Cytotoxic Effect of S-Petasin and Iso-S-Petasin on the Proliferation of Human Prostate Cancer Cells
Zhi-Hong Wang1,2, Hui-Wen Hsu3, Jou-Chun Chou1,2, Ching-Han Yu4,5 ,Da-Tian Bau6, Guei-Jane Wang7,8, Chih-Yang Huang1, Paulus S. Wang1,2,3,9,10 and Shyi-Wu
Wang11*
1Graduate Institute of Basic Medical Science, College of Medicine, and 7Graduate
Institute of Clinical Medical Science, China Medical University, Taichung, Taiwan, ROC;
2Medical Center of Aging Research, and 6Terry Fox Cancer Research Lab, China
Medical University Hospital, Taichung, Taiwan, ROC;
3Department of Physiology, School of Medicine, National Yang-Ming University, Taipei, Taiwan, ROC;
4Department of Physiology, School of Medicine, Chung Shan Medical University, Taichung, Taiwan, ROC;
5Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan, ROC;
8Department of Health and Nutrition Biotechnology, and 9Department of Biotechnology, College of Health Science, Asia University, Taichung, Taiwan, ROC;
10Department of Medical Research and Education, Taipei Veterans General Hospital,
Taipei, Taiwan, ROC;
11Department of Physiology and Pharmacology, College of Medicine, Chang-Gung
University, Taoyuan, Taiwan, ROC
Short Tile: S-Petasin and Iso-S-Petasin on Human Prostate Cancer Cells 1 2 3 4 5 6 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Correspondence to: Dr. Shyi-Wu Wang, Department of Physiology and
Pharmacology, College of Medicine, Chang-Gung University, Taoyuan 33333, Taiwan, ROC
Tel: +886 32118800 ext 5253, Fax: +886 33283031, e-mail: swwang@mail.cgu.edu.tw 1 2 3 4 5 6 7 8
Abstract. Background: Petasin (Petasides hybridus) is a perennial shrub that is
found in Europe as well as parts of Asia and North America. It has been used to treat hypertension, tumors and asthma. In a previous study, we reported that petasin possesses biological effects including inhibition of testosterone production and the release of corticosterone from rat zona fasciculata-reticularis cells, and antiproliferative effect on human T24 bladder carcinoma cells. Materials and Methods: In the present study, we assessed the effects of S-petasin and iso-S-petasin on the growth and proliferation of two hormone-independent DU145 and PC3 and one hormone-dependent LNCaP prostate cancer cell line at concentrations of 10-7-10-5 mol/l. The cell proliferation index, cell number index, expression of caspases and apoptosis-associated proteins and cell morphology were measured. Results: S-Petasin and iso-S-petasin reduced the viable cell number and increased the numbers of apoptotic cells in tested cell lines in a dose-dependent manner. Western blot analysis revealed that S-petasin and iso-S-petasin reduced the protein levels of procaspase 3, 8, and 9 and cleaved poly(ADP-ribose) polymerase (PARP) in all tested prostate cancer cell lines, and reduced that of procaspase 7 in LNCaP and PC3 cells. At the same time, S-petasin and iso-S-petasin increased mitochondrial membrane permeability and cytochrome c release from mitochondria to the cytosol via reducing the ratio of BCL2/BAX in DU145 and PC3 cells, and up-regulating the levels of p53 in DU145 cells but down-regulating it in PC3 cells. Conclusion: These results indicate that S-petasin and iso-S-petasin induce apoptosis via the activation of mitochondria-related pathways in prostate cancer cells, suggesting S-petasin and iso-S-petasin could be potential anti-cancer agents.
Key Words: Petasin, antiproliferative, apoptosis, prostate cancer
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Prostate cancer is a major health problem and type of age related cancer in men worldwide (1, 2). Furthermore, its prevalence has increased progressively in recent years. Since androgens play a role in promoting the development and progression of prostate cancer, the treatment of prostate cancer can be controlled by hormone manipulation. Hormonal manipulation, including surgical castration or other types of medical androgen- depletion strategy, has become the main treatment for prostate cancer for more than 60 years (3, 4). However, the major cause of mortality of this disease is the metastasis of cancer cells that escape hormone ablation therapy. Clinical evidence showed that in half of patients, disease recurred within 18-24 months after any type of hormone therapy and leading to a so-called hormone refractory condition. Therefore, it is necessary to develop newer and effective strategies for curing or controlling hormone-insensitive prostate cancer. In recent years, phytochemicals are agents that show potential chemopreventive or chemotherapeutic actions in the treatment of prostate cancer (5, 6). Petasites
hybridus (L.) is a widely used traditional Chinese herbal medicine. S-Petasin is the
most abundant bioactive compound isolated from P. hybridus. It has been shown that
S-petasin possesses many biological effects including reducing the severity of
tonsillitis, antispasmodic activity of the gastrointestinal tract, reducing asthmatic attacks and having antiproliferative activity toward human T24 bladder carcinoma cells (7, 8). In a study by Shih et al., S-petasin could suppressed ovalbumin- induced airway hyper-responsiveness via inhibition of phosphodiesterase activity (9). In Taiwan, S-petasin is used as folk medicine to treat hypertension, tumors and asthma (10). In our previous study, we found that S-petasin inhibits the production of testosterone from rat testicular interstitial cells (11). We demonstrated that S-petasin 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
reduced both basal and adrenocorticotropin- induced corticosterone release, and inhibited the release of corticosterone from rat zona fasciculata-reticularis (ZFR) cells via diminishing the activity of cholesterol side chain cleavage enzyme (cytochrome P450scc) and 11β-hydroxylase (12). Thus, based on these findings, it seems reasonable to speculate that S-petasin may find use in the treatment of human prostate cancer.
One of the hallmarks of cancer is the escape of cancer cells from apoptosis (13). Therefore, increasing apoptosis of cancer cells can be an effective strategy for treatment of all types of cancer. Apoptosis is controlled by two different pathways – the membrane death receptor-mediated pathway (14) and the mitochondria-mediated pathway (15). The death receptor pathway is mediated by the ligand binding to its receptor, which ultimately leads to apoptosis. Alternatively, the mitochondrial pathway is mediated by the change of mitochondrial membrane permeability, which promotes the release of cytochrome c from the mitochondria, thus resulting in apoptosis. Mitochondria-mediated apoptosis is also regulated by the B-cell leukemia/lymphoma 2 (BCL2) family of proteins (16).
In the present study, we hypothesized that there would be a cytotoxic effect or inhibitory role of S-petasin and iso-S-petasin (structure shown in Figure 1) on the prostate cancer cell lines (LNCaP, DU145 and PC3). The major objectives of the present study were to explore cell proliferation, cytotoxicity, and protein expression of apoptosis-related molecules in vitro in both dependent and androgen-independent prostate cancer cell lines. Under the conditions used in this study, S-petasin and iso S-S-petasin did not affect the viability of mouse TM3 Leydig cells and human adrenal cortical carcinoma cells (h295).
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24
Materials and Methods
Prostate cancer cell line culture. Human prostate carcinoma cell lines were
purchased from the Biosource Collection and Research Center of the Food Industry Research and Development Institute (Taiwan, ROC). LNCaP cells were maintained in RPMI-1640 medium (Gibco Laboratories, Buffalo, NY, USA) and DU145 and PC3 cells in Dulbecco’s modified Eagle’s medium (Gibco Laboratories Buffalo, NY, USA) with 2 mM L-glutamine, 2.0 g/l sodium bicarbonate, 0.11 g/l sodium pyruvate, 100 U/ml penicillin and 100 μg/ml streptomycin (BioSource International, Camarillo, CA, USA) with 10% fetal calf serum (Biological Industries, KBH, Israel). Cells were incubated in the condition of 5% CO2 in air at 37℃ as previously described (17). Cell proliferation assessment. Cell proliferation was determined with the use of the modified colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After cells were attached, they were challenged with different concentrations of S-petasin or iso-S-petasin that were kindly provided by Dr. Yun-Lian Lin from National Research Institute of Chinese Medicine, Taipei, Taiwan. The MTT (Sigma, St. Louis. MO, USA) assay was performed on days 1, 2, 3, and 4 as described previously (18). The proliferation index of each day referred to the optical density (OD) of that day divided by the OD of day 0. Each experimental setting was performed in three preparations and repeated three times.
Trypan blue dye exclusion assay. For trypan blue dye exclusion assay, 5x103 cells
were plated in 6-well plates, and allowed to attach overnight. The medium was replaced with fresh complete medium containing the desired concentrations of S-petasin or iso-S-S-petasin and the plates were incubated for 4, 8, 12, 18 and 24 hours at 37℃ in a humidified atmosphere of 95% air and 5% CO2. Both floating and adherent cells were collected and pelleted by centrifugation at 700 xg for 5 min. The cells were re-suspended in 25 ml phosphate buffered saline (PBS), mixed with 5 ml of 0.2% trypan blue (Sigma) solution and then counted using a hemacytometer.
Western blot analysis. Cells were incubated with S-petasin (10-7 to 10-5 M) or iso-S-petasin (10-7 to 10-5 M) for 8, 12 or 18 hours. After culture under the indicated conditions, the cells were harvested and washed twice in ice-cold PBS. The cell 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33
pellets were dissolved in RIPA lysis buffer (50 mM Tris–HCl, pH 7.4, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1 g/ml aprotinin), and the protein content of the lysate was determined using the Coomassie blue protein assay (Bio-Rad Laboratories, Inc. Richmond, CA, USA). Aliquots (80 μg) of cell lysate were resolved on 12% or 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by western blotting. The membranes were blocked with 5% nonfat milk in TBST [20 mM Tris– HCl (pH 7.6), 135 mM NaCl; 0.1% Tween 20]. The blots were incubated with the antibodies to cytochrome c, BCL2-associated X protein (BAX), BCL2, p53, caspase 3, 7, 8, 9 and their proenzyme forms and β-actin (Santa Cruz, CA, USA). The secondary antibodies used for the western blot were goat anti-mouse and goat anti-rabbit horseradish peroxidase-labeled antibodies. The signals were visualized by enhanced chemiluminescence detection (ECL Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK). The signal intensities of the western blots were calculated after their normalization to that of the internal control.
Cell morphology. The cells were plated into 24- wells plates at 37℃ under a humidified atmosphere of 5 % CO2. After the density reached 50-60 % confluence, the cells were treated with different concentrations of S-petasin or iso-S-petasin for 24 hours. For the cell morphological experiment, the culture plates were examined under a microscope (x20) and photographed.
Statistical analysis. All values are given as the mean±SEM. In some cases, the
treatment means were tested for homogeneity by the analysis of variance (ANOVA), and the difference between specific means was tested for significance by Duncan’s multiple- range test. In other cases, Student’s t-test was employed. A difference between two means was considered statistically significant when p<0.05.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
Results
Effect of S-petasin and iso-S-petasin on the proliferation of human prostate cancer cell lines. We first determined the antiproliferative effects of S-petasin and
iso-S-petasin on human prostate carcinoma cells. Proliferation status was assessed by both MTT assay (Figure 2) and trypan blue dye exclusion assay (Figure 3). The proliferation of LNCaP cells by MTT assay was significantly inhibited by S-petasin at doses of 8x10-6 to 10-5 M on day 4 (p<0.05). The proliferation of DU145 cells was significantly inhibited by S-petasin at 10-6 to 10-5 M on the day 3 and 4 of treatment (p<0.05). Similarly, the proliferation of LNCaP was significantly inhibited by iso-S-petasin at doses of 4x10-6 to 10-5 M on day 4 (p<0.05). The proliferation of PC3 cells was not significantly inhibited by S-petasin at any dose after treatment of 1 to 4 days. The proliferation of DU145 cells was significantly inhibited by iso-S-petasin at 2x10-6 to 10-5 M on day 3 and 4 of treatment (p<0.05). The proliferation of PC3 cells was not significantly inhibited by iso-S-petasin at any dose after treatment from 1 to 4 days.
The antiproliferative effects were also measured by trypan blue dye exclusion assay. The number of LNCaP cells was significantly reduced by both S-petasin and iso-S-petasin at doses of 10-6 to 10-5 M after treatment for 12 to 24 hours (p<0.05). The number of DU145 cells was significantly reduced by both S-petasin or iso-S-petasin at doses of 10-6 to 10-5 M after a 24-hour treatment (p<0.05). The number of PC3 cells was also significantly reduced by S-petasin at doses of 10-6 to 10-5 M after 18 to 24 hours` treatment (p<0.05) and to a greater extent by iso-S-petasin at doses of 10-7 to 10-5 M after 12 to 24 hours treatment (p<0.05).
Effect of S-petasin and iso-S-petasin on the expression of caspase proteins in human prostate cancer cell lines. Effects of S-petasin (10-7 to 10-5 M) and iso-S-petasin (10-7 to 10-5 M) on the expressions of caspases proteins were examined in LNCaP, DU145 and PC3 cell lines (Figures 4 and 5). Activation of caspase cascades results in a reduction of the proenzyme forms. In LNCaP cells, S-petasin reduced the amount of procaspase 3, 7, 8 and 9 proteins after 12 and 18 hours and iso-S-petasin treatments had similar effects (p<0.05). S-Petasin and iso-S-petasin also induced similar effects on DU145 and PC3 cell lines after 12 and 18 hours treatment (p<0.05), but the amount of procaspase 7 was not significantly different in DU145 cells after treatment. At the same time, active caspase cleaves cellular target 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34
proteins, including PARP, thus leading to cell death. Therefore, we also determined the effects of S-petasin and iso-S-petasin on the activation of PARP. Treatment of LNCaP cells with S-petasin (10-7 to 10-5 M) and iso-S-petasin (10-7 to 10-5 M) for 12 or 18 hours resulted in a dose- dependent increase in the cleavage of PARP (p<0.05). Similar effects were also observed for DU145 and PC3 cell lines (p<0.05)(Figures 4 and 5).
Effect of S-petasin and iso-S-petasin on the expression of apoptosis-associated proteins in human prostate cancer cell lines. An early event in apoptosis is the
disruption of the mitochondrial membrane permeability, which is induced by events including translocation of BAX from the cytosol to the mitochondria, and release of cytochrome c from mitochondria to the cytosol. Effects of S-petasin (10-7 to 10-5 M) and iso-S-petasin (10-7 to 10-5 M) on the protein expressions of p53, Bax, cytochrome c and Bcl-2 were examined in LNCaP, DU145 and PC3 cell lines (Figures 6 and 7). In LNCaP cells, both S-petasin and iso-S-petasin increased the release of cytochrome c from mitochondria after treatment for 8 and 12 hours (p<0.05). Treatment of S-petasin or iso-S-petasin for 8 or 12 hours also resulted in an increase of cytochrome c release, p53 expression and Bcl-2 down-regulation of BCL2 in PC3 cells (p<0.05). Similar effects with a concomitant of BAX translocation were also observed in DU145 cells (p<0.05).
Effect of S-petasin and iso-S-petasin on cell morphology. Morphological analysis of
cells treated with S-petasin and iso-S-petasin for 24 hours revealed cell heterogeneity as compared to control cells (Figure 8). In LNCaP cells, damaged cells became rounded and floating after treatment with S-petasin or iso-S-petasin for 24 hours. Similarly, treatment of DU145 and PC3 cell lines with S-petasin or iso-S-petasin for 24 hours led to shrunken, rounded and floating cells, while the untreated control cells were well spread.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
Discussion
Although an early diagnosis may contribute to curing prostate cancer, many patients still suffer from metastatic disease. Since the prostate is an androgen-dependent organ, androgen ablation becomes the major treatment of this cancer type (19, 20). However, prostate carcinoma may gradually become refractory to hormonal therapy after a period of treatment (21). Therefore, finding newer treatment strategies or promising agents is still needed.
P. hybridus (petasin) is a perennial shrub, found throughout Europe as well as
parts of Asia and North America, that has been used medicinally for centuries (22, 23). The use of plant-derived products has shown a great promise, owing to their being less costly and having fewer side effects. Therefore, increasing apoptosis or reducing proliferation can be an effective method for chemotherapeutic intervention in prostate cancer. In the present study, we evaluated the effects of S-petasin and iso-S-petasin in order to determine its potential in prevention of prostate cancer by studying proliferation of two hormone-independent (DU145 and PC3) and in one hormone-dependent (LNCaP) human prostate cancer cell lines. The results of our study indicate that S-petasin and iso-S-petasin exert significant dose- and time-dependent inhibitory effects on the proliferation of the tested prostate cancer cell lines. However, there is a slight inconsistency between tested assays for PC3 cells owning to the fluorescence property of petasin and this is in agreement with previous studies (24, 25). In our previous study, we found that petasin also inhibits the production of testosterone from rat testicular interstitial cells and has an inhibitory effect on corticosterone production from rat ZFR cells through reducing the activities of adenylyl cyclase, P450scc and 11β-hydroxylase (12). All of these results show that petasin indeed influences some biochemical processes via inhibition of enzymes expressed in prostate cancer.
The reduction of procaspase is a key step in apoptotic signal transduction, and caspases can be divided into two types of subfamilies: initiator caspases (including caspase 8 and 9) and effector caspases (including caspase 3, 6, and 7), which finally induce apoptosis (26, 27). Here we showed that protein levels of procaspase 3, 7, 8 and 9 in LNCaP and PC3 cells as well as procaspase 3, 8 and 9 in DU145 cells were reduced after S-petasin and iso-S-petasin treatments, indicating caspase cascades activation. Furthermore, treatments of S-petasin and iso-S-petasin resulted in cleavage of PARP in all tested cell lines. Apoptosis is tightly regulated by anti-1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34
apoptotic and pro-apoptotic effector molecules, including the BCL2 family proteins (28). The proteins of the BCL2 family either promote cell survival (e.g BCL2) or induce programmed cell death (e.g BAX) (29). The ratio of BAX to BCL2 is critical for apoptosis induction (30-32). An increase in the ratio of BAX to BCL2 stimulates the release of cytochrome c from mitochondria into the cytosol (33). The cytosolic cytochrome c then leads to the activation of caspase-3 and cleavage of PARP (34). In addition, the tumor suppressor protein p53 plays an important role in determining the response to DNA damage through its transcriptional activity (35, 36). After genotoxic stress, p53 protein functions as a transcription factor and up-regulates multiple downstream target genes, including BAX (29, 37). Our results showed that treatment with S-petasin and with iso-S-petasin resulted in up-regulation of p53 and its downstream regulator BAX with concomitant down-regulation of anti-apoptotic BCL2 in DU145 cells. In PC3 cells, we found treatments with S-petasin or iso-S-petasin resulted in down-regulation of p53 with concomitant down-regulation of BCL2 and no difference in BAX protein expression. In LNCaP cells, we found no difference in p53, BAX to BCL2 protein expression after treatment, but cytochrome c protein expression was still enhanced. These results show enhanced apoptosis with these treatments and indicate different mechanisms underlying the cytotoxicity of the tested prostate cancer cells.
Consistent with the previous findings in this study S-petasin and iso-S-petasin changed the morphology of human prostate cancer cells after 24 hours` treatment. This indicate that S-petasin and iso-S-petasin substantially influence several cell behaviors, such as cell proliferation, and induction of apoptosis, and ultimately induce morphological changes.
Conclusion
In summary, S-petasin and iso-S-petasin caused antiproliferative effects and cell apoptosis in androgen-dependent and -independent prostate cancer cells. Caspase activation, BAX translocation, and cytochrome c release were involved in the apoptotic pathway after treatment with S-petasin and iso-S-petasin. These findings suggest that S-petasin and iso-S-petasin could be potential anticancer agents.
Acknowledgements 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34
This work was supported in part by grants from DMR-102-124, China Medical University Hospital.
1 2 3
References
1. Rhim JS and Kung HF: Human prostate carcinogenesis. Crit Rev Oncog
8:305-328, 1997.
2. Jemal A, Center MM, DeSantis C and Ward EM: Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev
19:1893-1907, 2010.
3. Denmeade SR and Isaacs JT: A history of prostate cancer treatment. Nat Rev
Cancer 2:389-396, 2002.
4. Miyamoto H, Messing EM and Chang C: Androgen deprivation therapy for prostate cancer: Current status and future prospects. Prostate 61:332-353, 2004.
5. Kucuk O: Chemoprevention of prostate cancer. Cancer Metastasis Rev
21:111-124, 2002.
6. Barnes S: Role of phytochemicals in prevention and treatment of prostate cancer. Epidemiol Rev 23:102-105, 2001.
7. Debrunner B and Neuenschwander M: Sesquiterpenes of Petasites hybridus (L.)
G. M. et Sch.: Influence of locations and seasons on sesquiterpene distribution. Pharm Acta Helv 70:315-323, 1995.
8. Debrunner B: Untersuchungen zur Struktur und Analytik der Inhaltsstoffe von
Petasites hybridus (Petasin-Chemovarietät) Dissertation,. Berne: University of Berne 1994.
9. Shih CH, Huang TJ, Chen CM, Lin YL and Ko WC: S-Petasin, the main sesquiterpene of Petasites formosanus, inhibits phosphodiesterase activity and suppresses ovalbumin-induced airway hyperresponsiveness. Evid Based Complement Alternat Med 2011: Article ID 132374, 2011.
10. Sasaki SI: Taiwan Minkan Yakuyo Shoukubstsu Shi (A Manual of the Medicinal Plants of Formosa), Kobun Kan, Taipei, Taiwan, 1924.
11. Lin H, Chien CH, Lin YC, Chen CF and Wang PS: Inhibition of testosterone secretion by S-petasin in rat testicular interstitial cells. Chin J Physiol 43:99-103, 2000.
12. Chang LL, Tseng YC, Lin YL, Wun WS and Wang PS: Effects of S-petasin on corticosterone release in rats. Chin J Physiol 45:137-142, 2002.
13. Hanahan D and Weinberg RA: The hallmarks of cancer. Cell 100:57-70, 2000. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33
14. Ashkenazi A and Dixit VM: Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11:255-260, 1999.
15. Green DR and Reed JC: Mitochondria and apoptosis. Science 281:1309-1312, 1998.
16. Cory S, Huang DC and Adams JM: The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590-8607, 2003.
17. Yeh JY, Huang WJ, Kan SF and Wang PS: Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate 54:112-124, 2003.
18. Kan SF, Huang WJ, Lin LC and Wang PS: Inhibitory effects of evodiamine on the growth of human prostate cancer cell line LNCaP. Int J Cancer 110:641-651, 2004.
19. Marumo K, Baba S and Murai M: Erectile function and nocturnal penile tumescence in patients with prostate cancer undergoing luteinizing hormone-releasing hormone agonist therapy. Int J Urol 6:19-23, 1999.
20. Gleave M, Bruchovsky N, Goldenberg SL and Rennie P: Intermittent androgen suppression for prostate cancer: rationale and clinical experience. Eur Urol
34:37-41, 1998.
21. Ornstein DK, Oh J, Herschman JD and Andriole GL: Evaluation and management of the man who has failed primary curative therapy for prostate cancer. Urol Clin North Am 25:591-601, 1998.
22. Eaton J: Butterbur, herbal help for migraine. Nat Pharm 2:23-24, 1998.
23. Mauskop A: Petasites hybridus: ancient medicinal plant is effective prophylactic treatment for migraine. Townsend Lett 202:104-106, 2000.
24. Altman FP: Tetrazolium salts and formazans. Prog Histochem Cytochem 9:1-56, 1976.
25. Burdon RH, Gill V and Rice-Evens C: Reduction of a tetrazolium salt and superoxide generation in human tumor cells (HeLa). Free Radic Res Commun
18:369-380, 1993.
26. Mesner PW, Bible KC, Martins LM, Kottke TJ, Srinivasula SM, Svingen PA, Chilcote TJ, Basi GS, Tung JS, Krajewski S, Reed JC, Alnemri ES, Earnshaw WC and Kaufman SH: Characterization of caspase processing and activation in HL-60 cell cytosol under cell-free conditions: nucleotide requirement and inhibitor profile. J Biol Chem 274:22635-22645, 1999.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34
27. Shi Y: Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 9:459-470, 2002
28. Bremer E, van Dam G, Kroesen BJ, de Leij L and Helfrich W: Targeted induction of apoptosis for cancer therapy: current progress and prospects. Trends Mol Med 12:382-393, 2006.
29. Gross A, McDonnell JM and Korsmeyer SJ: BCL2 family members and the mitochondria in apoptosis. Genes Dev 13:1899-1911, 1999.
30. Reed JC: Regulation of apoptosis by BCL2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol 7:541-546, 1995.
31. Katiyar SK, Roy AM and Baliga MS: Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Cancer Ther 4:207-216, 2005.
32. Xiao D and Singh SV: Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res 62:3615-3619, 2002.
33. Wolf BB and Green DR: Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem 274:20049-20052, 1999.
34. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129-1132, 1997.
35. Huang TG, Ip SM, Yeung WS and Ngan HY: Changes in p21WAF1, pRb, Mdm-2, Bax and Bcl-2 expression in cervical cancer cell line transfected with a p53 expressing adenovirus. Eur J Cancer 36:249-256, 2000.
36. Park SY, Lee SM, Ye SK, Yoon SH, Chung MH and Choi J: Benzo [a]pyrene-induced DNA damage and p53 modulation in human hepatoma HepG2 cells for the identification of potential biomarkers for PAH monitoring and risk assessment. Toxicol Lett 167:27-33, 2006
37. Jin S, Mazzacurati L, Zhu X, Tong T, Song Y, Shujuan S, Petrik KL, Rajasekaran B, Wu M and Zhan Q: Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene 22:8536-8540, 2003.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31
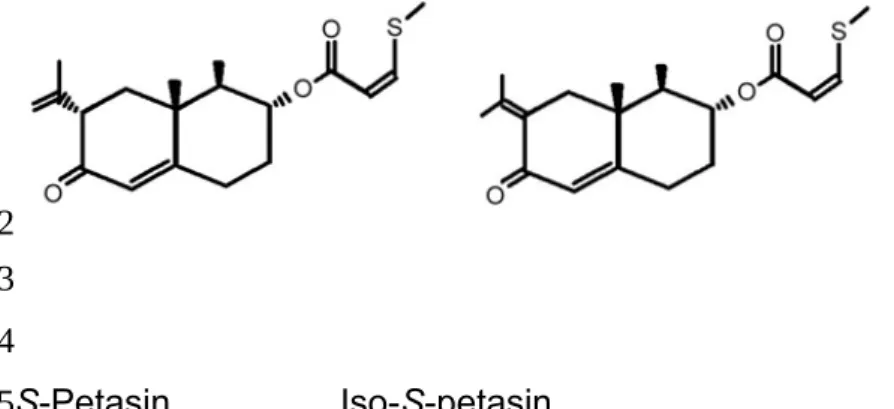
Figure 1. Chemical structure of S-petasin and iso-S-petasin (M.W = 334). S-Petasin Iso-S-petasin 1 2 3 4 5
Figure 2. Effects of S-petasin and of iso-S-petasin on the proliferation of LNCaP, DU145 and PC3 prostate cancer cells. The incubation period was from 1 to 4 days. Proliferation index was measured by MTT assay. Each value represents the meanSEM. *p<0.05 as compared with the control group for each incubation period. 1
2 3 4
2 x 10-6 M 4 x 10-6 M 8 x 10-6 M 10-5 M Control 10-8 M 10-7 M 10-6 M Control 10-8 M 10-7 M 10-6 M 2 x 10-6 M 4 x 10-6 M 8 x 10-6 M 10-5 M V a lu e o f C e ll P ro lif er a ti o n In d e x t o C o n tr o l C e lls a t D a y 0 0 2 4 6 8 10 12 LNCaP ( n = 3 ) LNCaP ( n = 3 ) 0 2 4 6 8 10 DU145 ( n = 3 ) 0 2 4 6 8 10 PC3 ( n = 3 ) S-petasin Iso-S-petasin DU145 ( n = 3 ) PC3 ( n = 3 )
Incubation Time ( day )
1 2 3 4 1 2 3 4 * * * * * * * * * * * * * * * * * * * * 1 2 3 4 5
Figure 3. Effects of S-petasin and of iso-S-petasin on the number of LNCaP, DU145 and PC3 prostate cancer cells. The incubation period was from 4 to 24 hours. Cell numbers were measured by trypan blue dye exclusion assay. Each value represents the meanSEM. *p<0.05 as compared with the control group for each incubation period. 1 2 3 4 5
0.0 0.5 1.0 1.5 2.0 2.5 3.0 0.0 0.5 1.0 1.5 2.0 2.5 0 4 8 12 18 24 0.0 0.5 1.0 1.5 2.0 2.5 S-petasin Iso-S-petasin V al u e o f C el l N u m b er In d ex t o C o n tr o l C el ls a t 0 H o u r 0 4 8 12 18 24
Incubation Time ( hours )
LNCaP ( n = 3 ) LNCaP ( n = 3 ) DU145 ( n = 3 ) DU145 ( n = 3 ) PC3 ( n = 3 ) PC3 ( n = 3 ) * * * * * * * * * * * * * * * * * * * * * * * * * * * * * 1 2 3 4 5
Figure 4. Effects of S-petasin and of iso-S-petasin on the protein expression of caspases and PARP in LNCaP, DU145 and PC3 prostate cancer cells. The cells were incubated with S-petasin or iso-S-petasin for 12 hours. Results are from a representative assay from three separate experiments.
1 2 3 4
Cell line LNCaP DU145 PC3 Incubation time 12h 12h 12h Conc. (M) β-Actin PARP S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Peptasin Iso-S-Peptasin 0 10-7 10-6 10-5 10-7 10-6 10-5 45 kDa 31 kDa 35 kDa 89 kDa 45 kDa 57 kDa 116 kDa Procaspase 8 Procaspase 9 Procaspase 3 Procaspase 7 Cleaved PARP 1 2 3 45 6 7
Figure 5. Effects of S-petasin and of iso-S-petasin on the protein expression of caspases and PARP in LNCaP, DU145 and PC3 prostate cancer cells. The cells were incubated with S-petasin or iso-S-petasin for 18 hours. Results are from a representation assay of three separate experiments.
1 2 3 4 5
Cell line LNCaP DU145 PC3 Incubation time 18h 18h 18h β-Actin PARP S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Peptasin Iso-S-Peptasin 0 10-7 10-6 10-5 10-7 10-6 10-5 45 kDa 31 kDa 35 kDa 89 kDa 45 kDa 57 kDa 116 kDa Conc.(M) Procaspase 8 Procaspase 9 Procaspase 3 Procaspase 7 Cleaved PARP 1 2 3 45 6
Figure 6. Effects of S-petasin and of iso-S-petasin on the protein expressions of p53, BAX, cytochrome c and BCL2 in LNCaP, DU145 and PC3 prostate cancer cells. The cells were incubated with S-petasin or iso-S-petasin for 8 hours. Cell lysates were separated as cytosolic and mitochondrial fractions. Samples were resolved on SDS-PAGE and analyzed by western blotting. Results are from a representation assay of three separate experiments.
1 2 3 4 5 6 7
Cell line LNCaP DU145 PC3 Incubation time 8h 8h 8h p53 BAX Cytochrome c Cytosol BAX S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 53 kDa 20 kDa 14 kDa 28 kDa 20 kDa BCL2 Conc.(M) 1 2 3 45 6
Figure 7. Effects of S-petasin and of iso-S-petasin on the protein expressions of p53, BAX, cytochrome c and BCL2 in LNCaP, DU145 and PC3 cells. The cells were incubated with S-petasin or iso-S-petasin for 12 hours. Cell lysates were separated as cytosolic and mitochondrial fractions. Samples were resolved on SDS-PAGE and analyzed by western blotting. Results are from a representation assay of three separate experiments. 1 2 3 4 5 6 7
Cell line LNCaP DU145 PC3 Incubation time 12h 12h 12h p53 Bax BCL2 S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 S-Petasin Iso-S-Petasin 0 10-7 10-6 10-5 10-7 10-6 10-5 53 kDa 20 kDa 14 kDa 28 kDa 20 kDa Cytosol BAX 20 kDa Cytochrome c 1 2 3 45 6
Figure 8. Effects of S-petasin and of iso-S-petasin on the morphology (at x200) of LNCaP, DU145 and PC3 prostate cancer cells for 24 hours. The cells were grown in 24-well culture dishes to near 50–60 % confluence and then treated with different concentrations of S-petasin or iso-S-petasin. Cell morphology was observed by microscopy. Results are from a representation assay of three separate experiments. 1 2 3 4 5 6
32
LNCaP
Control S-Petasin Iso-S-petasin
DU145 PC3 10-6 10-5 10-5 10-6 Conc.(M) 10-6 10-5 1 2 3 1 2 3 4