~. No coupling was observed between H - l f l and H-14c~, because these two protons were nearly orthogonal.
39.5 Compound 9 (C35H4409) gave rise to a molecular 32.3 ion [M] + at m / z 608.293 (calc. 608.297). The IR 36.1 spectrum showed absorptions at 1730, 1715 and 153.3 1630 cm -~ attributable to normal and conjugated ester 77.4 groups. In the ~H N M R spectrum, three acetoxy groups 26.4
29.0 appeared at 6 1.82 (s), 2.00 (s) and 2.06 (s). The 43.4 resonances at 8 7.74, 6.52 (AB quartet, J = 16 Hz) and 74.3 7 . 3 7 - 7 . 4 8 (5H) indicated the presence of a cinnamoyl 72.8 group. By comparison of the ~H and ~3C N M R spectra 136.1 of 9 with those o f 8 and 2ot-deacetoxytaxinine J, the 137.2 structure of 9 was assigned as 5 1 - ( c i n n a m o y l ) o x y - 7 f l - 70.2 hydroxy - 9t~,10fl,13ce - triacetoxytaxa - 4(20),11 - diene. 26.0 The chemical shifts of the 5fl-, 9fl- and 10a-protons 38.7 [8 5.55 (br s), 6.05 (d, J = 11.1 Hz) and 6.25 (d, J = 27.4
I 1.1 Hz)] were close to the values in 8 and 10. Because 32.3
15.8 the dihedral angle between H - 1 0 a and H-9fl was 17.1 nearly 180 °, these protons exhibited a large coupling 111.1 constant ( l l . 1 H z ) . The H - 7 ~ in 9 was geminal to a hydroxyl group and resonated at 8 4.32 (dd, J = 4.8 and 11.1 Hz), whereas the H-7ot in 10 geminal to an acetoxy group appeared at a lower field (8 5.70).
Compound 14
(C26H3807),
[M] + at m / z 462.260, gave ~H an d~3C N M R spectra similar to those of 13 (C24H3608). Compound 14 had three acetoxy groups as shown by the signals at 6 H 2.02 (s), 2.05 (s) and 2.07Taxanes from Taxus mairei 841
(s), whereas compound 13 had four acetoxy groups. The structure of 14 was assigned as 5ot-hydroxy- 9a,10fl,13ot-tdacetoxytaxa-4(20),ll-diene. The proton resonances at 8 5.09 (1H, br s) and 4.71 (1H, br s)
were attributable to the methylene group at C-4. The 9fl- and 10ot-protons appeared as an AB system at 8 5.73 and 6.07 with a large coupling constant (10.2 Hz). From the ~H-tH COSY spectrum, H-5fl and S 4.26 geminal to a hydroxyl group was correlated with H-6ot (at (5 1.75), h-6fl (at t~ 1.65) and H-20 ~at (5 5.09). The signal at t~ 5.67 (H-13fl) showed correlations with H-14ot (t~ 1.12), H-14fl (t~ 2.78) and H-18 ((5 2.08).
In summary, the twigs of T. mairei contain various taxane diterpenes. Isolation of taxanes 1-7 is signifi- cant since taxanes having substituents at C-14 are rare.
EXPERIMENTAL
General. Mp: uncorr; 1H NMR: 300 MHz; ~3C NMR 75 MHz; HPLC: Hibar Lichrosorb Si 60 column (10/~m or 7 ~m, 2 5 c r u x 1 cm i.d.).
Plant material. Twigs ( 1.2 kg) of T. mairei, collected in the remote mountains at an elevation of ca 2100 m (Tong-Shi, Taichung county), were exhaustively ex- tracted with Me2CO (7 1 × 3). The Me2CO extract was concd to give 100 g of residue, which was diluted with H20 and extracted ( × 3 ) with EtOAc. The combined EtOAc extracts were coned to give an oil (75 g), which was absorbed with 110 g silica gel and then subjected to CC on a column packed with 650 g silica gel. Eiution was with gradients of hexane, EtOAc and Me2CO. The portion obtained from elution with EtOAc-bexane (30-
AcO
18t~OAc
20
R 1OAc
I' 2'
2
OCOCHI~e2
1' 2' 5' 3' 4' 3 OCOCHMcCH2Me 4 OCOCHMeCH(OH)MeAcO
OAc
A c O , , . ~ 2H "
-
EH R1 H// bCOCH=CHC6H5
9 H H 10 H OH 11 H OAc 12 OAc OAcAcO
R1
~ O A c
5 OH OCOCHMeCH2Me 6 OAc OAc 7 OAc OCOCHMeCH2Me=AcO OAc OCOCsHs
'",,
U - ~ C O .,,.ll ICOCH=CHC6Hs
12
A c O ' " ' " ' ~
R1 R 2 13 H H OAc 14 H H OH 15 H OAc OAc 16 OAc OAc OH842 SHUNG-JIM YANG et al.
60%) was further subjected to flash chromatography and HPLC with elution with EtOAc-CHzCI 2 (20- 40%) or Me:CO-hexane (30-50%) to give 2 (12 mg), 3 (80mg), 1 (75mg), 7 (28mg), 13 (21mg), 6 (55mg), 10 (90mg), 11 (10mg), 15 (79mg), 4 (22 rag), 5 (15 rag), 14 (11 mg), 8 (15 rag), 9 (30 mg), 12 (13mg) and 16 (12mg) in ascending order of polarity.
9et - Hydroxy- 14B - ( 2- methylbutyryl )oxy- 2tx,5ot, l O[3- t r i a c e t o x y t a x a - 4 ( 2 0 ) , l l - d i e n e (5). Amorphous solid, [a] 23 +65.8 ° (CHC13; c 0.55). FABMS (NBA) m / z
(rel. int.): 585 [M + Na] ÷ (15), 503 (4), 461 (10), 443
K B r
(6), 401 (6), 341 (16), 281 (30), 57 (100). IR ~'m,x
- - I
cm : 3456, 1725, 1620.
2ct,5ot,9ot,1013,1413 - Pentaacetoxytaxa - 4(20),11 -
diene (6). Needles, mp 132-133 °, [a] 25 +26 ° (CHCL3; c 2.4). FABMS (NBA) m / z (rel. int.): 562 [M] + (3), 503 (5), 443 (14), 383 (9), 323 (12), 263 (40), 135 (100). IR v,,a~Km cm-l. 2929, 1730, 1234, 1021.
5 ce - ( C innamoy l )oxy - 7f3 - hydroxy - 9ct,1013,13ct - tri- a c e t o x y t a x a - 4 ( 2 0 ) , l l - d i e n e (9). Yellow gum. [a]~) 5
+20 ° (CHC13; c 0.5). FABMS (NBA) m / z (rel. int.): 608 [M] + (20), 590 (13), 548 (100), 488 (30), 428 (16). IR v~,~' x' c m - ' : 3469, 1730, 1631, 1234, 1159, 1021.
5e~ - Hydroxy - 9e~, 10f~, 13~x - triacetoxytaxa - 4(20), 11 -
diene(14). Needles, mp 204-206 °, [a]~ 6 +266 ° (CHC13; c 0.9). FABMS (NBA) m / z (rel. int.): 462 [M] + (6), 444 (20), 403 (70), 343 (63), 283 (100), 265
- - 1
(58). IR /:maxKar cm : 3485, 1734, 1235, 1019, 898.
REFERENCES
1. Appendino, G. (1995) Nat. Prod. Rep. 349. 2. Kingston, D. G. I., Molinero, A. A. and Rimoldi, J.
M. (1993) Progress in the Chemistry o f Organic
Natural Products, Vol. 61 (Herz, W., Kirby, G. W., Moore, R. E., Steglich, W. and Tamm, Ch., eds), p. 1. Springer, New York.
3. Chen, W.-M. (1990) Acta Pharm. Sin. 25, 227. 4. Wani, M. C., Taylor, H. L., Wall, M. E., Coggon, P.
and McPhail, A. T. (1971) J. Am. Chem. Soc. 93, 2325.
5. Yeh, M.-K., Wang, J.-S. and Chen, F.-C. (1988) J.
Chin. Chem. Soc. 35, 309.
6. Min, Z.-D., Jiang, H. and Liang, J. Y. (1989) Acta Pharm. Sin. 24, 673.
7. Ma, W., Stahlhut, R. W., Adams, T. L., Park, G. L., Evans, W. A. Blumenthal, S. G., Gomez, G. A., Nieder, M. H. and Hylands, P. J. (1994) J. Nat. Prod. 57, 1320.
8. Chen, W.-M., Zhang, P.-L., Wu, B. and Zheng, Q.-T. (1991) Acta Pharm. Sin. 26, 747.
9. Zhang, H., Takeda, Y., Minami, Y., Yoshida, K., Matsumoto, T., Xiang, W., Mu, O. and Sun, H. (1994) Chem. Letters 957.
10. Yeh, M.-K., Wang, J.-S. and Chen, F.-C. (1988)
Phytochemistry 27, 1534.
11. Zhang, Z. and Jia, Z. (1991) Phytochemistry 30,
2345.
12. Liang, J.-Y., Min, Z.-D. and Niwa, M. (1988) Acta. Chem. Sin. 46, 1053.
13. Yoshizaki, F., Fukuda, M., Ishida, T. and In, Y. (1988) Chem. Pharm. Bull. 36, 2098.
14. Erdtmam, H. and Tsuno, K. (1969) Phytochemistry
8, 931.
15. Ho, T.-I., Lee, G.-H., Peng, S.-M., Yeh, M.-K., Chen, F.-C. and Yang, W.-L. (1987) Acta Cryst.
C43, 1378.
16. Della Casa De Marcano, D. P. and Halsall, T. G. (1969) J. Chem. Soc., Chem. Commun. 1282. 17. Kingston, D. G. I., Hawkins, D. R. and Ovington,