Research Express@NCKU Volume 4 Issue 5 - May 16, 2008 [ http://research.ncku.edu.tw/re/articles/e/20080516/1.html ]
Linear adsorption of nonionic organic
compounds from water onto hydrophilic
minerals: silica and alumina
Yu-Hong Su
1,2, Yong-Guan Zhu
1, Guangyao Sheng
3, and Cary T.
Chiou
4,5,*1Research Center for Eco-environmental Sciences, Chinese Academy of Sciences, Beijing 100085, People’s Republic of China
2Department of Chemistry, Xinjiang University, Urumqi, Xinjiang 830046, People’s Republic of China
3Department of Crop, Soil, and Environmental Sciences, University of Arkansas, Fayetteville, Arkansas 72701
4U.S. Geological Survey, Box 25046, MS 408, Federal Center, Denver, Colorado 80225
5Department of Environmental Engineering and Sustainable Environment Research Center, National Cheng Kung University, Tainan 70101, Taiwan
Email:carychio@ncku.edu.tw
Environmental Science and Technology, v. 40, pp. 6949-6954 (2006)
S
oil/solid organic-matter (SOM) content dictates the extent and mode of uptake of organic compounds by geologic materials. Normally, if natural solids contain more than 0.1-0.2 % of SOM and little charcoal-like substance or soot, adsorption of nonionic organic solutes from water by solid surfaces is insignificant compared to the concurrent solute partition into SOM; this is attributed to the strong competitive adsorption of water for usually polar mineral/inorganic surfaces. However, for solids containing little or no SOM, adsorption with mineral/inorganic surfaces becomes
the major/dominant process, even if the overall uptake is weak. Although a significant advance has been achieved in modeling the uptake of organic ligands and ionizable solutes (e.g., carboxylic acids) by some hydrophilic minerals, the mechanism for relatively weak adsorption of nonionic organic solutes by these minerals remains to be elucidated. The latter subject has not been well attended in conventional
adsorption studies, where such organic solutes or vapors normally condense under strong adsorptive
Research Express@NCKU - Articles Digest
solute-mineral systems water must be preferentially adsorbed, thus making the competitive solute adsorption weak and linear. The specific solute-mineral layout that yields linear solute adsorption, which resembles superficially the linear solute partition with SOM, has been a subject of interest to be further resolved, since the conventional adsorption isotherms are typically nonlinear and no significant solute partition with solid inorganic material is anticipated.
To characterize the linear adsorption phenomena in aqueous nonionic organic solute-mineral systems, the adsorption isotherms of some nonpolar organic solutes [1,2,3-trichlorobenzene (TCB), lindane (LIN), phenanthrene (PHN), and pyrene (PYN)] and polar nonionic solutes [1,3-dinitrobenzene (DNB) and 2,4-dinitrotoluene(DNT)] from single- and binary-solute solutions on hydrophilic silica and alumina were established. Toward this objective, the influences of temperature, ionic strength, and pH on
adsorption were also determined. Table 1 lists the physicochemical properties of the test compounds.
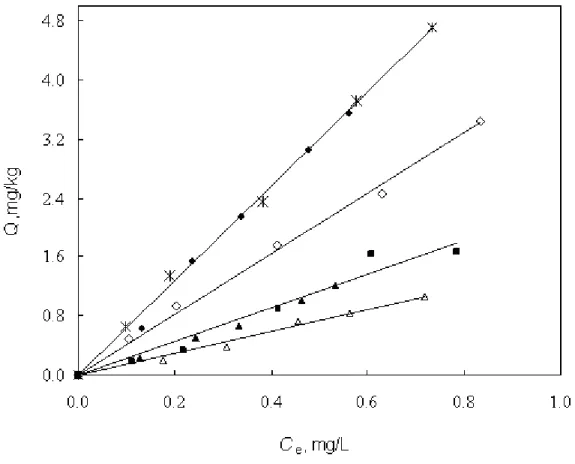
The representative adsorption isotherms of nonpolar PHN and polar DNT on silica and alumina at 25 (±1) ˚C and 45(±1) ˚C are shown in Figures 1 and 2, in which the mass of adsorbed solute per unit weight of the solid (Q) is plotted against the equilibrium solute concentration in water (Ce). The
isotherms are all practically linear and extend to high relative concentrations (Ce/Sw). Measured linear adsorption coefficients (Kd) of all solutes on silica and alumina at different temperatures and ionic strengths are listed in Table 2.
Table 1.Physicochemical properties of the organic compounds used in adsorption experimentsa
a MW = molecular weight;ρ = density at room temperature (g/mL); mp = melting point (˚C); Sw = water solubility at 25˚C (µg/mL);∆ = molar heat of solution in water at 25˚C (kJ/mol); Kow = octanol-water partition coefficient.b NA = not available.
Figure 1. Adsorption isotherms of PHN from 0.01M CaCl2 on silica (pH = ~ 5.25) and alumina (pH = ~ 6.28): ◆ 25˚C on silica; ▲ 25˚C on alumina; ◊ 45˚C on silica; ∆ 45˚C on alumina; * 25˚C on silica with PYR; ■ 25˚C on alumina with PYR.
Research Express@NCKU - Articles Digest
Figure 2. Adsorption isotherms of DNT on alumina (pH = ~ 6.28): ▲ 25˚C in 0.01M CaCl2; * 25˚C in 0.01M CaCl2 with DNB; ◊ 25˚C in 0.01M CaCl2 with PHN; ∆ 25˚C in 0.05M CaCl2
Table 2.Adsorption coefficients of selected organic solutes on silica and alumina
Research Express@NCKU - Articles Digest
hypothesized. For nonpolar solutes, the adsorption occurs presumably by London (dispersion) forces onto a water film above the mineral surface. For polar solutes, the adsorption is also assisted by polar- group interactions. The reduced adsorptive forces of solutes with hydrophilic minerals due to physical separation by the water film and the low fractions of the water-film surface covered by solutes offer a theoretical basis for linear solute adsorption, low exothermic heats, and no adsorptive competition.
The postulated adsorptive forces are supported by observations that ionic strength or pH poses no effect on the adsorption of nonpolar organic compounds on hydrophilic minerals while it exhibits a significant effect on the uptake of polar organic compounds. The model effectively consolidates the outstanding characteristics of nonionic compounds with natural hydrophilic minerals. The noncompetitive adsorption as illustrated has an important consequence to the transport and fate of individual contaminants in multi-contaminant natural aquatic environments.