行政院國家科學委員會補助專題研究計畫成果報告
※※※※※※※※※※※※※※※※※※※※※※※※※
※ ※
※ 室內空氣清淨機(過濾興靜電集塵) ※
※
效能特性探討(2/2)
※
※※※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:■個別型計畫
□整合型計畫
計畫編號:NSC89-2314-B-002-472-
執行期間:2000 年 08 月 01 日至 2001 年 07 月 31 日
計畫主持人: 陳志傑
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
執行單位: 台灣大學公共衛生學院職衛所
中
華
民
國 90 年 10 月 31 日
行政院國家科學委員會專題研究計畫成果報告
計畫編號:NSC89-2314-B-002-472-
執行期限:89 年 08 月 01 日至 90 年 07 月 31 日
主持人:陳志傑 台灣大學公共衛生學院
計畫參與人員:黃盛修 台灣大學公共衛生學院
一、中文摘要 在理論上,對於微粒帶電的機制已經建立得 相當完整,其中一些外在的因素包括:充電時間、 離子濃度、微粒粒徑、電場強度、微粒之介電常數、 離子的種類特性以及溫度等對於微粒充電量的影 響雖然在過去的文獻中已有詳細的探討與瞭解,但 是微粒的濃度對於其本身的充電過程之影響卻鮮 少有人做過研究。一旦微粒的帶電存在著所謂的遮 蔽效應,意味著過去所發展出來的理論在應用上勢 必須對微粒的濃度作適當的限制。因此,在本研究 中,使用了兩個自製的微粒充電器:擴散充電器以 及電場充電器,藉以進一步瞭解微粒在高濃度下其 充電是否有遮蔽的效應,以及其影響的程度與範 圍。 實驗的結果發現,當超細微粒在濃度小於 1 × 105 #/cm3時,於擴散充電的過程中並未發現有遮蔽 效應的產生;相對地,微米粒徑的微粒在電場充電 中,當微粒的表面積濃度大於 2 × 104µ m2/cm3時, 即可明顯地看出遮蔽效應的產生,而且該表面積濃 度之臨界值,並不因為微粒的粒徑而改變。研究中 亦發現,除了表面積濃度之外,微粒的帶電量以及 微粒的穿透率亦可以作為遮蔽效應產生的指標。 關鍵詞:氣膠充電、遮蔽效應、靜電集塵器
Abstr actParticle charging theories are well established. Although the effect of external
conditions including charging time, ion concentration, particle diameter, electric field strength, dielectric constant of the particle, the nature of ions and temperature has been thoroughly examined, the effect of particle concentration on particle charging has not been investigated in a systematic manner. However, due to the masking effect on particle charging, these theories may not be applicable in an environment where particle concentration is high, in particular in the field charging regime. To further understand the masking effect and thus study the effect of particle concentration on the particle charge, two homemade chargers, a diffusion charger and a field charger, were used herein.
Experimental results indicate that, when charged by the diffusion charger, the masking effect was not observed for ultrafine particles with a number concentration less than 1 × 105 #/cm3. However,
with the field charger the masking effect was obvious when it was challenged with micrometer particles that had a surface area concentration of above 2 × 104
µm2/cm3. This value did not vary with particle size. Furthermore, both the number of particle charge and the aerosol penetration could be used as an index to gauge the masking effect.
Keywor ds: Aerosol Charge, Masking Effect,
Electrostatic Precipitator
Intr oduction
Charging aerosol particles with unipolar ions is an essential physical process as well as a significant phenomenon in aerosol physics that has numerous practical applications. It can occur either with or without an applied electric field. For example, the former is used in electrostatic precipitators (ESPs) to remove particles from effluent gas streams, while the latter is used in Electrical Aerosol Analyzer (Liu and Pui, 1975) to measure the size distribution of aerosol particles.
Particles are charged when ions bombard its surface. Historically, two mechanisms, diffusion and field charging, have been considered to govern the charging process. Diffusion is a result of collisions of ions and particles, which have motion due to their thermal kinetic energy. Alternately, field charging occurs when ions follow electric field lines until they terminate on a particle or on the collection surface of opposite polarity.
The theory for the charging of particles has been well developed. In 1923, Rohmann presented the initial fundamental studies of the phenomena involved therein (Clements and Yu, 1991). In 1932, however, Pauthenier and Moreau-Hanot re-derived the field charging equation in an alternate form (Liu and Kapadia, 1978; Clements and Yu, 1991; Zhuang et al., 2000). White (1963), Oglesby and Nichols (1978), Jantunen and Reist (1983), and Hinds (1999) have presented a more recent analysis of field charging. In field charging, the following equation can be
employed to express the particle charge as a function of time (Hinds, 1999):
+
+
=
t
N
eZ
K
t
N
eZ
K
e
K
Ed
t
n
i i E i i E Eπ
π
ε
ε
1
4
2
3
)
(
2 (1)where ε is the relative permittivity of particle, E is the electrical field strength, d is the particle diameter, KE
is the electrostatic constant of proportionality, e is the charge of an electron, Zi is the mobility of the ions,
and Ni is the ion concentration.
The theory for diffusion charging has been developed by Fuchs in 1947 (Liu and Kapadia, 1978; Zhuang et al., 2000), Bricard in 1949 (Liu and Kapadia, 1978; Lawless, 1996; Zhuang et al., 2000), and Whits in 1963 (Smith and McDonald, 1975, 1976; Lawless, 1996). An approximate expression for the particle charges due to diffusion charging during time t is (Hinds, 1999):
+
=
kT
t
N
e
c
d
K
e
K
dkT
t
n
E i i E2
1
ln
2
)
(
2 2π
(2)where k is the Boltzmann’s constant, T is the
temperature, and
c
iis the mean thermal speed of the ions.Accordingly, in field and diffusion charging, the particle charge is proportional to d2 and d,
respectively. Therefore, for particles larger than 1 µm (Smith and McDonald, 1975; Kim and Yoon, 1997; Hinds, 1999) in high electric field, the field charging is dominant. Furthermore, the prediction based on the field charging theory agrees reasonably well with experiments (Smith and McDonald, 1975; Clements and Yu, 1991). In contrast, diffusion charging is the dominant mechanism for particles that are less than 0.1
µm (Hinds, 1999).
Strictly speaking, both diffusion and field charging mechanisms operate at the same time on all particles, however neither mechanism can fully explain the charges measured on the particles. Many studies confirm that field charging is important for large particles and higher electrical fields, whereas diffusion charging is dominating for small particles or when the electric field is low or equal to zero (Smith and McDonald, 1975; Liu and Kapadia, 1978; Lawless, 1996). In intermediate cases, neither is adequate and must be replaced by a model that combines field and diffusion charging (Smith and McDonald, 1975) or by a numerical solution of the field-diffusion problem (Liu and Kapadia, 1978). Moreover, Lawless (1996) presented approximations for charging rates that are applicable to diffusion, field and combined
charging. However, sum of the charges that equations (1) and (2) predict independently, produce a superior approximation for the measured charge (Turner et al., 1995; Anthony and Wayne, 1992).
Charging time, ion concentration, particle diameter, electrical field strength and, to a lesser extent, dielectric constant of the particle, nature of the ions and the temperature at which charging occurs control the amount of charge that a particle acquires. To date, many studies have examined how these external conditions affect the charging of particles. Notably, many of these discussions focus only on a single particle condition or low particle concentration. We shall now have to consider how this picture alters when the charging zone is filled with numerous particles. Zukeran et al. (1999) revealed that when using an ESP to collect incense-combustion -generated particles, its number collection efficiency decreased with a particle concentration that exceeded 4.5 × 1010 particles/m3. The efficiency of an ESP mainly depends on its ability to charge particles. Therefore, it is believed that the decrease of ESP’s collection efficiency at high particle concentration condition might be mostly due to the decrease of particle charges, i.e., particles might mask each other when acquiring charges under high concentration condition. In order to further explore and establish a firm understanding of the masking effect on particle charging, this study undertook a controlled experiment.
Mater ials and methods
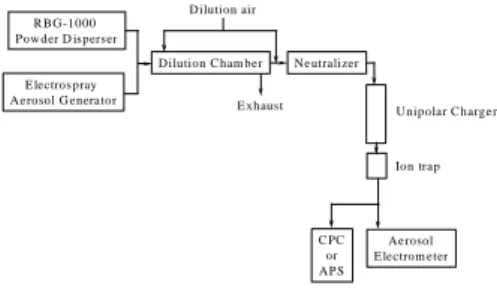
Figure 1 schematically depicts the experimental system setup that was used to study the masking effect on particle
E lectros pray Ae rosol G enerator
R BG-1000 Pow der D isperser
Dilution air
E xhaust Ne utralizer Dilution C ham ber
Ae rosol E lectrom eter C PC or AP S Unipolar C harge r Ion trap
Figure 1. Schem a tic diagram of the e xperim ental setup to evaluate the m asking effe ct on particle charging.
c harging. An electrospray aerosol generator (model 3480, TSI, Inc.) was employed to produce
monodispersed nanometer-sized sucrose particles. A Palas powder disperser (model RGB-1000, Germany) was used employed to generate narrowly distributed micrometer-sized acrylic particles (MX-series, Soken Chemical & Engineering Co. Ltd., Japan). The generated aerosols were then delivered into a dilution chamber. The dilution air was controlled based on the desirable particle concentration. The test aerosol was transported through a neutralizer prior to entering the unipolar charger.
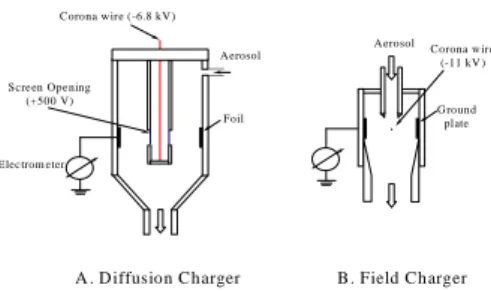
Corona w ire (-6.8 kV) Screen Opening (+500 V) A erosol Electrom eter Foil Corona w ire (-11 kV ) A erosol G round plate ·
A . D iffusion Charger B. Field Charger
Figure 2 . Schema tic sketch o f the particle chargers: A) diffus ion c ha rger, B) fie ld charg er.
To investigate the masking effect on particle charging, two homemade unipolar chargers, diffusion and field, were employed herein. The geometrical configuration of the diffusion charger (Figure 2A) was in the form of two concentric metal cylinders with a 0.3-mm copper wire and was placed along the axis of the cylinders. Notably, it is similar to the one that both Liu and Pui (1975) and Buscher et al. (1994) used. A negative high voltage was applied to the wire to produce ions from a corona discharge. A slight positive voltage was applied to the inner cylinder to force the ions through its screen opening and into the region between the cylinders. The electric field between the cylinders was extremely weak, thus there was no field charging effect. An insulated foil on the inner side of the outer cylinder was connected to an electrometer. Thus, the ion current that flowed between the cylinders was measured and
monitored. Alternately, a small-scale wire-plate ESP was used as a field charger (Figure 2B). A 0.3-mm copper wire with dimensions of 15 × 13 mm was placed between the two grounded plates. The distance between the wire and each grounded plate was 6.5 mm. The total airflow rate through the diffusion charger and the field charger was 5.3 and 6 L/min, respectively. To get rid of the high mobility negative ions, a small ion trap was used directly behind the outlet of the chargers.
To measure the aerosol concentration of nanometer and micrometer sized particles a
condensation particle counter (CPC, model 3022, TSI Inc.) and an aerodynamic particle sizer (APS, model 3320, TSI Inc.) were used, respectively. Particle number concentration data obtained in the power-on and power-off modes were used to evaluate the aerosol penetration through the charger. The total aerosol charge was measured with an aerosol electrometer (model 3068, TSI, Inc.). The number of elementary charges per particle, Ne, was then calculated through the following equation:
e
N
F
I
N
e×
×
=
(3) where I is the electrometer current, F is the sampling flow rate of the electrometer, N is the particle concentration, and e is the elementary unit of charge. Herein, the sampling flow rate of the aerosol electrometer was 5.0 L/min.Results and Discussions
Figure 3 presents the aerosol penetration through the diffusion charger for neutralized particles as a function of particle size that was measured without the ion
source.
Particle diam eter, nm
1 0 1 00 1 00 0 A e ro s o l pe ne trat io n , % 0 2 0 4 0 6 0 8 0 1 00
Air flow rate = 5.3 L /m in (Coron a disc ha rg e o ff)
Figure 3. Pa rtic le p en etration throug h the d iffusion charge r for ne utra l partic le s as a functio n of partic le s ize .
Above 40 nm, the charger approaches practically 100 % penetration and below it decreases gradually. At 10 nm particle diameter, aerosol penetration through diffusion charger reaches 80 %. The decrease in aerosol penetration was due to diffusion loss (Buscher et al., 1994; Chen and Pui, 1999). However, when the corona discharge was turned on, the particles became charged, and electrostatic forces removed a portion of them. At lower concentration conditions, the charger achieved only 50 % penetration of the charged nanoparticles, as shown in Figure 4. The value of aerosol
Particle num ber concentration, 1/cm3 0 1 e+5 2 e+5 3 e+5 4 e+5
A e ro s o l p e n e tr a ti o n , % 0 1 0 2 0 3 0 4 0 5 0 6 0
Pa rticle dia m eter, n m 4 4 5 0 6 0
C ha rg ed pa rticles
Figure 4. Particle penetration through the diffusion charge r a s a function of particle number concentration when corona discharge was turned on.
penetration did not vary with particle size. As the concentration increased, aerosol penetration decreased. For example, when the particle
concentration increased from 5 × 104 to 3 × 105 #/cm3, the aerosol penetration decreased from 50 % to 40 %. The decreases in aerosol penetration might be due to the effect of particle space charge. Increasing the particle space charge enhanced the electric field strength, (Bohm, 1982; Park and Kim, 1998) which propelled the particles toward the grounded surface at a higher speed, and the aerosol penetration thus decreased (Huang and Chen, 2001). Although the loss of charged particles will result in underestimating the number of charges on each particle when they are partially charged, it will not change the trend if masking effect occurs. Therefore, the output efficiency of the charger was not improved intensely.
Figure 5. Experimental results of the particle charges versus particle number concentration. Particle num ber concem tration, 1/cm3
0 1 e+5 2 e+5 3 e+5 4 e+5
N u m b e r o f e le m e n ta ry c h a rg e s 0 .0 0 0 .0 2 0 .0 4 0 .0 6 0 .0 8 0 .1 0 0 .1 2 0 .1 4 4 4 5 0 6 0 Pa rticle dia m eter, n m
Figure 5 illustrates the experimental results of particle charges as a function of aerosol number concentrations. The graph indicates that the particle charges increase with particle size. However, for a given size, the particle charge did not change significantly with the increase in the aerosol number concentration. Thus, the test conditions that were employed herein failed to reveal the masking effect of nanoparticles through diffusion charging. Owing to the limitation of our testing system, a higher test aerosol concentration was not generated. However, our experimental results do not guarantee that the masking effect in the diffusion charger will not occur when the aerosol concentration exceeds 3 × 105 #/cm3. That is, further investigation is needed.
Particle num ber concentration, #/cm3 10 100 1000 E le m e n ta ry n u m b e r o f c h a rg e s 0 100 200 300 400 500 600 1.98 3.92 6.26 P article diam eter, µm
Figure 6. Particle charges acqu ire d by m icrom eter-sized partic les in the field charger.
Nu mb e r o f e le me n ta ry c h a rg e s
In contrast, Figure 6 demonstrates the number of elementary charges that were a function of aerosol number concentration for micrometer-sized
particles. Obviously, the particle charges increased with an increase in particle size. At lower aerosol concentrations, the particle charge was independent of particle concentration. However, when the aerosol concentration increased to a critical value, the particle charges decreased significantly. For example, the number of elementary charges of 6.26 µm particles was 474, which did not change until the aerosol number concentration exceeded 100 #/cm3. When it was increased to 1,000 #/cm3, the particle charges decreased to 120 elementary charges. Similarly, the critical value for 1.98 and 3.92 mm particles was 1,500 and 500 #/cm3, respectively. The decreasing of particle charge indicates the masking effect.
Within the field charging mechanism, the free ions travel along the field lines and collide with particles where the field lines intersect
them. Therefore, masking effect is dependent on the amount of surface area. Figure 7, which is modified from Figure 6, presents the surface area concentration versus
Su rface a rea co ncentratio n, µm2/cm3 1e+2 1e+3 1e+4 1e+5
N um be r of el em en ta ry c ha rge s 10 100 100 0
P article diam eter, µm 1.96 3.92 6.26
Figu re 7. Particle cha rg e ve rsus surface area con ce ntratio n fo r micrometer particles in field cha rg er.
the particle charges. As mentioned previously, the particle charge decreased as the surface area increased to a critical value regardless of which particle size was employed. For all particle sizes used in this study, the particle charge decreased significantly when the surface area concentration exceeded 2 × 104µm2/cm3 (Figure 7). Notably, the field charger used in this study is was a small-scaled ESP. Thus, high particle loss in the charger, particularly micrometer particles, was unavoidable. However, since monodispersed aerosols were employed herein, the loss will not bias the calculation of particle charge.
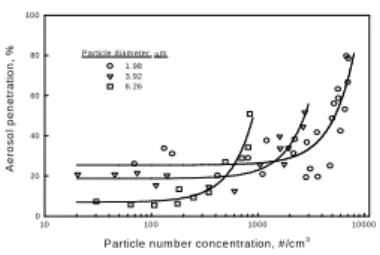
Particle num ber concentration, #/cm3 10 100 1000 10000 A er o s o l p en et rat ion , % 0 20 40 60 80 100 1.98 3.92 6.26 Particle diam eter, µm
Figu re 8. The aero so l p enetratio n o f m icrometer particles throu gh the field charger as a fun ctio n o f particle numb er con centratio n..
Figure 8 demonstrates that the charged particle penetration through the field charger as a function of particle number concentration. As expected, when the number concentration was less than 100 #/cm3 the penetration was only 25 % for 1.98 µm
particles. However, for 3.92 µm and 6.26 µm particles, the penetration was 20 % and 8 %, respectively. Due to the masking effect on particle charging, the aerosol penetration increased
significantly as the particle number concentration increased to a critical value. These experimental results coincide with those in Figure 6. Based on Figure 7, the surface concentration, instead of the number concentration was employed in Figure 8. The trends of aerosol penetration increase in Figure and the particle charge decrease in Figure were symmetrical. Furthermore, the aerosol penetration increased significantly as the surface concentration exceeded 2 × 104µm2/cm3, which was mainly due to the decrease in particle charges, i.e., masking effect on particle
charging.
Conclusions
This study examined the masking effect on particle charging with a diffusion charger and a field charger for monodispersed nanometer and micrometer particles, respectively. The conditions employed in this work failed to prove the masking effect in the diffusion charger. However, in the field charger, the masking effect was obvious when the surface area concentration of particles exceeded 2 × 104
µm2/cm3. Therefore, as with particle charging theories, particle concentration should also be
considered when the concentration is high. Moreover, both the particles and the aerosol penetration can be used as an index to gauge the masking effect.
Acknowledgment
The authors would like to thank the
National Science Council of Taiwan for
financially supporting this research under
Contract No. NSC 90-2320-B-002-039.
Refer ences
Anthony, J.B. and Wayne, T.D. (1992). Air pollution engineering manual / Air & Waste Management Association. New York: Van Nostrand Reinhold. Bohm, J. (1982). Electrostatic precipitators, Elsevier,
New York, 1982.
Buscher, P., Schmidt-Ott, A., and Wiedensohler, A. (1994). Performance of a Unipolar “Square Wave” Diffusion Charger with Variable nt-Product. J. Aerosol Sci. 25(4): 651-663. Clements, J.C. and Yu, K.K. (1991).
Continuum-Regime Field Charging in an Electron Swarm. IEEE Trans. Ind. Appl. 27(6):
1225-1232.
Hauan, S.H. and Chen, C.C. (2001). Ultra-fine Particles Penetration through ESPs. Environ. Sci. Technol. (submitted, 2001).
Hinds, W.C. (1999). Aerosol technology: properties, behavior, and measurement of airborne particles. New York: Wiley.
Kim, K.B. and Yoon, B.J. (1997). Field Charging of Spherical Particles in Linear Electric Field. J. Colloid Interface Sci. 186: 209-211.
Lawless, P.A. (1996). Particle Charging Bounds, Symmetry Relations, and an Analytic Charging Rate Model for the Continuum Regime. J. Aerosol Sci. 27(2): 191-215.
Liu, B.Y.H. and Kapadia, A. (1978). Combined Field and Diffusion Charging of Aerosol Particles in the Continuum Regime. J. Aerosol Sci. 9: 227-242. Liu, B.Y.H. and Pui, D.Y.H. (1975). On the
Performance of the Electrical Aerosol Analyzer. J. Aerosol Sci. 6: 249-264.
Oglesby, S. and Nichols, G.B. (1978). Electrostatic precipitation. New York: Dekker.
Park, S.J. and Kim, S.S. (1998). Effects of Particle Space Charge and Turbulent Diffusion on Performance of Plate-plate Electrostatic Precipitators. J. Electrostst.45: 121-138.
Smith, W.B. and McDonald, J.R. (1975). Calculation of the Charging Rate of Fine Particles by
Unipolar Ions. J. Air Pollut. Control Assoc. 25(2): 168-172.
Smith, W.B. and McDonald, J.R. (1976).
Development of a Theory for the Charging of Particles by Unipolar Ions. J. Aerosol Sci. 7: 151-166.
Turner, J.H., Lawless, P.A., Yamamoto, T., Coy, D.W., Greiner, G.P., McKenna, J.D., and
Vatavuk, W.M. (1995). Electrostatic Precipitators. From
http://www.epa.gov/ttn/catc/dir1/chpt6a.pdf. White, H.J. (1963). Industrial Electrostatic
Precipitation. Addison-Wesley, New York. Zhuang, Y., Kim, Y.J., Lee, T.G., and Biswas, P.
(2000). Experimental and Theoretical Studies of Ultra-fine Particle Behavior in Electrostatic Precipitators. J. Electrostst.48: 245-260. Zukeran, A., Looy, P.C., Chakrabarti, A., Berezin,
A.A., Jayaram, S., Cross, J.D., Ito, T., and Chang, J.S. (1999). Collection Efficiency of Ultrafine Particles by an Electrostatic Precipitator Under DC and Pulse Operating Models. IEEE Trans. Ind. Appl. 35(5): 1184-1191.