行政院國家科學委員會專題研究計畫 期中進度報告
子計畫四:奈米生物性氣膠採樣與分析探討(II)(1/2)
計畫類別: 整合型計畫 計畫編號: NSC92-2621-Z-002-013- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學公共衛生學院環境衛生研究所 計畫主持人: 李芝珊 計畫參與人員: 曾俊傑 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 5 月 21 日
行政院國家科學委員會補助專題研究計畫期中進度報告
奈米生物性氣膠採樣與分析探討 (II) (1/2)
計畫類別:□ 個別型計畫
■
整合型計畫
計畫編號:NSC
92-2621-Z-002-013
執行期間:
92 年 8 月 1 日至 93 年 7 月 31 日
計畫主持人:
李芝珊
執行單位:台灣大學環境衛生研究所
成果報告類型(依經費核定清單規定繳交):
■
精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:
中 華 民 國 93 年 5 月 21 日
行政院國家科學委員會專題研究計畫成果報告
奈米生物性氣膠採樣與分析探討
Sampling and Analysis for Nano Bioaerosol
計畫編號:NSC 92-2621-Z-002-013
執行期限:92 年 8 月 1 日至 93 年 7 月 31 日
主持人:李芝珊 國立臺灣大學環境衛生研究所
E-mail: csli@ccms.ntu.edu.tw
一、中文摘要 隨著奈米科技的進步,新技術與 新知識提供了大家看問題的新方向與解決 問題的新途徑。目前已有許多流行病學研 究指出許多傳染性疾病是經由環境中的病 毒氣膠傳播傳播,基於人類健康危害觀 點,感染性的生物氣膠不論在疾病的傳輸 與健康風險上都扮演著極重要的角色,因 此為了瞭解病毒氣膠所可能帶來的健康衝 擊,有必要對於病毒氣膠做進一步的描述 與評估。本實驗利用噬菌體來代替實際會 對人體產生健康影響的病毒,並評估常用 的 生 物 氣 膠 採 樣 器 : Andersen impactor, AGI-30 impingers, gelatin filter 和 Nuclepore filter 對 於 病 毒 氣 膠 的 採 樣 效 率。經由本研究得知,病毒的外型構造以 及是否具有套膜均會影響其採樣效率。對 於 親 水 性 的 病 毒 ( 不 具 套 膜 ) 來 說 , Andersen impactor, impinger, and gelatin filter 均 能 有 效 的 捕 集 病 毒 氣 膠 , 至 於 Nuclepore filter,由於其在採樣以及萃取的 過程中會使病毒去活化,因此其採樣效率 較低。另外,對於具有套膜的病毒來說, 本研究中各種採樣器對其採樣效率均相當 低 , 主 要 是 因 為 套 膜 對 於 環 境 壓 力 (Environmental Stress)相當敏感,採樣過程 中的所產生的採樣壓力會對病毒造成去活 化的影響。 關鍵詞:生物氣膠、病毒氣膠、噬菌體、 Andersen impactor; Impinger; Gelatin filter; Nuclepore filterAbstract
Rencently, nanotechnology draws scientists’ eye. The great progresses are in both knowledge and technology. In aerosol studies, particle size distribution always plays a very important role in particle’s behavior, related to both particle transmission and health issues. As a consequence, it is essential to find out the whole story of smaller particles- nanoparticles. In order to understand health risk from virus exposure, it is important to characterize virus aerosols. In this study, bacteriophages were surrogates for mammalian viruses in assessing sampling efficiency of Andersen impactor, impingers, gelatin filter and Nuclepore filter, as well as storage effects of virus aerosols in AGI-30 impinger. Our results demonstrated virus particle morphology and with/without envelope could significantly affect virus sampling performance. For hydrophilic virus, Andersen impactor, impinger, and gelatin filter are likely to perform better than Nuclepore filter. The recovery of lipid-envelope virus sensitive to sampling stress was indicated to be very low.
Keywords: bioaerosols; virus aerosol;
bacteriophage;
Andersen impactor; Impinger; Gelatin filter; Nuclepore filter
二、緣由與目的
Most viruses are obligate parasites and considered to be pathogenic to humans or animals by air, food, water and vectors. By air pathway, airborne and droplet transmission are the major spreading methods for viral diseases, such as smallpox, influenza, measles and mumps virus. Recently, Severe Acute Respiratory Syndrome (SARS) and influenza virus attracted public attention and both were transmitted by virus aerosol. From the aerosol point of view, the droplet diameters in the range of 1~100 µm will completely evaporate in few seconds even at high relative humidity environment. Therefore, the generated droplet will immediately decrease the diameter size and remain virus itself. Although virus particles can remain airborne for long periods of time and have the potential for retention in the respiratory track (Ijaz, et al., 1987), virus concentrations will be diluted by the airflow, and environmental stress would make virus lose its infectivity.
A number of studies indicated that virus aerosol below 2 µm are found to be especially prominent and important (Couch et al., 1965). It was indicated the mean size of airborne virus aerosol was 1.3~2.3 µm (Edward et al., 1943). The overall sampling efficiency of bioaerosol samplers with different designs may differ significantly from one another because of the different physical collection efficiency and the stress imparted to the microorganisms. The selection of sampler, microorganism hardiness, sampling time, and sampling flow rate are considered to be the most important factors to affect microbial collection and survival in bioaerosol samplers (Macher & Willeke 1992; Nevalainen et al. 1993). Among these factors, the selection of sampler,
sampling procedure, and sampling flow rate are considered to be the most important factors to affect bioaerosol collection and survival in bioaerosol samplers (Lin & Li, 1998; Li & Lin, 1999a).
For sampling evaluations of virus aerosol, the commonly assessed virus targets were human/animal virus harmful for human health, such as poliovirus, human coronavirus, rotavirus and adenovirus (P 2 virus or P 3 virus). Some of the studies used bacteriophage to substitute for human/animal virus (Harstad, 1965; Hatch & Warren, 1969; Trouwborst & de Jong, 1972), however, the evaluated target virus did not consist of all kinds of structure and nucleic acid types of virus. Using Andersen 6- STG sampler, more than 87 % of infectious virus were found to be smaller than 2.1 µm (Ijaz et al., 1987). In addition, impinger demonstrated much higher virus viable recovery those of filter (Hatch & Warren, 1969; Dubovi & Akers, 1970; Trouwborst, et al., 1972). Moreover, virus sampling efficiency was observed to highly depend on RH and stress during sampling and extraction process (Harstad, 1965; Ijaz et al., 1987). However, there are considerable variations, which may be influenced by virus target, aerosol generation, virus assay, the definition of sampling efficiency (Harper, 1963).
In this current study, the virus sampling performance of most commonly used bioaerosol sampler, Andersen one-stage impactor, AGI-30 impingers, Gelatin filter and polycarbonate filtration, were investigated. For safety concern, bacteriophage was a suitable surrogate for mammalian viruses. For understanding all kinds of viruses, single strand DNA (phi x174), single strain RNA (MS2), double strand DNA (T7) and double stain RNA (phi 6) bacteriophges were investigated.
三、結果與討論
Test Microorganisms
In our current study, single strand DNA (phi x174, ATCC 13706-B1), single strain RNA (MS2, ATCC 15597-B1), double strand DNA (T7, ATCC 11303-B1) and double stain RNA (phi 6 with envelope lipid, ATCC 21781-B1) bacteriophges were investigated. The host bacteria are Escherichia coli for coliphages phi x174, MS2, and T7 (ATCC 13706, 15597 and 11303, respectively) and
Pseudomonas syringae (ATCC 21781) for
phi 6. A high titer stock of bacteriophages was made by plate lysis and elution. For allowing the phage attached to the host, the bacteriophages were mixed with its own host. After cultivation, 5 ml top agar was added to the sterile tube of the infected cells. The contents of the tube were mixed by gentle tapping for 5 sec and poured onto the center of a labeled agar plate. Finally, the plate was incubated for 24 h at 37 °C for coliphages and 26 °C for phi 6, respectively. After cultivation, 5 ml SM buffer was pipetted on to a plate showing confluent lysis. Then, the plate was slowly rocked for 40 min and the buffer was transferred to a tube for centrifuge at 4,000 x g for 10 min. After removing the supernatant, the resulting phage stock was stored at 4 °C. To quantify the bacteriophages, plaque assay were performed as described by Adams (Adams, 1959).
Aerosol Generation and Test system
The virus sampling test chamber is 29 cm in diameter with a height of 32 cm. A Collison thee-jet nebulizer (BGI Inc.,Waltham, MA) was used for nebulization of the microbial phages suspension at 3 L/min of dry, filtered, and compressed laboratory air, then passed though a Kr-85 particle charge neutralizer
suspension was then diluted with filtered and compressed air at 57 L/min. The stock solution of bacteriophage MS2, Phi x174 and T7 were diluted in sterile deionized water for nebulization. In addition, phi 6 phage was diluted in the sterile deionized water with 0.03 % tween 80.
An aerodynamic particle sizer (APS, Model 8000, API Inc., Hadley, MA) were used to determine real-time number concentration and size distribution of viral bioaerosols in the range of 0.5 µm to 30 µm. In addition, an Andersen six-stage viable impactor (Andersen Samplers, Inc., Atlanta, GA) was used to measure size distributions of the evaluated viable virus.
Test Samplers
Andersen 1-STG sampler is the sixth stage of the Andersen six-stage sampler with 400 0.25-mm holes, drawing air at a flow rate of 28.3 L/min (the corresponding velocity is 24 m/s) by using 20 ml LB Broth with 3 % gelatin plates. The calculated and reported cut-point diameters of this sampler are 0.57 µm and 0.65 µm, respectively.
The AGI-30 (Ace Glass Inc.) of an all-glass impinger with a 30-mm jet-to-plate distance was operated at sampling flow rate at 12.5 L/min for 5 min. Moreover, sterile deionized water was chosen for different relative humidity of AGI-30 sampling.
A Nuclepore filter consists of a polycarbonate membrane with a 0.4-µm pore size and a 37-mm diameter supported by cellulose pads loaded into open-face and thee-piece plastic cassettes. Filters and support pads were autoclaved, and plastic cassettes were sterilized with ethylene oxide before sampling. The Nuclepore filter was operated at 2 L/min for sampling time 20 min.
80-mm diameter) (Sartorius, Gottingen, Germany) was placed in a sterile filter holder by carefully letting the filter slide out of the pocket onto the filter support of the aluminum filter holder. The gelatin filter was operated at 30 L/min for 5 min. After sampling, the filter could dissolve on the agar surface at the temperature of 35 to 40 °C.
For comparison of samplers, the parameter, Ctest /Csusp (colony survival, CS; Csusp: PFU/m3 by the evaluated sampler, Csusp: PFU/ml in the suspension), was used as a reference.
Results and Discussion
Characteristics of aerosolized virus
Our results demonstrated that virus infectivity of our evaluated strains in spray suspension and aerosol phase (at RH 20 %, 55 % and 85 %) could be maintained up to 90 min with coefficient of concentration variation below 25 %. By using APS, geometric mean aerodynamic diameters of MS2 (as shown in Fig 1), phix174, phi6, and T7 were observed to be 1.23 µm, 1.25 µm, 1.25 µm, and 1.24 µm with geometric standard deviation of 1.5, respectively. By Andersen 6-STG sampler, more than 95 % of recovered PFU plaques were found to be less than 2.1 µm.
Bioefficiency of tested samplers
Andersen 1-STG sampler:
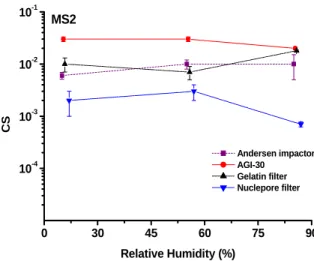
The CS values of MS2 and phi x174 were observed to be 0.01 at three RHs (as shown in Fig. 2, 3). The CS values of T7 (as shown in Fig. 4).at RH 85 % were similar to those of MS2 and phi x174 , but found to be much lower (1 log decrease) at RH 55 % and 20 % (as shown in Fig 4). In regard to phi 6, the CS value was observed to be much lower (10-4) than the other three virus at all RHs(as shown in Fig 5). These differences might be explained by that MS2 and phi
x174 phage are icosahedral viruses without lipids and result in more resistant to sampling stress than those of T7 (tail fiber) and phi 6 (with lipid envelope). Regarding tailed phage T7, the observed higher recoveries at high RH could be explained by the moisture film formation to protect the delicate fibers (tail fiber) of tailed phage from breakage from sampling stress (Hatch & Warren, 1969). In addition, the lipid content of phi 6 was indicated to be essential for infection and extremely sensitive to environmental stress (Woolwine & Gerberding, 1995). The low recoveries of phi 6 might be related to the lipid content affected by the sampling stress like impaction and dehydration.
AGI-30 impinger
For AGI-30, the CS values of MS2, phi x174 at three RHs, and T7 at 85 % RH were observed to be 10-2. The virus recoveries were found to be similar to those for Andersen sampler. For T7, there is a 2 log crease in recovery at RH 55 % and 20 %. Our finding agreed well with those found for tailed phage T1 and T3 by impinger (Harstad, 1965; Hatch & Warren, 1969). This significant lower recovery of T7 might be associated to the nature of protein or nucleic acid submitted to an instant reconstitution in impinger fluids and causes a molecular configuration not compatible with adsorption, penetration, or multiplication (Hatch & Warren, 1969). In regard to phi 6, the CS values were 10-3 at all RHs which is higher than those in Andersen sampler.
Gelatin filter
For gelatin filter, average CS values for MS2 and phi x174 were 10-2 at different RHs. For hydrophilic virus (MS2 and phi x174), the recoveries among Andersen 1-STG sampler, AGI-30, and gelatin filter were similar. In addition, their performance is similar to those of hardy endospore B.
subtilis and yeast cells (Li et al., 1999; Lin &
Li, 1999b). For phi 6 and T7, their recoveries were found to be still low which was similar to fragile bacteria. From the previous studies (Crook 1995, Li et al., 1999), gelatin filter is not satisfactory for collecting airborne fragile bacteria because the gelatin dried out during extended sampling and placing additional dehydration stresses in the collected microorganisms. This reason might apply for sensitive virus like phi 6 and T7, because of higher biological stress by filtration with related dehydration stress.
Nuclepore filter
Regarding the Nuclepore filter, average CS values for MS2 and phi x174 were 10-3, that was lower than the other three evaluated samplers. In addition, there is no virus recovery (close to zero) for phi 6 and T7 by Nuclepore filter. For the Nuclepore filter, the evaluated virus aerosols in this study were larger than 0.4 µm pore size and the virus aerosol penetration through filter should be negligible. Therefore, the observed virus infectivity loss from Nuclepore filter should be primarily related to the biological stress during filtration, dehydration during sampling, and extraction process (Li, et al., 1999).
In summary, our results strongly demonstrated that virus particle morphology and with/without envelope would significantly affect virus sampling performance. For hydrophilic virus, Andersen impactor, impinger, and gelatin filter are likely to perform better than Nuclepore filter. In regard to lipophilic virus (with a lipid envelope), the virus recoveries were found to be lower than those of the hydrophilic virus, because lipid is extremely sensitive to sampling stress. Additionally, recoveries of MS2, phi x174, and phi 6 did not depend on RH, but T7 with
tail fiber has high sensitivity to RH. These findings demonstrated that RH plays different role in recovery of different microorganism (Ijaz et al., 1987; Cox, 1995).
四、計畫成果自評 本計畫已在實驗室中建立病毒生物氣 膠採樣分析技術的評估系統,並己運用此 系統評估四種病毒採樣技術之採集效能, 此成果可運用於環境採樣,並提供更完整 的病毒生物氣膠暴露危害評估之採樣技 術。 五、參考文獻 [1] Adams, M. H. (1959). Bacteriophages. Interscience Publishers Inc., New York, 450-456
[2] Akers, T. G., Prato, C. M., & Dubovi, E. J. (1973). Airborne stability of simian virus 40. Applied Microbiology, 26, 146-148.
[3] Benbough, J. E. (1971). Some factors affecting the survival of airborne viruses.
Journal of General Virology, 10,
209-220.
[4] Cox, C. S. (1995). Stability of Airborne Microbes and Allergens. Bioaerosols
Handbook, (Edited by Cox, C. S. and
Wathes, C. M.), pp. 77-99. Lewis Publishers, Boca Raton, FL.
[5] Couch, R. B., Gerone, P. J., Cate, T. R., Griffith, W. R., Alling, D. W., & Knight, V. (1965). Preparation and Properties of a Small-Particle Aerosol of Coxsackie A21. Proceedings of the Society for
Experimental Biology and Medicine,
118, 818-822.
[6] Crook, B. (1995). Non-Inertial Samplers: Biological Perspectives. Bioaerosols
Handbook, (Edited by Cox, C. S. and
Wathes, C. M.), pp. 269-283. Lewis Publishers, Boca Raton, FL.
Airborne stability of tailless bacterial viruses S-13 and MS-2. Applied
Microbiology, 19, 624-628.
[8] Edward, D. G., Elford W. J., & Laidlaw, P. O. (1943). Studies on sirborne virus infectious. I. Experimental technique and preliminary observations on influenza and infectious ectromelia.
Journal of Hygiene, 43, 1-10.
[9] Forade K. K., Myers, E. A., Hanley, J. T., Ensor, D. S., & Roessler, P. F. (1999). Methodology to perform clean air delivery rate type determinations with microbiological aerosols. Aerosol
Science and Technology, 30, 235-245.
[10] Harper, G. J. (1963). The influence of environment on the survival of airborne virus particles in the laboratory. Arch
Gesamte Virusforsch, 13, 64-71.
[11] Harstad, J. B. (1965). Sampling submicron T1 bacteriophage aerosols.
Applied Microbiology, 13, 899-908.
[12] Hatch, M. T., & Warren, J. C. (1969). Enhanced recovery of airborne T3 coliphage and Pasteurella pestis bacteriophage by means of a presampling humidification technique.
Applied Microbiology, 17, 685-689.
[13] Ijaz, M. K., Karim, Y. G., Sattar, S. A., & Johnson-Lussenburg, C. M. (1987). Development of methods to study the survival of airborne viruses. Journal of
Virological Methods, 18, 87-106.
[14] Kowalski W. J. & Bahnfleth W. (1998). Airborne Respiratory Diseases and Mechanical Systems for Control of Microbes. Heating/Piping/Air Conditioning HPAC Engineering, 70,
34-48
[15] Li, C. S., Hao, M. L., Lin, W. H., Chang, C. W., & Wang, C. S. (1999). Evaluation of microbial samplers for bacterial microorganisms. Aerosol
Science and Technology, 30, 100-108.
[16] Li, C. S., & Lin, Y. C. (1999a). Sampling performance of impactors for bacterial bioaerosols. Aerosol Science
and Technology, 30, 280-287.
[17] Li, C. S., & Lin, Y. C. (1999b). Sampling performance of impactors for fungal spores and yeast cells. Aerosol
Science and Technology, 31, 226-230.
[18] Li, C. S., & Lin, Y. C. (2001). Storage effects on bacterial concentration: determination of impinger and filter samples. Science of the Total Environment, 278, 231-237.
[19] Lin, W. H., & Li, C. S. (1998). The effect of sampling time and flow rates on the bioefficiency of three fungal spore sampling methods. Aerosol
Science and Technology, 28, 511-522.
[20] Lin, W. H., & Li, C. S. (1999a). Collection efficiency and culturability of impingement into a liquid for bioaerosols of fungal spores and yeast cells. Aerosol Science and Technology, 30, 109-118.
[21] Lin, W. H., & Li, C. S. (1999b). Evaluation of impingement and filtration methods for yeast bioaerosol sampling. Aerosol Science and Technology, 30, 119-126.
[22] Lin, W. H., & Li, C. S. (2003). Influence of storage on the fungal concentration determination of impinger and filter samples. American Industrial
Hygiene Association Journal, 64,
102-107.
[23] Macher, J. M., and Willeke, K. (1992).
Performance Criteria for BioaerosolSamplers, Journal of Aerosol
Science 23, 647-650.
[24] Nevalainen, A., Willeke, K., Liebhaber, F., Pastuszka, J., Burge, H., and Henningson, E. (1993). Bioaerosol
Sampling. Aerosol Measurement:
Principles, Techniques, and Applications, (Edited by Willeke, K. and
Baron, P. A.), pp. 471-492. Van Nostrand Reinhold, New York.
[25] Thorne, P. S., Kiekhaefer, M. S., Whitten, P., & Donham, K. (1992). Comparison of bioaerosol sampling methods in barns housing swine.
Applied and Environmental Microbiology, 58, 2543-2551
[26] Trouwborst, T., & de Jong, J. C. (1972). Mechanism of the inactivation of the bacteriophage T 1 in aerosols. Applied
Microbiology, 23, 938-941.
[27] Trouwborst, T., & de Jong, J. C. (1973). Interaction of some factors in the mechanism of inactivation of bacteriophage MS2 in aerosols. Applied
Microbiology, 26, 252-257.
[28] Trouwborst, T., de Jong, J. C., & Winkler, K. C. (1972). Mechanism of inactivation in aerosols of bacteriophage T1. Journal of General Virology, 15, 235-242.
[29] Tyrrell, D. A. J. (1967). The spread of viruses of the respiratory tract by the airborne route. Symposia of the
Society for General Microbiology, 17,
286-306.
[30] Woolwine, J. D., & Gerberding, J. L. (1995). Effect of testing method on apparent activities of antiviral disinfectants and antiseptics. Antimicrob
0.1 1 10 0.0 0.2 0.4 0.6 0.8 1.0
Aerodynamic Particle Diameter,Da,µm
... APS GMD=1.23µm, GSD=1.49 Andersen 6-stage GMD=1.28µm, GSD=1.54 S T A NDE RI Z E D S IZ E DI S T RI BUT IO N, ∆ C / ∆ log D a
Fig. 1. The particle size distributions of MS2 virus in the test chamber measured by APS and an Andersen six-stage impactor. Each particle size distribution represents the mean of at least three trials. 0 30 45 60 75 90 10-4 10-3 10-2 10-1 MS2 CS Relative Humidity (%) Andersen impactor AGI-30 Gelatin filter Nuclepore filter
Fig. 2. The effects of relative humidity on colony survival of Andersen impactor, AGI-30 impinger, Nuclepore and gelatin filter for MS2 virus.
0 30 45 60 75 90 10-4 10-3 10-2 10-1 phi x174 CS Relative Humidity (%) Andersen impactor AGI-30 Gelatin filter Nuclepore filter
Fig. 3. The effects of relative humidity on colony survival of Andersen impactor, AGI-30 impinger, Nuclepore and gelatin filter for phi x174 virus.
0 30 45 60 75 90 10-4 10-3 10-2 10-1 Ansersen impactor AGI-30 Gelatin filter Nuclepore filter T7 CS Relative Humidity (%)
Fig. 4. The effects of relative humidity on colony survival of Andersen impactor, AGI-30 impinger, Nuclepore and gelatin filter for T7 virus.
0 30 45 60 75 90 10-4 10-3 10-2 10-1 phi 6 Relative Humidity (%) CS Andersen impactor AGI-30 Gelatin filter Nuclepore filter
Fig. 5. The effects of relative humidity on colony survival of Andersen impactor, AGI-30 impinger, Nuclepore and gelatin filter for phi 6 virus.