國 立 交 通 大 學
材料科學與工程學系
博 士 論 文
層狀矽酸鹽補強聚丙烯晴-丁二烯橡膠
與聚丁二烯橡膠奈米複合材料之性質
研究
Mechanical and Morphological Effects of Layered silicates
on Nitrile-Butadiene and Butadiene Rubber
Nanocomposites
研究生: 黃為國
(Wei-Gwo Hwang)
指導教授: 韋光華
(Kung-Hwa Wei)
層狀矽酸鹽補強聚丙烯晴
-丁二烯橡膠與聚丁二烯橡膠奈米
複合材料之性質研究
Mechanical and Morphological Effects of Layered silicates
on Nitrile-Butadiene and Butadiene Rubber
Nanocomposites
研 究 生:黃為國 Student:Wei-Gwo Hwang
指 導 教 授:韋光華 Advisor:Kung-Hwa Wei
國 立 交 通 大 學
材 料 科 學 與 工 程 系
博 士 論 文
A ThesisSubmitted to Department of Materials Science and Engineering College of Engineering
National Chiao Tung University in partial Fulfillment of the Requirements
for the Degree of Doctor of Philosophy
in
Materials Science and Engineering Oct 2005
Hsinchu, Taiwan, Republic of China
Abstract
In this thesis, a variety of methods are used to prepare the elastomer
nanocomposites comprised of inorganic or organo-modified layered silicates and rubber matrices. Excellent mechanical , thermal and barrier properties can be obtained for each nanocomposite by performing proper process design, material selection, physical and/or chemical reactions by the addition of compatibilizers or surfactants, etc.
In chapter 2, elastomer nanocomposites consisting of nitrile butadiene rubber (NBR) latex and layered silicates are prepared by a modified latex shear blending process aided with ball milling. The mode of dispersion of layered silicates in NBR is partially exfoliated and intercalated when the concentration of layered silicates is below 7.5 wt %, as evidenced by transmission electron microscopy and X-ray diffraction results. The tensile and tear properties are much higher than that of neat NBR. Specifically, the tensile and tear mechanical properties of the NBR / layered silicates can increase by 200% and 60%, respectively. The decomposition
temperature of the nanocomposites increases slightly.
Different methods to form the nanocomposites of intercalated and exfoliated organosilicates in acrylonitrile butadiene rubber (NBR) are carried out by a solution blending process in chapter 3. The dispersion and intergallery spacings of
organosilicates in these nanocomposites are examined by transmission electron microscopy and X-ray diffraction. Dramatic enhancements in the mechanical and thermal properties of NBR are found by incorporating less than ten parts of
organosilicates. The fluid impermeability is also improved significantly. Nanocomposites of intercalated and exfoliated organosilicates in butadiene rubber (BR) have also been prepared by using a two-stage melt blending process in
chapter 4. X-ray diffraction and transmission electron microscopy are used to examine, respectively, the intergallery spacing of the organosilicates and their dispersion in the BR. Dramatic enhancements in the mechanical and thermal properties of BR occur when it incorporates less than 10 parts of organosilicates and the loading ratio of the organosilicate to dicarboxylic acid-terminated butadiene oligomer is about 3. In addition, the relative water vapor permeability of the BR nanocomposites containing 10 parts of organosilicate—both in the presence and absence of the compatibilizer—reduce largely compared to that of the neat BR.
It can be concluded that the successfully prepared NBR(BR)/organosilicate nanocomposites having intercalated and partially exfoliated structures can be obtained by different blending methods in proper process design and material selection. As a result of significantly improved compatibility and the strong molecular chains interactions between layered silicates and rubber matrices, the mechanical, thermal, and many other properties of these nanocomposites containing a low weight percent of layered silicates can be increased substantially.
摘要
本論文主旨為探討利用不同組成之有機或無機層狀矽酸鹽黏土強化橡膠材 料所形成之奈米橡膠複材的性能改變結果,研究顯示經由適當之製程設計、材 料選配與利用可產生化學或物理反應之界面活性劑、共溶劑等寡分子體之導入 效果,可獲得極佳之機械性能改良,熱性能與阻斷性能(barrier property)提昇等。 本文第二章,探討利用乳化相摻混程序,並結合高剪力摻合及球磨分散方 式可將無機層狀矽酸鹽黏土均勻混入NBR 橡膠乳膠中,經由 X 光繞射檢測及 TEM 觀察結果顯示,當層狀矽酸鹽黏土含量低於 7.5 wt %時,可形成分散良好 之部分剝層與插層結構之奈米橡膠複材,其機械性能如拉力與抗撕裂強度均比 NBR 原材料性能提昇相當多,分別提昇逾 200%與 60%,此外其熱分解溫度也 相對提昇。 在第三章中,探討以溶液摻合作業方式製備有機改質之層狀矽酸鹽補強 NBR 橡膠所形成之奈米橡膠複材,經由 X 光繞射檢測及 TEM 觀察結果顯示, 此一複材之結構為大部分插層與局部剝層之分散相,只需添加少於10 份矽酸鹽 黏土,即可製備具相當高強度與熱性質之奈米橡膠複材,其抗水滲透等性能也 有顯著提昇。 在第四章中,探討經由二階段熔融摻合作業方式製備有機改質之層狀矽酸 鹽補強BR 橡膠所形成之奈米橡膠複材,經由 X 光繞射檢測及 TEM 觀察結果 顯示,其結構為大部分插層之分散相,審慎選用有機黏土與共容劑,在含量少 於10 份之有機矽酸鹽黏土與約 3 份之共容劑可獲得最佳之機械與物理性能提 昇,且抗水滲透等性能也有相對提昇。 本論文的研究結果可得到以下結論,經由適當之製程設計、材料選配,可以 有許多不同途徑用以產製具有大部分插層與局部剝層型態學之奈米橡膠複材,這主要是基於經有機改質或特殊表面處理的橡膠高分子與矽酸鹽補強材料之間 所產生很強的分子間作用引力,與改善界面相容性之效果所致。
誌謝
論文口試結束,又立即要趕回台中,像這樣新竹-台中兩地來回的在職研 究生生涯五年,心中感慨良多,有獲得就要有付出與犧牲。論文之順利完成, 首先要感謝指導教授韋光華老師的指導,四年多來韋老師在專業學養的能力與 氣質,相當令人佩服,也適時的提供我研究方向與方法之修正及研究內涵之提 昇與指導,順利本論文之付梓,僅致萬分之謝意。 感謝口試委員薛敬和老師、戴念華老師、黃華宗老師、林宏洲老師,在他 們的指導下,讓我獲益良多,期望日後有機會再多多請教! 論文研究過程中,相當感謝中科院前副所長張元彰博士之大力協助,順利 本研究之完成、此外更感謝力特光電公司吳昌謀博士、明鑫科技公司沈賢博士 等在實驗研究與討論上的協助,另外航材組實驗室劉峻博、邱秋月、夏芝庭、 陳淑芬等同事在實驗研究執行上之協助也一倂致謝。 在身兼在職工作與研究生生涯期間,多蒙本校實驗室小小學弟妹們之多方 協助,包括實驗上與校方雜務處理及連絡上的幫助,解決我不少新竹-台中兩地 來回奔波之困擾,僅致謝意 ,於此特別感謝奇明、孝蔚、錦成三位博士及孟婷、 中斌、耀德、清茂、守謙及其他許多小學弟們之多方協助。 另外感謝各實驗室曾經在我研究生生涯出現過的大大小小學長、學弟妹 們,逢甲大學貴儀中心之季元貞小姐,塑發中心之劉崇輝組長、朱美珍小姐等, 也感謝他們在這過程中的協助。 最應感謝的是我親愛的老婆,美雲,與孩子,玠維、則銘,感謝你們這幾 年來的犧牲、包容與支持,有你們真好!。遠在台南的父親,嬸嬸,弟妹們, 感謝您們的支持鼓勵,感謝母親在天之靈庇祐! 還有所有的親友們,岳父、岳 母,感謝你們支持鼓勵! 謝謝大家!!Table of Content
Chapter 1: Introduction
1-1 Reinforcement of Nanoparticles ... 1-1 1-2 Materials ... 1-5 1-3 Research Motivation and Purpose... 1-10 Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene
Rubber/Silicate Nanocomposites
2-1 Introduction ... 2-1 2-2 Experimental Methods and Analysis ... 2-2 2-3 Results and Discussions ... 2-4 A. Layered silicates dispersion in NBR nanocomposites... 2-5 B. Mechanical and thermal properties of NBR nanocomposites ... 2-6 C. Schematic drawings of latex blending mechanism ... 2-7 2-4 Conclusions ... 2-8 Chapter 3: Mechanical, Thermal, and Barrier Properties of
NBR/Organosilicate Nanocomposites
3-1 Introduction ... 3-1 3-2 Experimental Methods and Analysis ... 3-3 3-3 Results and Discussions
A. Layered silicates dispersion in NBR nanocomposites... 3-7 B. Mechanical, thermal and barrier properties of NBR
nanocomposites ... 3-9 3-4 Conclusions ... 3-14 Chapter 4: Synergistic Effect of Compatibilizer in Organo-modified
Layered Silicate Reinforced BR Nanocomposites
4-1 Introduction ... 4-1 4-2 Experimental Methods and Analysis ... 4-3 4-3 Results and Discussions ... 4-7 A. Layered silicates dispersion in BR nanocomposites... 4-7 B. Mechanical, thermal and barrier properties of BR
nanocomposites ... 4-11 4-4 Conclusions ... 4-16 Chapter 5: Conclusions ... 5-1
Figure Lists
Chapter 1: Introduction
Figure 1 Structure of montmorillonite (Na+-MMT). [reproduced according to ref.: M. Alexandre and P. Dubois, Mater. Sci. Eng., 28, 1 (2000)]... 1-15
Figure 2 Layered silicate (Na+-MMT) basically composed of aggregates that is associated by many primary particles, which, in turn, are consisted of lots of superimposed lamellae. ... 1-16
Figure 3 (a) Orientations of alkylammonium ions in the galleries of layered silicates
with different layer charge densities [Lagaly, 1986]. (b) The
alkylammonium ions arranged in bilayers between the silicate layers, their chain axes orienting perpendicular to the silicate sheets [reproduced according to Lagaly, 1975]. ... 1-17
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate Nanocomposites
Figure 2A-1 X-ray diffraction patterns for layered silicate (Na+-MMT ) and for NBR/silicate nanocomposites ... 2-11
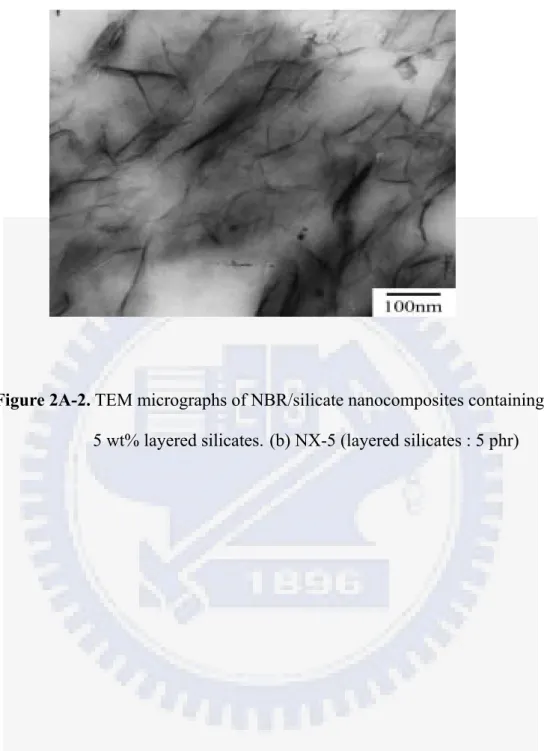
Figure 2A-2 TEM micrographs of NBR/silicate nanocomposites containing 3 wt%
layered silicates. (a) NX-3 ... 2-12
Figure 2A-2 TEM micrographs of NBR/silicate nanocomposites containing 5 wt%
layered silicates. (b) NX-5... 2-13
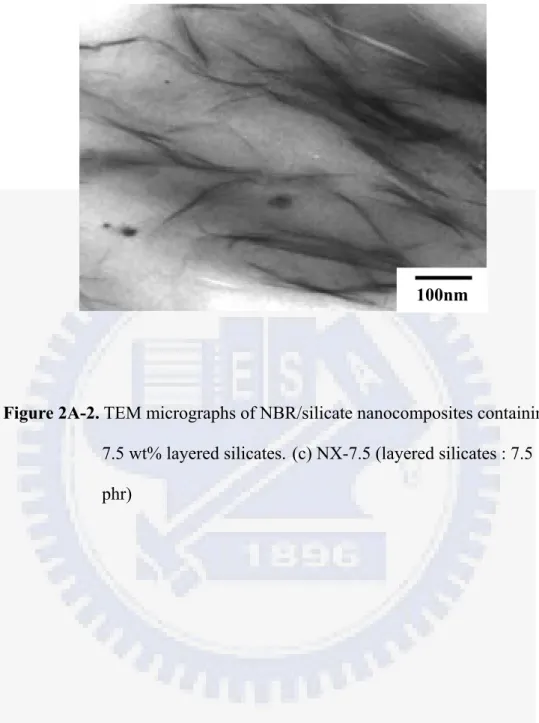
Figure 2A-2 TEM micrographs of NBR/silicate nanocomposites containing 7.5 wt%
layered silicates. (c) NX-7.5 ... 2-14
Figure 2B-1 Typical stress-strain behavior of various NBR/silicate nanocomposites.
... 2-15
Figure 2B-2 Curves of tensile properties versus layered silicate content (wt%) of
NBR/silicate nanocomposites. a. Tensile strength ... 2-16
NBR/silicate nanocomposites. b. M500 (engineer modulus). . 2-17
Figure 2B-3 Curves of tear strength versus layered silicate content (wt%) of
NBR/silicate nanocomposites ... 2-18
Figure 2B-4 Comparative TGA results (5% weight loss decomposition temperature,
Td, oC) of NBR/silicate nanocomposites with neat NBR ... 2-19
Chapter 3: Mechanical, Thermal, and Barrier Properties of NBR/Organosilicate Nanocomposites
Figure 3A-1 X-ray diffraction patterns of NBR nanocomposites with various
organosilicate. Content... 3-18
Figure 3A-2 TEM image taken on the NBR nanocomposite containing 7.5 phr
organosilicates. ... 3-19
Figure 3B-1 Typical tensile stress/strain curves of the NBR nanocomposites with
various organosilicate content... 3-22
Figure 3B-2 Typical tensile stress/strain curves demonstrate the reinforcing effect in
NBR/organosilicate nanocomposites. ... 3-23
Figure 3B-3 FTIR spectra of organosilicate, NBR nanocomposite(10 wt%
organosilicate) and pure NBR... 3-24
Figure 3B-4 Possible reaction mechanism of ammonium salt intercalant and
zinc-sulfur-amine complexation between organosilicate and highly polarized NBR. ... 3-25
Figure 3B-5 Relative vapor permeability of NBR nanocomposites containing
various amounts of organosilicate. Note: The reference values for water and methanol are 967 and 5111 g/m2/day, respectively. ... 3-26
Chapter 4: Synergistic Effect of Compatibilizer in Organo-modified Layered Silicate Reinforced BR Nanocomposites
Figure 4A-1 X-ray diffraction patterns of BR nanocomposites containing various
Figure 4A-2 TEM image of the BR nanocomposite containing 10 phr organosilicate
and 3 phr CTB... 4-23
Figure 4A-3 Locally enlarged TEM image of the BR nanocomposite containing 10
phr organosilicate and 3 phr CTB... 4-24
Figure 4A-4 FE-SEM image of the NBR nanocomposite containing 10 phr
rganosilicate and 3 phr CTB. ... 4-25
Figure 4A-5 FE-SEM image of the BR nanocomposite containing 10 phr
organosilicate (without CTB) . ... 4-26
Figure 4B-1 Typical tensile stress–strain curves of the BR nanocomposites
containing 10 phr organosilicate in the presence and absence of compatibilizer... 4-28
Figure 4B-2 Typical tear strength curves of the NBR nanocomposites containing 10
phr organosilicate in the presence and absence of compatibilizer.4-29
Figure 4B-3 The synergistic effect of the CTB/organosilicate ratio on the tensile
stress–strain curves of the NBR nanocomposites containing various organosilicate contents (neat BR has a tensile strength of 201 ± 14 Psi). . ... 4-30
Figure 4B-4 The synergistic effect of the CTB/organosilicate ratio on the tear
strength of the BR nanocomposites containing various organosilicate contents (neat BR has a tear strength of 22.83 ± 0.85 lb/in)... 4-31
Figure 4B-5 Water vapor permeabilities of the BR nanocomposites containing
Scheme & Table Lists
Chapter 1: Introduction
Scheme 1 Different types of composites arising from the interaction of layered
silicates and polymers: (a) phase-separated microcomposite, (b)
intercalated nanocomposite, and (c) exfoliated nanocomposite. … ... 1-13
Scheme 2 Surface modification via compatibilizer. … ... 1-13 Table 1 Characteristic properties of layered silicates: (a) Typical example of structure
and chemistry of smectites commonly used. (b) Examples of layered silicic acids. ... 1-14
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate Nanocomposites
Scheme 1 Formation of exfoliated layered silicates... 2-20 Scheme 2 Latex blending process ... 2-21
Chapter 3: Mechanical, Thermal, and Barrier Properties of NBR/Organosilicate Nanocomposites
Table 3A-1 Formulation of NBR compounds ... 3-17 Table 3A-2 Vulcanization characteristics of NBR compounds... 3-17 Table 3B-1 Mechanical properties of NBR compounds ... 3-20 Table 3B-2 Crosslinking density and thermodynamical characteristics of NBR
compounds... ... 3-21
Table 3B-3 Thermal properties of NBR compounds... 3-21
Chapter 4: Synergistic Effect of Compatibilizer in Organo-modified Layered Silicate Reinforced BR Nanocomposites
Table 4A-1 Formulation of BR/organosilicate nanocomposites.. ... 4-20 Table 4A-2 Representative vulcanization characteristics of BR/organosilicate
Table 4A-3 X-Ray diffraction data of BR/organosilicate nanocomposites... 4-21 Table 4B-1 Crosslinking densities and thermodynamic characteristics of
BR/organosilicate nanocomposites... 4-27
Chapter 1: Introduction
1-1 Reinforcement of Nanoparticles
Nanocomposites are a new class of materials that comprise a dispersion of at least one dimension with nanometer-size particles classified as lamellar, fibrillar, shell-like, spherical and others in a matrix with single or multi-component. Three types of nanocomposites are acknowledged dopending on the dimension numbers of the dispersed particles being in nanometer range. Isodimensional nanoparticles are the first type which have three dimensions being in the order of of nanometers, such as spherical silica obtained by in-situ sol-gel methods [1-2] or by (living free radical) polymerization promoted directed from their surface [3-4]. In general, the
interactions between the polymer and the silanol groups would generate during the sol-gel process, a macrophase separation should be avoided, and the resulted materials would have a high degree of homogeneity and optical transparency. However, these properties are highly dependent on the reaction condition of the sol-gel process, e.g. the pH value. The second type, an elongated structure formed by two dimensions being in nanometer scale and one dimension being larger are nanotubes or whiskers, such as carbon nanotubes. The third type of nanocomposites is characterized by only one dimension in the nanometer range, usually named as polymer/layered silicate nanocomposites, where the filler is present in the form of sheets of one to several nanometers thick and hundreds to thousands nanometers long.
On recent developments, polymer/layered silicate nanocomposites have attracted the attention of government, academic, and industrial researchers [5-11]. This new type of composites, based on nanometer range of organic or inorganic clays dispersed in a variety of polymer matrices, i.e. thermoplastics, thermosets, resins, and
elastomers, prepared via various synthetic routes, including in-situ intercalative polymerization, exfoliation-adsorption (from polymers or prepolymers in solution, emulsion polymerization), template synthesis, and melt intercalation. The highly enhanced mechanical and thermal properties of the new class polymer
nanocomposites are usually reinforced by only small amount (e. g. lower than 10 weight percentage) of nanoscopically dispersed intercalated and/or exfoliated layered silicates relative to the unfilled polymer. Once the nanolayer exfoliation has been achieved, these remarkably improved properties of the nanocomposites can be manifested, including tensile strength, modulus or stiffness, heat distortion
temperature, flame retardance, barrier properties, and so forth, which result from the special structure and morphology of these nanocomposites with respective to
conventional composites [12].
Among several reinforcements and fillers, carbon black is the most important reinforcing agent used in elastomeric industry. It produces a remarkable reinforcing effect on vulcanized rubber because it has a variety of active functional groups such as carboxyl, phenolic, and quinine groups on the surface of the particles that leads to strong interaction with the rubber chains. However, carbon black causes pollution, gives black color, increases the compound viscosity and impairs the processibility of the compound when it is incorporated in a large amount into rubber. Many light or pale color reinforcing agents, such as kaolin, precipitated silica, and sepiolite, etc. were researched to replace carbon black. Nevertheless, their reinforcing properties are lower than those of carbon black because of their hydrophilic nature which leads to poor compatibility and decreases the intermolecular force between those fillers and polymer matrices. Conventional polymer/clay composites containing aggregated and non-homogeneous nanolayer tactoids ordinarily can improve rigidity, but they often sacrifice elongation, toughness, and strength.
The complete dispersion of clay nanolayers, also termed as layered silicates, with the single layered silicate or bundles reduced in size from 100 microns to several nanometers is called as exfoliation in a polymer matrix. The structure of
polymer/layered silicate nanocomposite would optimize the chain reaction cites (e.g. high aspect ratio and the whole surface area effect) of layered silicates and confine the polymer molecules to isolate and force them into an orderly arrangement in these two-dimensional spaces, which, in turn, results in significant improvement of the combined physical and mechanical properties of the polymer nanocomposite. Depending on the nature of the components used (layered silicate, compatibilizer or surfactant, organic cation, and polymer matrix) and the preparation method, three main types of composites may be obtained when a layered silicate is associated with a polymer as shown in Scheme 1 [13].
Clay mineral/polymer composites can be prepared in several ways. However, true nanocomposites are obtained by methods that can overcome the tendency of layered silicates to form face-face aggregates. The most serious obstacle in
reinforcing practicable polymers by delaminated and dispersed the silicates into their matrices is the incompatibility between the layered silicates and polymers.
Therefore, the appropriate modification of external and internal surfaces of the layered silicates would generate an organophilic interaction with the hydrophobic polymers and provide the most promising ways to prepare nanocomposites, e.g. Scheme 2. According to Akelah and Moet [14], for preparing polymer/layered silicate nanocomposites, several attempts have been applied to incorporate the layered silicates with the organic polymers, depending on their physical, chemical, or
mechanical interactions. The physical interaction includes method of impregnation of monomers followed by polymerization, direct adsorption of linear polymers. The chemical interaction includes grafting of polymers via coupling agents, interactions of
polymer functional groups with clays, hydrolysis of organometallics within polymeric matrices, and intercalation of polymers with organophilic clays. Basically, Akelah and Moet emphasize synthesis method to prepare nanocomposites. For example, the cation exchange grafting of polymers through chemical bonds to the layered silicates to produce nanophase organic-inorganic hybrids has been developed. This technique may involve either by producing monomer-impregnating organophilic silicates first, followed by polymerized the monomers to grow the polymeric chains in the
interlayers space; or by direct intercalation of positively charged polymer chains from solution to the anionic silicate layers. Two intercalation processes, including
solution polymerization and melt polymerization were adapted for producing these nanocomposites. At present, these synthesis methods for preparing polymer nanocomposites introduced by Akelah and Moet are still adopted widely in many different polymer industries, including plastics, resins, and elastomers.
Besides synthesis or in-situ polymerization methods, now-a-days, there are many other methods to prepare the polymer nanocomposites. Many related researches have been reported. These methods, according their process design, are emulsion polymerization or latex blending process, solution process either by prepolymers or polymers, and melt intercalation process with internal mixers or extruders in proper formulation and process design.
Since Toyota Motor Company, a pioneered researcher of polymer
nanocomposites, created Nylon 6-clay hybrid (NCH), used to make a timing-belt cover, they have had a lot of reports to focus on these fields of preparing practicable nanocomposites [15, 16]. Actually, nano-scale reinforcement should enable parts and system design of polymer composites that will be cost-competitive with other polymers, and eventually replace metals and glass, thus enabling the automotive or sporting industries to capture a leadership position in fuel-efficient, higher-quality,
and durability.
1-2 Materials
1-2-1 Nitrile rubber (NBR) and butadiene rubber (BR)
Nitrile rubber, an elastomeric copolymer of butadiene and acrylonitrile, is synthesized generally by emulsion polymerization and forms either in case a latex or a dry rubber. The important properties trends of the copolymer are influenced by acrylonitrile content, a polar component that resulting in a high polar NBR elastomer. Nitrile rubber is available in several grades of oil resistance based on the
acrylonitrile content of the elastomer raging from about 53 to 18%. On the contrary, polybutadiene rubber, a nonpolar elastomer, can be prepared by either emulsion or solution polymerization. Most is produced by solution polymerization in which a variety of controlled structure BR, e.g. a configuration of cis, trans, and vinyl components, etc., can be made. Both rubbers are widely used in machinery, automobiles, and sporting industries today.
Elastomers are entropic systems characterized by a network structure (through a path of vulcanization process) which includes flexible segments of molecular chain (acting as springs), connected by chemical or physical crosslinks(acting as knots). The flexible segments are commonly in the disordered random coil conformation in the unstretched state and assume extended conformation upon stretching. The presence of network knots prevents the material from flowing during applying tensile or shear force. Hence, to prepare an elastomer
nanocomposite, the melt intercalation process should be properly controlled to obtain a homogeneous and well dispersed phase of layered silicate-rubber molecular chain before mature vulcanization or simultaneously.
1-2-2 Layered silicates
1-2-2.1 Structure and type
The layered silicates (usually referred to as clay minerals) are composed of stacks of hydrated alumino-silicate, in particular montmorillonite (MMT). Their crystal structure consists of two dimensional layers with thickness about 1 nm and lateral width 200-300 nm that is made up of two silica tetrahedral sheets with an edged-shared octahedral sheet of either alumina or magnesia as shown in Figure 1 [14]. Stacking of these layers results in intergalleries or interlayers due to strong van der Waals force. The interlayers of MMT are separated by sheets of hydrated inorganic cations, typically Na+ or Ca+, which balance the oxide layers charge and are readily ion exchanged with a wide variety of positively charged species. MMT is basically composed of aggregates having 0.1 to 10 µm particle size range that is associated by many primary particles, which, in turn, are consisted of lots of superimposed lamellae, as shown in Figure 2. Each primary particular contains approximately 8 lamellae or 16 planes. Among all the layered silicates, Na+-MMT having the optimum cation exchange capacity (CEC), 90-120 meq/100 g, is the most frequently used nano-filler.
Variation in the amount, type, and crystallographic origin of the excess layer charge results in a large family of natural (e. g., MMT. hectorite, saponite beidellite) and synthetic (e. g., laponite, fluorohectorite) layered silicates exhibiting different characteristics, like layer size, stacking perfection, reactivity, and Lewis acidity. Two types of the major inorganic layered silicates include smectites and layered silicic acids, and the typical chemical formulas of some layered silicates are shown in Table 1.
2-2-2 Hydrated swelling and functionality modified
The physical and mechanical properties of polymer nanocomposites are significantly improved when the grains of layered silicates are reduced in size from
100 µm to several nanometer range. These nanocomposites are multilayered sandwich-like materials in which polymer chains are intercalated between ultrathin sheets of layered silicates. However, due to its hydrophilic nature, layered silicate is generally swelled in water to expand its intergallery space to be blended with the aqueous polymer or modified by alkylammonium surfactants to achieve enough hydrophobicity and expanding gallery to be well miscible with the organophilic polymer matrix.
Hydrated swelling The driving force for the swelling of MMT is caused by hydration of the interlayer inorganic cations. Water can associate with the cations to form hydration shells and complete the filling of the interlayer space. The interlayers expansion and water packing density are affected by the density and type of cations, layer charge and its distribution, and the strength of the liquid structure rather than by the MMT silicate surface. Water molecules having strong salvation forces and low self-preservation tendency would distribute well around the cations. In presence of larger amounts of water, the MMT becomes fully expanded and dispersed with the spacings exceed 2 nm, where fewer regular gallery can be measured by the X-ray pattern. It also means that single silicate layer can be obtained in indefinite swelling of MMT in water and the interlayer cations no longer reside at the silicate surface. Hence, exfoliating MMT layers dispersing well in water-soluble polymer or aqueous polymer latex would be expected.
Functionality modified Due to most of the polymers, including plastics, elastomers, and resins are hydrophobic and water-insouble, the hydrophilic inorganic layered silicates need to be modified with the functional organomodifier to become miscibly with the polymers. To enhance the compatibility of layered silicates with
organophilic polymers, the interlayer cations are generally replaced with organic
cations (e.g., quarternary ammonium ion), called intercalation, functionalizing the inter and outer surface of aluminosilicate. Some type of quarternary ammonium used to modify Na+-MMT or other layered silicates are by replacing the sodium ion with the following organic species [17]:
(1) n-alkylammonium ions(n=1-18) CnH2n+1-NH3+ (2) N-n-alkylpyridinium ions (n=1-18) (3) N,N,N-trimethyl-N-n-alkyl-ammonium ions (n=12, 14, 16, 18) CnH2n+1-+N-(CH3)3 (4) N,N-dimethyl-N,N-di-n-alkyl-ammonium ions(n=8, 10, 12, 14, 16, 18) (CnH2n+1)2-+N-(CH3)2
Example of cation exchange reaction is as the follow: Na 2Si14O29.11H2O + x M + → M xNa2-xSi14O29.z H2O + x Na + +(11-z)H 2O x : the fraction of sodium interlayer cations replaced
M+ : (1) and (2) are nearly quantitative ion exchange (3) are markedly reduced for long chain
Actually, at present, several routes have been used to intercalate layered silicate particles, including intercalation by solvents and solutions, intercalation by organic cations, intercalation by organic liquids, by inorganic intercalants, and by melt intercalation method. During intercalation the intercalant molecules diffuse to the galleries between individual silicate layers, and the basal spacings are expanded.
CnH2n+1- +
N
The process depends on the balance of forces, the size and geometry of gallery, the type, size, and structure of intercalant molecules, the matrix viscosity, etc.
Depending on the charge density of the clay, the intercalant onium ions may lie parallel to the clay surface as a monolayer, a lateral bilayer, a pseudo-trimolecular layer, or an inclined paraffin structure as illustrated in Figure 3 (a) [18]. In general the basal spacing increases most nearly linearly with the chain length of the cation. According to Lagaly et al [17], the high basal spacings of alkylammonium-magadiite have been interpreted by the following model: the alkylammonium ions are arranged in bilayers between the silicate layers, their chain axes orienting perpendicular to the silicate sheets as shown in Figure 3 (b).
1-2-3 elastomer nanocomposites
For several years, many researchers have done a lot of efforts, attempted to develop elastomer/layered silicate nanocomposites, and expected to obtain high
performance nanocomposites that having superior mechanical and physical properties. However, much of such devotion has failed to produce true elastomer nanocomposites with layered silicates to replace the traditional carbon black. There are the reasons that to achieve a true layered silicate exfoliation is difficult and there is only a week interaction force occurred between both phases of silicate and rubber matrix.
Actually, most of the rubber chains are partially intercalated into the silicate galleries as the scheme 1 (b) shown, and a few are exfoliated as the scheme 1 (c) shown, which could be due to the following reasons:
•The viscosity of the rubber is too high due to its higher molecular weight, that restricts the movement of the molecular chains and prevents them from moving into the silicate galleries.
•The vulcanization reaction is too fast and restricts the movement of the rubber chains into the silicate galleries because of the formation of network. Thus, the
intercalation only occurs at the entrance of the galleries.
•The shear force is insufficient during blending progress, and cause the ‘partial’ intercalation, which could be due to too higher temperature, poor process design or improper equipment.
•There is little chemical reaction happened between the surface of silicate and elastomer molecular chains, which may result from non-enough compatibility of both phases.
During last decade, elastomer/layered silicate nanocomposites have been prepared by many different process methods, including emulsion polymerization or latex blending, melt intercalation, and solution process [19-24]. In this thesis, some new strategy based on the similar process was developed to prepare disorderly
exfoliated nanocomposites, in which proper selection of layered silicates and rubbers
are considered carefully. Concerning the compatibility, there also have some factors to be considered, e.g. polar effect, coupling agent or surfactant with reactive
functional group, compatibilizer selection, and so forth.
1-3 Research Motivation and Purpose
The interaction between inorganic or organo-modified layered silicates and the elastomer chains to enhance the physical and mechanical properties of the elastomer nanocomposites is interesting. It needs to modify inorganic silicates to change its surface state for improving the compatibility of layered silicates with the
hydrophobic elastomer. Different processes can be designed to create a true elastomer nanocomposite, depending on polymer type and phase state, proper formulation design (material selection), proper selection of surfactant or compatibilizer, equipments used, and thermodynamics available. When a elastomer nanocomposite is prepared, besides of the mechanical, thermal, and
barrier properties are tested, the morphology and thermodynamic properties of the nanocomposite sample would also be interested to be performed. A true elastomer nanocomposite can exhibit superior mechanical, thermal, and processing properties and is expected to be applied suitably for sporting goods, surgical hoses or gloves, and replace metals in automotive and other application [15]. Currently, the global expectations for fuel economy and low emission for manufacturing and
transportation are requested seriously and immediately. A demand for new low-cost, high-performance, and light-weight functions would be needed, and this could also be predicted to create a series of novel polymer nanocomposites in the near future.
Reference
[1] J. E. Mark, Polym. Eng. Sci., 36, 2905 (1996).
[2] L. Matejka, K. Dusek, J. Plestil, J. Kriz, and F. Lednicky, Polymer, 40, 171 (1998).
[3] Z. Ahmad, M. I. Sarwar, and J. E. Mark, J. Mater. Chem., 7, 259 (1997). [4] T. von Werne, T. E. Patten, J. Am. Chem. Soc., 121, 7409 (1999).
[5] B. Finnigan, D. Martin, P. Halley, R. Truss, and K. Campbell, J. Appl. Polym. Sci., 97, 300 (2005).
[6] M. W. Weimer, H. Chen, E. P. Giannelis, and D. Y. Sogah, J. Am. Chem. Soc.,
121, 1615 (1999).
[7] A. Usuki, A. Tukigase, and M. Kato, Polymer, 43, 2185 (2002).
[8] M. Maiti, S. Sadhu, and A. K. Bhowmick, J. Polym. Sci. Part B: Polym. Phys., 42, 4489 (2004).
[9] A. Ranade, N. D’Souza, C. Thellen, and J. A. Ratto, Polym. Int., 54, 875 (2005).
[10] H. A. Stratz, D. R. Paul, and P. E. Cassidy, Polymer, 46, 3818 (2005).
[11] M. Krook, G. Morgan, and M. S. Hedenqvist, Polym. Eng. Sci., 45, 135 (2005). [12] G. Kickelbick, Prog. Polym. Sci., 28, 83 (2003).
[13] M. Alexandre and P. Dubois, Mater. Sci. Engng., 28, 1 (2000).
[14] A. Akelah and A. Moet, J. Appl. Polym. Sci.: Appl. Polym. Sympo., 55, 153 (1994)].
[15] J. M. Garcés, D. J. Moll, J. Bicerano, R. Fibiger, and D. G. McLeod, Adv. Mater., 12,1835 (2000).
[16] T. W. Lee, O. O. Park, J. Yoon, and J. J. Kim, Adv. Mater., 13, 211 (2001). [17] G. Lagaly, K. Beneke, and A. Weiss, Am. Mineral. 60, 642 (1975).
[18] G. Lagaly, Solid State Ionics, 22, 43 (1986).
[19] M. Song, C. W. Wong, J. Jin, A. Ansarifar, Z. Y. Zhang, and M. Richardson, Polym. Int., 54, 560 (2005).
[20] K. G. Gatos, N. S. Sawanis, A. A. Apostolov, R. Thomann, and J. Karger-Kocsis, Macromol. Mater. Eng., 189, 1079 (2004).
[21] W. F. Dong, Y. Q. Liu, X. H. Zhang, J. M. Gao, F. Hwang, Z. H. Song, B. H. Tan, and J. L. Qiao, Macromolecule, 38, 4551 (2005).
[22] Z. F. Wang, B. Wang, N. Qi, H. F. Zhang, and L. Q. Zhang, Polymer, 46, 719 (2005).
[23] Z. J. Zhang, L. Zhang, Y. Li, and H. D. Xu, Polymer, 46, 129 (2005). [24] M. Y. Liao, W. Shan, J. D. Zhu, Y. Li, and H. D. Xu, J. Polym. Sci : Part B:
Polym. Phys., 43, 1344 (2005).
Scheme 1. Different types of composites arising from the interaction of layered
silicates and polymers: (a) phase-separated microcomposite; (b) intercalated nanocomposite and (c) exfoliated nanocomposite. [Referring to : M. Alexandre and P. Dubois, Mater. Sci. Eng., 28, 1 (2000)]
Scheme 2. Surface modification via compatibilizer
Table 1 : Characteristic properties of layered silicates
(a) Typical example of structure and chemistry of smectites commonly used.
Montmorillonite Hectorite Saponite Fluorohectorite Laponite Type Dioctahedral Trioctahedral Trioctahedral Trioctahedral Trioctahedral
Substitution Octahedral Octahedral Tetrahedral Octahedral Octahedral Unit Cell Mx[Al4-xMgx] Mx[Mg6-xLix] My-x[Mg6-xFex3+] Li1.12[Mg4.88]
Li1.12
Na0.7[Mg5.4] Li0.4
Formula (X~0.67)
Si8O20(OH)4 Si8O20(OH)4 Si8-yAlyO20(OH)4[Si8O20]F4 Si8O20(OH)4 M: exchangeable cation, like Na+.
(b) Examples of layered silicic acids.
Layered silicic acids Chemical composition Interlayer spacing, d001, nm
Kanemite NaHSi2O5.7H2O ~1.03 Makatite Na2Si4O9.5H2O ~0.903 Octosilicate Na2Si8O17.9H2O ~1.10 Magadiite Na2Si14O29.11H2O ~1.56 Kenyaite Na2Si20O41.10H2O ~1.20 . Chapter 1: Introduction
Figure 1. Structure of montmorillonite (Na+-MMT). [reproduced according to A. Akelah and A. Moet, J. Appl. Polym. Sci.: Appl. Polym. Sympo., 55, 153 (1994)]
Figure 2. Layered silicate (Na+-MMT) basically composed of aggregates that is associated by many primary particles, which, in turn, are consisted of lots of superimposed lamellae.
Agglomerate consisting of microaggregate
Microaggregate consisting of primary particles
Simply intercalate tactoids (primary particles)
How to obtain exfoliated layered silicates ?
Figure 3. (a) Orientations of alkylammonium ions in the galleries of layered
silicates with different layer charge densities [18].
(b) The alkylammonium ions arranged in bilayers between the silicate layers, their chain axes orienting perpendicular to the silicate sheets [17]. Chapter 1: Introduction
Silicate Layer
NH3 ♁ NH2 NH3 ♁ NH2 NH3 ♁ NH ♁ NH ♁ NH NH NHSilicate Layer
(b) The alkylammonium ions arranged in bilayers between the silicate layers, their chain axes orienting perpendicular to the silicate sheets [reproduced according to Lagaly et al, 1975].
(a) Orientations of alkylammonium ions in the galleries of layered silicates with different layer charge densities [Lagaly, 1986].
Chapter 2: Preparation and Mechanical Properties of Nitrile
Butadiene Rubber/Silicate Nanocomposites
2-1 Introduction
Layered silicates/polymer nanocomposites combine the easy processing of polymers with the strength of nanometer-sized layered silicates having one dimension about 1 nm. These nanocomposites can exhibit synergistic properties from
individual components without causing large detrimental effect to their original properties by incorporating only a small percentage of layered silicates, thus
providing a new technological and economic route for the production of potentially valuable materials. Nylon 6-silicate is the first example of those hybrid composites [1]. Other polymer-layered silicate nanocomposites, involving materials such as polyimide [2-4], polyurethane [5-7], polyolefins [8-10], polyisoprene rubber [11] and silicone elastomer [12], have also been reported recently.
Only a few cases of elastomer nanocomposites consisting of various rubbers and layered nano-silicates have ever been reported [13-15]. Different methods of
intercalation of layered silicates, such as melt intercalation, in-situ polymerization and latex shear blending methods, have been adopted to prepare the elastomer
nanocomposite. Specifically, some organo-modified silicate reinforced elastomer nanocomposites, obtained from a melt intercalation method, have been reported by the high shear force generated in a plasticorder or a twin-screw extruder [15-22]. For instance, ethylene propylene diene methylene linkage rubber-clay hybrid has been prepared successfully by melt mixing of EPDM and organophilic clay in a
plasticorder followed by vulcanization process [16]. On the other hand, a shear blending process on elastomer latex produces a poor intercalation and dispersion
morphology of silicate layers [23-26].
Nitrile butadiene rubber (NBR), in the solid or latex state, is one of the most widely used, commercialized and mass-productive elastomers that is manufactured mostly by the emulsion polymerization method. In the present study, NBR/silicate nanocomposites are prepared using a modified latex shear blending method. The source of layered silicate utilized in this study is Na+-montmorillonite (MMT), which consists of a lamellar structure that is constructed from an octahedral alumina sheet sandwiched between two tetrahedral alumina sheets. The surfaces of these silicate layers contain negative charges with strong van der Waals interaction forces that are balanced by Na+ cations absorbed on the near- surface to compensate for the net negative charge. When the inorganic layered silicates are dispersed in water, the interlayer spacing largely expand due to hydrogen bonding, which enables relatively larger-sized molecules, such as surfactants, to penetrate into their galleries [27-29]. Then, the enlarged galleries could allow even larger molecules to diffuse and
intercalate into the interlayer space. NBR/silicate nanocomposites could not be easily prepared by melt intercalation as a result of the poor miscibility of NBR with layered silicates. In the present study, a new intercalation process is used to produce nanocomposites by shear blending of nitrile latex with surfactant-treated hydrophilic layered silicates, and the resulting mechanical properties of these nanocomposites are investigated.
2-2. Experimental Methods and Analysis
2-2.1 Materials
Nitrile butadiene rubber latex (NBR latex, Nancar 1052) containing 31-33% acylonitrile was obtained from Taiwan Nancar Co. Ltd., with a solid content of about 27%. Clay Swy-2 (Wyoming Na+-montmorillonite, i.e. Na+-MMT), having a
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
cationic exchange capacity (CEC) of 76.4 meq./100g, was provided from the Clay Minerals Depository at the University of Missouri, Columbia, MO. Aromatic polyglycol ether (Emulvin W), a nonionic emulsifier acting as a emulsifying,
stabilizing and wetting agent for latexes, was acquired from Mobay Co. Ltd. Other ingredients, such as the surfactant (sodium salt of methylene- bis-naphthalene
sulphonic acid), dispersing agent and curing agent were supplied from a local agency (R.T. Vanderbilt Co. Ltd., Taiwan).
2-2.2 Latex blending and sample preparation
The last number behind the sample notation, NX-, stands for different content of layered silicates, which was added to NBR latex. For example, NX-0 is neat NBR, and NX-10 contains 10wt% layered silicates in NBR. Typical industrial
formulations were used in this study. A mixture of Na+-MMT , dispersing agent, emulsifier, potassium hydroxide (electrolyte) and deionized water was placed in a ball-mill tank to produce a homogeneous, swelled and intercalated layered silicate solution. The cure agents, including 0.5 phr (parts per hundred rubber) sulfur and proper amounts of accelerator, zinc oxide, oleic acid, dispersing agent, emulsifier, and potassium hydroxide, were also mixed and prepared as a slurry at the same time. Subsequently, each clay solution was individually added to the nitrile butadiene
rubber latex and blended for 48 hours to form a homogeneous latex composite. Then, each batch of curing slurry was added to the latex composite and blended an
additional 48 hours. Afterward, each batch of latex composite was poured directly into a flat plate vessel, dried for 24 hours in a 50 oC hot air oven, washed with water and cured for 4 hours at 110 oC to obtain a rubber slab. Each slab was controlled to an approximate thickness of 1 mm.
In the present study, a well-dispersed layered silicate water slurry was prepared by mechanical ball milling of an emulsified solution with an adjustment of its pH
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
value. Then, it was blended with the prepared nitrile latex, using normal stirring for a proper period of aging time. It was found that most of the homogeneous
nanocomposite latexes were stable even after one month when stored at room temperature.
2-2.3 Characterization
Wide-angle X-ray diffraction (XRD) measurements were carried out with a Mac Science M18 X-ray diffractometer. The X-ray beam was produced by
nickel-filtered Cu Kα radiation with wavelength λ = 0.154 nm operated at 30 kV and 25 mA. The diffraction curves were obtained within the range of scattering angles (2θ) of 2-10o at a scan rate of 1 o/min. The TEM used is a JEOL JEM 2000FX electron microscope operating with an acceleration voltage of 100 kV. Ultrathin sections of the cured samples were microtomed using Leica Ultracut Uct into about 100-nm thick slices with a diamond knife; subsequently, a layer of carbon was deposited onto these slices and placed on 400 mesh copper nets.
Thermogravimetric analysis (TGA) of each sample was carried out under nitrogen purge in a Perkin-Elmer TGA-7. About 10 mg of cured sample was heated from 50 to 700 oC at 10 oC / min. Both the tensile and tear strength of the cured rubber slab were tested on a MTS tensile tester, Sintech 1-D, according to the ASTM standard D412-97 method, die C and D624-95 method, die B, respectively.
2-3 Results and Discussions
The dispersion image of layered silicates on the NBR matrix are shown and discussed individually in the section 2-3-A for the NBR-layered silicate
nanocomposites. The mechanical properties and the mechanism scheme of the NBR-layered silicate nanocomposites are discussed in the section 2-3-B and 2-3-C, respectively.
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
2-3-A. Layered silicates dispersion in NBR nanocomposites
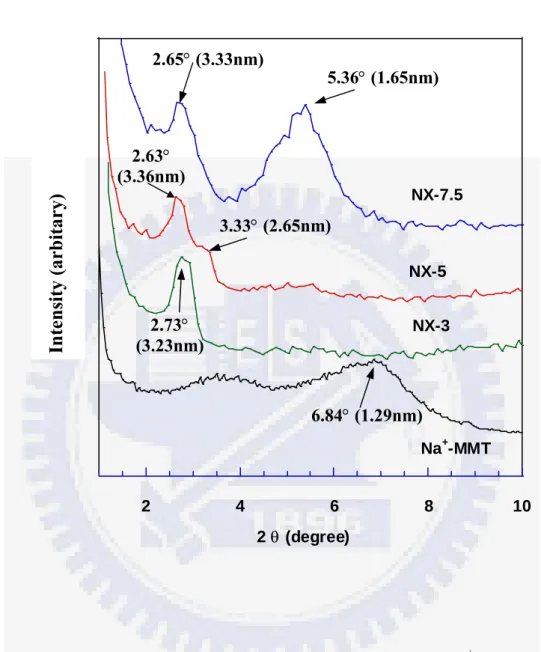
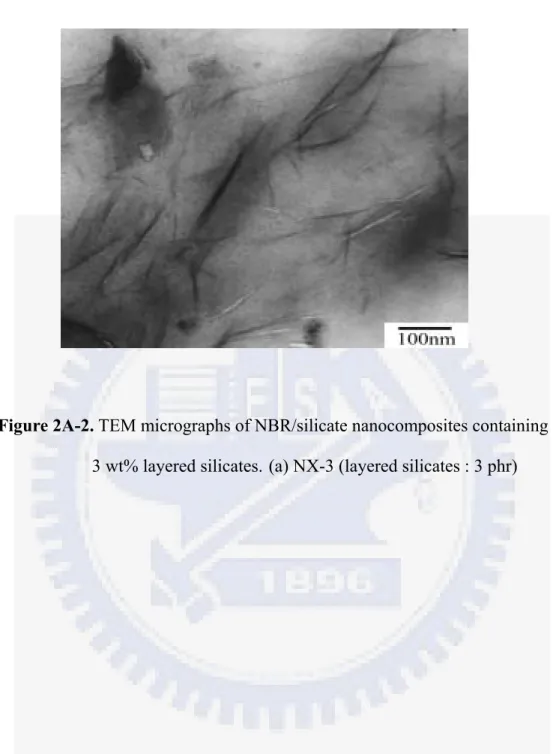
Figure 2A-1 shows the X-ray diffraction curves for the cured NBR/silicate nanocomposites. The X-ray diffraction peaks at 2θ = 6.84o , 2.73o, 2.63o and 2.65o represent the diffraction of the (001) crystal surface of layered silicates in the nanocomposites, corresponding to d-spacings of 1.29, 3.23, 3.36 and 3.33 nm, respectively. This indicates that a relatively large gallery expansion in layered silicates has been obtained in the case of NX-3 , NX-5 and NX-7.5. For the NX-5 sample, the major peak appears around 2.63o, and a blurred peak appears at about 3.33o (2.65nm), which might be caused by non-uniform expansion of layered silicates. For the NX-7.5 sample, other than the diffraction of X-ray by the (001) plane at about 2θ = 2.65o, another strong diffraction peak can be observed at about 5.36o (1.65nm). This probably indicates that there has a bimodal structure for layered silicates in NBR when the amount of layered silicates is over about 5 wt%. Figure 2A-2 presents TEM micrographs of the dispersion of nanometer-sized layered silicates in the NBR film. Single silicate layers in the rubber matrix and a few multi-layer bundles in the case of NBR containing 3 and 5 wt% layered silicates can be found in Figure 2A-2 (a) and (b). In both cases, the exfoliated layered silicates, where the interlayer d-spacing was larger than 3.2 nm, can be observed; whereas, in Figure 2A-2 (c), the dispersion of silicates in NBR can be found to actually adopt a bimodal structure, consisting of both intercalated and exfoliated states. This reveals that a higher silicate loading would lead to poorer dispersion and more aggregated bundles in the rubber film. A critical concentration of 7.5 wt% layered silicate in NBR/silicate nanocomposites can be utilized to exert the exfoliated nanocomposite using the modified latex shear blending method. The results of the TEM analysis correspond to those of the X-ray analysis quite well. Hence, based upon the results of XRD and TEM, it is likely that
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
in the nanocomposites of NBR with silicate concentrations between 3 and 7.5 wt%, exfoliated and partially intercalated layered silicates coexist .
2-3-B. Mechanical and thermal properties of NBR nanocomposites
2-3-B.1 Mechanical properties
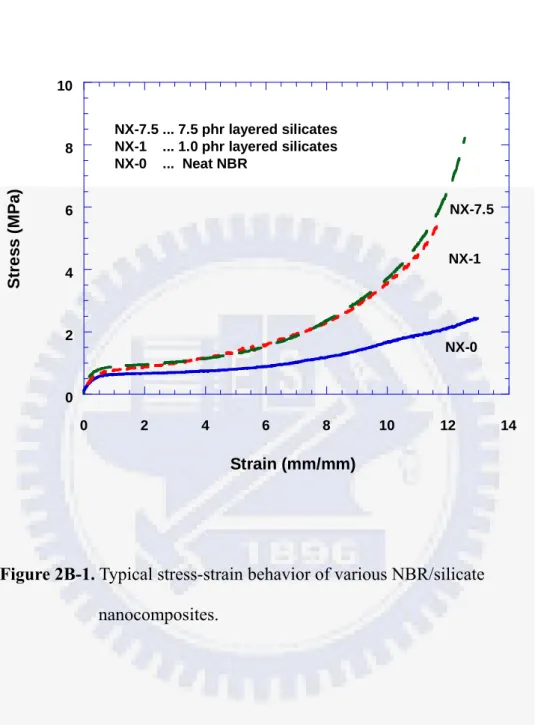
The stress-strain curves of these crosslinked NBR/silicate nanocomposites are shown in Figure 2B-1. At low strains, these materials behave similarly, but the tensile modulus of NX-7.5 increases dramatically at high strains, which is different from that of neat NBR (NX-0). The low strain behavior results from somewhat low sulfur content (low crosslink density). The addition of nano-silicate can enhance the tensile modulus of NBR. It can also be reasonably assumed that the dramatic increase in stress of NX-7.5 in the high strain region results from the effect of molecular chain orientation and the resultant orientation of layered silicates brought about by the rubber molecular orientation.
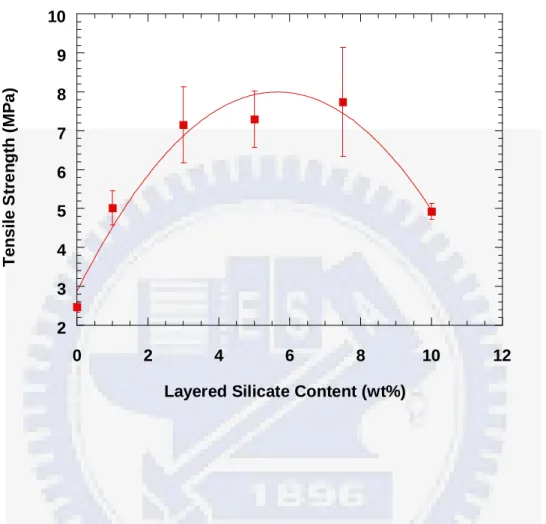
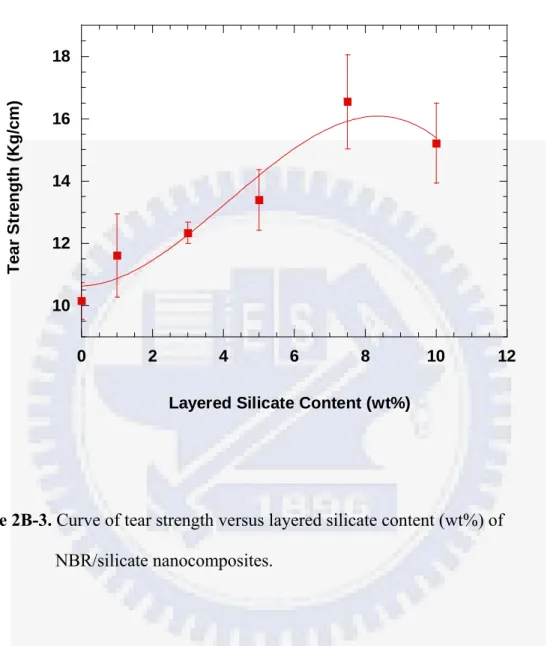
The effects of layered silicates content on the tensile and tear mechanical properties of the crosslinked NBR/silicate nanocomposites are illustrated in Figure 2B-2 and 2B-3. The tensile strength increases by more than 200 %, with a slight effect on the elongation, and the tear strength also increases considerably for these NBR/silicate nanocomposites, compared to neat NBR. The tear properties of these crosslinked NBR/silicate nanocomposites display the similar trend to that of their tensile mechanical properties. Additionally, the tensile strengths at 500% elongation (M500, engineering modulus) of the NBR/silicate nanocomposites are also much higher than that of neat NBR. The engineering modulus is an indicator of the stiffness of the rubber compounds. The tensile and tear strengths of the NBR/silicate nanocomposites increase with the amount of layered silicates up to 7.5 wt%, but decrease at 10 wt% concentration. The maximum increases in the tensile
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
and tear strength are about 200% and 60%, respectively. It can therefore be concluded that tensile and tear properties of NBR can increase dramatically with a layered silicates content up to 7.5wt% using a modified shear blending system, where the molecular chain motion of NBR into the galleries of largely exfoliated nano-silicate platelets prepared by a ball milling process. Second, exfoliated layered silicates with finer dispersion can be established with the assistance of small molecular, non-ionic and ionic surfactants and through the use of high shear forces in the ball milling process.
2-3-B.2 Thermal properties
Thermal degradation temperatures (at 5% weight loss) of NBR/silicate nanocomposites also increase with the content of layered silicates. Figure 2B-4 gives a detailed thermal gravimetric analysis of neat NBR and two nanocomposites. In addition to improving the initial decomposition temperature, the weight loss due to the thermal pyrolysis of NBR is nearly constant, until a temperature of about 450 oC is reached. At higher temperature, the layered silicates inhibit weight loss in NBR. This is possibly due to the presence of layered silicates dispersed
homogeneously in the NBR matrix that could extend the total immigration out-paths of small molecules, as well as inner volatiles, and would reduce the permeability of oxygen into the bulk of the nanocomposite. Therefore, it could inhibit the
occurrence of NBR chain scission and improve the thermal stability.
2-3-C. Schematic drawings of latex blending mechanism
Schematic drawings of the ball milling and latex blending mechanism are provided in scheme 1 and 2, respectively. In scheme 1, the intergallery space of layered silicates in water is largely expanded since the appearance of the silicate solution is homogeneous and transparent. Then, the ball milling process generates
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
a proper shear force to delaminate the layered silicate and allow the surfactant molecules to move into the interspace of layered silicate. In scheme 2, after mixing with the rubber latex, the emulsified and well-expanded layered silicates can allow the elastomer molecular chains to diffuse and intercalate into the silicate galleries. After coagulating, nearly exfoliated and partly intercalated elastomer nanocomposite can form.
2-4 Conclusions
NBR/silicate nanocomposites, with a mostly exfoliated and partially intercalated coexisting structure, were successfully prepared by ball milling of surfactant-treated layered silicates in emulsified solution, followed by latex shear blending. The tensile mechanical properties and tear strengths of these
nanocomposites increase with the amount of layered silicates, as compared to that of neat NBR. Additionally, these nanocomposites display higher thermal stabilities than neat rubber.
Acknowledgements
We appreciate the financial support provided by the Ministry of Economic Affairs through Project 92FCA12V and the National Science Council through Project NSC91-2120-M-009-001
References
[1] A. Usuki, M. Kawasum, Y. Kojima, Y. Fukushima, A. Okada, T. Kurauchi, and O. Kamigaito, J. Mater. Res., 8, 1179 (1993).
[2] H. L. Tyan, Y. C. Liu, and K. H. Wei, Chem. Mater., 11, 1942 (1999). [3] T. Agag, T. Koga, and T. Takeichi, Polymer, 42, 3399 (2001).
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
[4] L.Y. Jiang, C. M. Leu, and K. H. Wei, Adv. Mater., 14, 426 (2002). [5] T. K. Chen, Y. I. Tien, and K. H. Wei, Polymer, 41, 1345 (2000). [6] Y. I. Tien and K. H. Wei, Macromolecules, 34, 9045 (2001). [7] J. Ma, S. Zhang, and Z. Qi, J. Appl. Polym. Sci., 82, 1444 (2001).
[8] M. Kawasumi, N. Hasegawa, M. Kato, A. Usuki, and A. Okada, Macromolecules,
30, 6333 (1997).
[9] E. Passaglia, W. Bertuccelli, and F. Ciardelli, Macromol. Symp., 176, 299 (2001). [10] E. A. Manias, L. Touny, K. E. Wu, B. L. Strawhecker, and T. C. Chung, Chem.
Mater., 13, 3516 (2001).
[11] Y. T. Vu, J. E. Mark, L. H. Phom, and M. Engelhardt, J. Appl. Polym. Sci., 82, 1391 (2001).
[12] K. S. Kwan, D. A. Harrington, P. A. Moore, J.R. Hahn, J. V. Degroot, and G. T. Burns, Rubber Chem. Technol., 71, 630 (2001).
[13] J. T. Kim, T. S. Oh, and D. H. Lee, Polym. Int., 52(7), 1058 (2003). [14] J. T. Kim, T. S. Oh, and D. H. Lee, Polym. Int., 52(7), 1203 (2003). [15] S. Varghese and J. Karger-Kocsis, J. Appl. Polym. Sci., 91(2), 813 (2004). [16] A. Usuki, A. Tukiguse, and M. Kato, Polymer, 43, 2185 (2002).
[17] C. Nah, H. J. Ryu, S. H. Han, J. M. Rhee, and M. H. Lee, Polym. Int., 50, 1265 (2001).
[18] Y. W. Chang, Y. Yang, S. Ryu, and C. Nah, Polym. Int., 51, 319 (2002). [19] S. Joly, G. Garnaud, R. Ollitrault, and L. Bokobza, Chem. Mater., 14, 4202
(2002).
[20] Z. Wang and T. Pinnavaia, Chem. Mater., 10 3769- (1998).
[21] T. K. Chen, Y. I. Tien, and K. H. Wei, J. Polym. Sci.: Chem., 37, 2225 (1999). [22] A. Akelah and A. Moet, J. Appl. Polym. Sci., 55, 153 (1994).
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
[23] Y. Wang, L. Zhang, C. Tang, and D. J. Yu, Appl. Polym. Sci., 78, 1879 (2000). [24] M. Xiong, L. Wu, S. Zhou, and B. You, Polym. Int. 51, 693 (2002).
[25] L. Zhang, Y. Wang, Y. Sui, and D. Yu, J. Appl. Polym. Sci., 78, 1873 (2000). [26] Y. P. Wu, L. Q. Zhang, Y. Q. Wang, Y. Liang, and D. S. Yu, J Appl. Polym. Sci.,
82, 2842 (2001).
[27] K. E. Strawhecker and E. Manias, Chem. Mater., 12, 2943(2000).
[28] T. Yui, H. Yoshida, H. Tachibana, D. A. Tryk, and H. Inoue, Langmuir, 18, 891 (2002).
[29] Y. K. Kim, Y. S. Choi, K. H. Wang, and I. J. Chung, Chem. Mater., 14, 4990 (2002).
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2A-1. X-ray diffraction patterns for layered silicate (Na+-MMT ) and for NBR/silicate nanocomposites.
NX-7.5 2 4 6 8 10 2 θ (degree) Na+-MMT NX-3 NX-5 Intensity (arbitary) 6.84° (1.29nm) 2.73° (3.23nm) 3.33° (2.65nm) 2.63° (3.36nm) 5.36° (1.65nm) 2.65° (3.33nm)
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2A-2. TEM micrographs of NBR/silicate nanocomposites containing
3 wt% layered silicates. (a) NX-3 (layered silicates : 3 phr)
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2A-2. TEM micrographs of NBR/silicate nanocomposites containing
5 wt% layered silicates. (b) NX-5 (layered silicates : 5 phr)
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2A-2. TEM micrographs of NBR/silicate nanocomposites containing
7.5 wt% layered silicates. (c) NX-7.5 (layered silicates : 7.5 phr)
100nm
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2B-1. Typical stress-strain behavior of various NBR/silicate nanocomposites. 0 2 4 6 8 10 0 2 4 6 8 10 12 14 St re s s ( M P a ) Strain (mm/mm) NX-7.5 ... 7.5 phr layered silicates NX-1 ... 1.0 phr layered silicates NX-0 ... Neat NBR NX-1 NX-7.5 NX-0
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2B-2. Curves of tensile properties versus layered silicate content (wt%) of
NBR/silicate nanocomposites. a. Tensile strength. 2 3 4 5 6 7 8 9 10 0 2 4 6 8 10 12 Tens ile S tre n g th ( M P a )
Layered Silicate Content (wt%)
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2B-2. Curve of tensile properties versus layered silicate content (wt%) of
NBR/silicate nanocomposites. b. M500 (Engineering modulus). 0.6 0.8 1 1.2 1.4 1.6 0 2 4 6 8 10 12 S tr e ngt h a t 5 0 0 % Eb ( M Pa )
Layered Silicate Content (wt%)
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2B-3. Curve of tear strength versus layered silicate content (wt%) of NBR/silicate nanocomposites. 10 12 14 16 18 0 2 4 6 8 10 12 Tea r S trengt h ( K g /c m )
Layered Silicate Content (wt%)
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
Figure 2B-4. Comparative TGA results (5 % weight loss decomposition
temperature, Td, oC) of NBR/silicate nanocomposites with neat NBR. 200 400 600 10 20 30 40 50 60 70 80 90 100
NX-0
NX-5
NX-7.5
5% weight loss
450
oC
Compd. Onset temp.,oC (5% weight loss) NX-0 412.5 NX-5 420 NX-7.5 421
W
eight (%
)
Temperature (
oC)
Chapter 2: Preparation and Mechanical Properties of Nitrile Butadiene Rubber/Silicate
※ Enlargement in local domain as the following After ball milling
Surfactant molecule Na+ Na+ Na+ Na+ Na+ Na+ Hydrophilic site Hydrophobic site Media : water Surfactant Emulsifier Na+ Na+ Na+ Na+ Na+ Na+
Scheme 1. Formation of exfoliated layered silicates
Electrolyte
Micelle Formation of layered silicate Chapter 2: Preparation and Mechanical Properties
Scheme 2. Latex blending process Shear blending Micelle of rubber latex Micelle of layered silicate Elastomer nanocomposite Chapter 2: Preparation and Mechanical Properties
Chapter 3: Mechanical, Thermal, and Barrier Properties of
NBR/Organosilicate Nanocomposites
3-1 Introduction
The pioneering work conducted by Toyota Central Research on nylon-6/silicate nanocomposites has inspired great interest in polymer nanocomposites over the past decade [1-3]. This particular interest results from the highly efficient enhancement of the barrier and mechanical properties of a polymer by the nanometer-sized silicates when they are well-dispersed in the polymer matrix. The ideal case would have the layered silicates completely separated from one another (termed delaminated or exfoliated) in the polymer matrix. In the other case, there would be a slight increase in the intergallery spacing of the layered silicates but the same orientation for most of layered silicates (termed intercalated) is retained in the matrix. Exfoliation, however, can hardly be achieved in silicates without modification by small organic molecules, which diffuse readily into the intergallery of the silicate layers and open up with enough space between silicates to allow further penetration of the polymer molecules. The driving force for exfoliation or intercalation depends upon the thermodynamic interaction between polymer and silicate as well as the diffusion of polymer chains into the intergallery of the silicate layers. In most cases, proper chemical treatment and optimized processing are key to the formation of nanocomposites. The interlayer distance of the montmorillonite-based silicates when modified with alkylammonium cations is between 1.5 and 3.5 nm. This space is usually large enough to produce intercalated and exfoliated structures upon further processing. In-situ
intercalation have been developed to prepare polymer-layered silicate nanocomposites [4, 5].
While organosilicates have been used in various thermoplastics and thermoset resins, a fairly small number of studies have been reported on the use of
organosilicates to reinforce rubber. “Bound rubber”, a traditional concept, can be used to demonstrate the reinforcing behavior of rubber with carbon black or layered silicates, which is an undissolved composition of rubber compound by solvent [6-8]. Bound rubber based on carbon black would cause a considerable increase in
compounding viscosity and is disadvantageous in the mixing process. Another drawback of carbon black is that it has a black color and is usually used in high amounts, which is detrimental to the final performance of rubber formulations. A variety of white or pale fillers have been chosen to replace carbon black, such as silica or clay minerals. The reinforcing effect of NBR nanocomposites containing a few weight percent of layered silicates is similar to NBR compounds having a much higher loading of carbon black. When using properly treated organosilicate, however, it can provide an effective physical reinforcement, helping to reduce the viscosity of the uncured elastomeric compound and also retain transparency [8-10]. Additionally, the efficiency of the silicates in modifying the properties of the rubber is largely determined by the degree of their dispersion and the extent of exfoliation in the rubber. Rubbers are more hydrophobic than some of thermoplastics, such as nylon 6, and therefore, it is difficult to achieve dispersion of layered silicates in a rubber matrix by treating montmorillonite with alkyl ammonium ions alone. There are, however, some advantages for rubber/silicate nanocomposites. First,
amine-compounds are widely used in sulfur curing rubber formulations as
accelerators, and hence the ammonium molecule in the montmorillonite might be involved in the sulfur curing reaction. Besides, the very high molecular weight
Chapter 3: Mechanical, Thermal, and Barrier Properties of NBR/Organosilicate Nanocomposites