行政院國家科學委員會專題研究計畫 期中進度報告
子計畫五:疾病動物模式奈米微粒毒性探討(II)(1/2)
計畫類別: 整合型計畫 計畫編號: NSC92-2621-Z-002-014- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學公共衛生學院職業醫學與工業衛生研究所 計畫主持人: 鄭尊仁 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 5 月 25 日
行政院國家科學委員會補助專題研究計畫期中進度報告
奈米微粒與健康風險研究-子計畫五:疾病動
物模式奈米微粒毒性探討(II)(1/2)
計畫類別:□ 個別型計畫
■
整合型計畫
計畫編號:NSC
92-2621-Z-002-014
執行期間:
92 年 8 月 1 日至 93 年 7 月 31 日
計畫主持人:
鄭尊仁
執行單位:台灣大學職業醫學與工業衛生研究所
成果報告類型(依經費核定清單規定繳交):
■
精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:
中 華 民 國 93 年 5 月 25 日
行政院國家科學委員會專題研究計畫成果報告
疾病動物模式奈米微粒毒性探討
計畫編號:NSC 92-2621-Z-002-014
執行期限:92 年 8 月 1 日至 93 年 7 月 31 日
主持人:鄭尊仁 國立臺灣大學職業醫學與工業衛生研究所
E-mail: tcheng@ntu.edu.tw
一、中文摘要 近年來,許多流行病學研究指 出,空氣污染中氣懸微粒的增加,與呼吸 系統疾病和心血管疾病死亡率增加有關, 這些死亡大都發生於已經有心肺疾病的個 人,然而確實致病機轉仍不清楚。流行病 學研究亦指出,長期暴露於懸浮微粒會增 加肺癌死亡的風險。相關研究指出,奈米 微粒(ultrafine particle, <0.1µm)可能是呼吸 與心血管疾病死亡率及致病率增加的重要 原因之一。根據微粒成分與毒性研究顯 示,相同重量下,同材質的奈米微粒毒性 較粗微粒高,可能與奈米微粒的數目及總 表面積皆較粗微粒高出許多,引起氧化壓 力相對較大有關;此外,奈米微粒在肺泡 具有高沈積率,亦具有穿透肺泡間質,經 血液循環通透至身體其他器官之特性,因 此奈米微粒的暴露在健康風險上扮演極重 要之角色。近年,已有一些研究針對奈米 微粒與細胞毒性的關係進行探討,主要著 重在奈米微粒造成的發炎反應,但是對於 奈米微粒引起的呼吸道變化及心血管系統 效應之間的關係並不清楚。本研究目的 為:(1)、探討肺部上皮內襯液體對於奈米 微粒引起細胞氧化傷害的影響,評估奈米 微粒與細胞反應後產生之ROS 及評估細胞 DNA 單股斷裂情形。(2)、以糖尿病大鼠模 式進行奈米微粒毒性試驗,以氣管灌注方 式進行125 及 500µg/ml 奈米碳黑暴露,觀 察其肺部發炎及周邊血液發炎反應、內皮 細胞功能標記之變化。研究結果顯示,在 非細胞系統中,奈米碳黑產生的ROS 有隨 暴露濃度及暴露時間增加呈現上升的趨 勢。碳黑微粒在添加ELF 的環境下能顯著降低ROS 產生,同時,ELF 亦能減少 DNA 單股斷裂的情形。在糖尿病大鼠模式中, 我們發現暴露於奈米碳黑導致顯著的肺部 發炎及傷害反應,奈米碳黑在糖尿病大鼠 造成顯著的周邊發炎反應增加,血管內皮 素升高及血液一氧化氮降低,奈米微粒暴 露可能與糖尿病有共同的的病理生理作用 途徑,造成心血管疾病增加的風險。 關鍵詞:奈米微粒、糖尿病大鼠、肺部上 皮內襯液體、 反應性氧化物種、心肺疾 病 Abstract
Epidemiologic studies have shown consistent associations between the exposure to particulate air pollution and acute increase in morbidity and mortality, especially for susceptible subjects with pre-existing respiratory and cardiovascular disease. Furthermore, it has been reported that long-term exposure to combustion-related fine particles may be associated with lung cancer mortality. However, the exact mechanism remains unclear. It is believed that ultrafine particles may have a greater inflammatory effect than larger particles at the same mass concentration because of larger surface area and oxidative stress. In addition, ultrafine particles deposited in alveolar region may translocate into other organs. Thus, ambient ultrafine particles play critical role in health risk assessment. However, the role of ultrafine particles on cardiopulmonary events is not clear.
The goal of this study was: (1) to investigate the effect of epithelium lining fluids (ELF) on ultrafine particle-induced ROS generation. Ultrafine carbon black induced-ROS generation and DNA single strand breaks
were evaluated. (2) to evaluate the effect of ultrafine carbon black on lung inflammation, systemic inflammation and endothelial dysfunction in STZ-diabetic rats. Our results revealed that in cell free system, the amounts of ROS increased with exposure concentration and exposure time. ELF significantly decreased ROS 90% as compared to culture medium after ufCB exposure. Furthermore, ELF also decreased DNA single-strand breakage after ufCB exposure. In diseased animal study, we found ufCB caused significant increase in pulmonary inflammation. We also observed significant alteration in systemic inflammation and endothelial dysfunction in diabetic rats exposed to ufCB. We suggest that ufCB and diabetes may share the common pathway which may be related to cardiovascular events.
Keywords: ultrafine particles, diabetic rats, epithelium lining fluid, reactive oxygen species, cardiopulmonary disease
二、緣由與目的
Epidemiologic studies have shown consistent associations between the exposure to particulate air pollution and acute increase in morbidity and mortality, especially for susceptible subjects with pre-existing respiratory and cardiovascular disease (Pope and Dockery 1999; Samet et al., 2000; Pope et al., 2002). These epidemiologic studies also document that particles with a diameter below 2.5µm (PM2.5) have greater adverse
health outcomes as compared to particles with a diameter below 10µm (PM10). Recent
studies further indicate that the ultrafine particles (< 100 nm) may have an increased toxicity relative to larger particles under the same mass concentration (Ferin et al., 1992; Oberdorster et al., 1995; 2001). Many mechanisms of ultrafine particle-induced toxicity have been proposed, including greater number concentration, larger surface area and transition metals on particles (Oberdorster, 2001).
Since ultrafine particles have greater surface area compared to fine or coarse particles of same mass, greater reactive oxygen species (ROS) may be generated by the ultrafine particles. Ultrafine particles have been shown to generate ROS and cause oxidative stress (Stone et al., 1998). Several studies have demonstrated that ultrafine particles induce production of ROS both in vitro and in vitro (Stone et al., 1998; Wilson et al., 2002). Furthermore, various ultrafine particle have been demonstrated to generate more free radicals and ROS than fine particles (Stone et al., 1998; Wilson et al., 2000). Then, ROS may elicit transcription of proinflammatory cytokines and result in a cascade of inflammation events (Stone et al., 1998).
In order to investigate the effect of ultrafine particles on health effects, an in
vitro study and an in vivo study were conducted in this report.
First, we investigated if airway lining fluid affects the ROS generated by ultrafine particle and subsequent DNA damage. Pulmonary epithelial lining fluid ( ELF ) consists of various antioxidants, which can protect lung cells against the oxidative damage of PM. However the exact mechanism remains unclear. The aim of this study was to determine the effect of ELF on PM-induced oxidative damage. Studies have found that susceptible subjects tend to have lower antioxidant in the peripheral blood and airway lining fluid. Animal studies also demonstrated that those deficient in antioxidants are more susceptible to PM exposure (Norwood et al., 2001). Those with deficiency in antioxidants are more likely to be affected by PM. Airway lining fluid contains antioxidants including Vitamin C and E and glutathione as well as enzymes including superoxide dismutase, glutathione peroxidase and catalase. Previous studies have shown that antioxidants in lining fluid decreased after PM exposure. Although the antioxidation effects have been demonstrated in cell free system, it is not clear if these changes also result in the reduction in the subsequent DNA damage. In this study, we investigated if airway lining fluid affects the ROS generated by ultrafine particle and subsequent DNA damage.
Second, we used diabetic rats to investigate the cardiovascular effect of ultrafine particles. The association between ambient particulate matter (PM) and cardiovascular diseases has been demonstrated in epidemiological studies (Pope and Dockery, 1999; Samet et al., 2000; Pope et al., 2002). Subjects with existing cardiovascular diseases including ischemic heart disease and congestive heart failure are
found to be more susceptible to ambient PM exposure (Pope et al., 2002). Recently, some researchers have also suggested that diabetic patients are another sub-population at risk for PM-associated cardiovascular events (Goldberg et al., 2001; Zanobetti et al., 2001, 2002). Diabetes has been reported as an effect modifier of air pollution related hospital admissions for heart disease in elder person (Zanobetti et al., 2001), doubling the risk of a PM10-associated cardiovascular
admission compared with non-diabetics (Zanobetti et al., 2002). Diabetes mellitus is a common disease, and has been associated with cardiovascular morbidity and mortality (Resnick et al, 2001). PM is also reported to affect cardiovascular diseases (Pope et al., 2004). However, the mechanisms through which ultrafine particle enhances the risks of cardiovascular disease in diabetics remain unclear. We hypothesize that diabetes and ultrafine particles may share common pathway and act synergistically in the development of cardiovascular diseases.
Hyperglycemia of diabetics has been associated with increased reactive oxygen species (ROS) formation (Maritim et al., 2003). Diabetic patients usually have significantly elevated concentrations of 8-OHdG in their serum (Nishikawa et al., 2003) and decreased levels of glutathione (GSH) (Dincer et al., 2002). It is proposed that increased ROS may induce inflammation in endothelium, alter endothelium function, and increase coagulability (Beckman et al., 2002). Inflammation activity also increased in individuals with diabetes, as shown by increased levels of C-reactive protein (CRP) (Jager et al., 1999; Schalkwijk et al., 1999), interleukin-6 (IL-6)(Schram et al., 2003), and tumor necrosis factor (TNF-α) (Lechleitner et al., 2000). Hyperglycemia also inhibits the production of nitric oxide (NO) by blocking
eNOS synthase activation and increasing the production of ROS in endothelial and vascular smooth muscle cells (De Vriese et al., 2000). In addition to reducing concentration of NO, diabetes increases the production of vasoconstrictors, most importantly, endothelin-1 (ET-1). Reports on endothelial dysfunction in patients with diabetes have been widely studied, including increased ET-1 and decreased NO (Haak et al., 1992; Williams et al., 1996).
The exact mechanisms through which ambient PM causes cardiovascular diseases remain unclear. PM exposure is associated with increased generation of ROS (Tao et al., 2003). It is proposed that PM may increase the oxidative stress related to cardiovascular disease in peripheral blood (Sorensen et al., 2003). PM exposure is also associated with elevated levels of C-reactive protein (Peters et al., 2001; Pope et al., 2003), enhanced production of proinflammatory cytokines (Ghio and Devlin 2001; Seaton et al., 1999; Schwartz 2001; Peters et al., 2001) and increased blood viscosity (Seaton et al., 1995; Peters et al., 1997) in epidemiological studies. In animal studies, the association between PM exposure and increased ET-1 has also been reported (Bouthillier et al., 1998; Vincent et al., 2001). It appears that PM and diabetes share common pathway in the development of cardiovascular diseases. Thus, we hypothesize exposure to PM may potentiate the cardiovascular diseases of diabetes through the enhanced production of oxidative stress and endothelial dysfunction.
In order to test the effects of ambient particles on diabetics, we exposed streptozotocin (STZ)-induced diabetic rats to PM. Streptozotocin is a metabolite of the soil organism streptomyces achromogenes and was first reported to be diabetogenic in studies of dogs and rats in 1963 (Bell et al.,
1983). Diabetes is caused by a direct toxic effect of streptozotocin on the pancreatic beta cell. After the administration of streptozotocin, there is a characteristic increase in blood glucose, which is maintained at the level of 400mg/dL or greater. This diabetic animal model has been used in many studies of diabetes pathophysiology for years (Vural et al., 2002; Ryu et al., 2003; Zang et al., 2003)
三、材料與方法
In vitro study
Chemical reagents
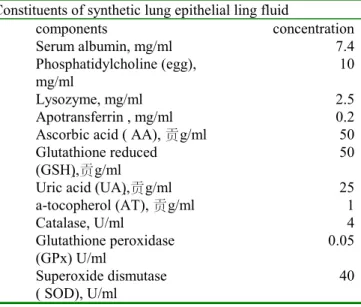
Albumin (BSA), lysozyme (chicken egg white), apotransferrin (human), glutathione (GSH), uric acid (UA), α-tocopherol (AT), ascorbic acid (AA), catalase (CAT, bovine liver), superoxide dismutase (SOD, in bovine erythrocytes), glutathione peroxidase (GPx, bovine erythrocytes), Phosphatidylcholine (egg) , 2,7-dichlorofluorescin diacetate (DCFH-DA)were obtained from Sigma Chemical (St. Louis, MO). All chemicals were reagent grade or of higher purity.
A549 cell culture
The human A549 cell line was from American Type Culture Collection and cultured in a humidified 37oC environment in F12-K medium supplemented with 10% fetal calf serum and 1% penicillin and streptomycin. This cell line, derived from a patient with alveolar cell carcinoma of the lung, has been used as a model of human alveolar type II cells. A549 cells were cultured in six well transwell.
Particles preparation
The particles used in this study was ultrafine carbon black particles (14 nm, Degussa, Printex 90), suspended in culture medium in 50 and 150 µg/ml
Preparation of ELF
ELF was formulated based on method of
Guobin (2001) and Andre (1990). The components in ELF were listed in Table 1. Apotransferrin was used as a surrogate for lactoferrin because it has similar iron-binding qualities yet is more readily available. The following is the procedure for preparation of 100 ml of complete ELF. 1.0 ml of AT in chloroform (0.1 mg/ml) was added to a 100ml glass tube that contained 40ml idd water. The mixture was evaporated under nitrogen at room temperature. Next, 50 ml of medium were added, and the mixture was ultrasonicated in a water-ice bath., a solution of proteins (740 mg of albumin, 240 mg of lysozyme, and 20 mg of apotransferrin in 30 ml of medium) was added to this solution slowly. Finally, UA (0.5 mg/ml, 5.0 ml), AA (5 mg/ml, 1.0 ml), CAT(4 U/ml), SOD (40U/ml), GPx (0.05U/ml) and GSH (5 mg/ml, 1.0 ml) were added, and RPMI1640 was added to a final volume of 100 ml. The ELF was adjusted to pH 7.4 using NaOH (0.2 M) and H3PO4 (0.2 M).The ELFs were
stored at -80°C.
Determination of ROS generation
Generation of ROS in the exposed solution of cell and free system was determined using the probe DCFH-DA (2,7-dichlorofluorescin diacetate ) . The diacetate form of the probe is both lipid soluble and nonfluorescent. Incubation of DCFH-DA with serum containing medium results in cleavage of the diacetate group by esterase enzymes to yield a relatively lipid insoluble, nonfluorescent probe (DCFH). Subsequent oxidation of DCFH by ROS yields the highly fluorescent compound 2,7-dichlorofluorescein (DCF), which can be quantified by microplate reader with excitation wavelength at 485nm(bandwidth 20nm)and an emission wavelength at 530 nm ( bandwidth 25nm ) DCFH-DA (final concentration 20M) was incubated for 15 min
at 37°C in cell culture medium (10% FBS) in order to cleave the DCFH-DA precursor to DCFH. Suspensions of particles were prepared in RPMI1640 medium, mixed by vortexing, and then sonicated for 10 min. After exposure of the DCFH to the particles for 30 min ( 37℃ ) , DCF fluorescence intensity was determined by Cytofluor 2300 microplate reader (Millipore, Bedford, MA, USA).
Determination of DNA single strand breaks by comet assay
Quantities of 20 µl of A549 suspensions (105 cells/ml) were mixed with 1 ml 1.5% low melting agarose and gelled to frost slides pretreated with 0.1 % normal melting agarose on ice. After gelling, the slides were immersed in lysis buffer then washed with PBS. They were then placed in alkaline buffer for electrophoresisat 21 V and 190 mA for 20 min. Slides were rinsed and stained in sybr green. Individual cells were examined under microscope with photomicrographic system. All slides were coded and read blindly. The images were then recorded for further image analysis.
A proprietary image processing software, which has been used in previous studies (Ma et al., 1996) was applied to calculate the distribution of DNA on the agarose. Images of 100 randomly selected cells from each set of experiment were analyzed under a fluorescence microscope adapted with an excitation of 515-560 nm and a barrier filter of 590 nm. Parameters used in the analysis were tail length (%), tail intensity (%) and tail moment. Tail length (%) was defined as the propertion of tail length in whole comet length, tail intensity (%) was defined as the proportion of tail intensity in total comet intensity, and tail moment was defined as the product of tail length and tail intensity. We used % of the tail length and intensity
because of their ease in measurement (Ma et al., 1996).
In vivo studies
Particles preparation
The particles used in this study was ultrafine carbon black particles (14 nm, Degussa, Printex 90), suspended in culture medium in 125 and 500 µg/ml
Diabetic animals and intratracheal instillation of ufCB
Male Sprague-Dawley rats, weighing 200~250 g, were obtained from the National Laboratory Animal Breeding and Research Center, Taiwan. They were housed in plastic cages on Aspen chip bedding, and provided with Lab Diet 5001, water ad lib, except during the exposure. Animals were maintained on a 12-hour light/dark cycle at 22 + 1°C and 55 + 10 % relative humidity. A single intraperitoneal (IP) injection of streptozotocin (STZ, Sigma Chemical Co., 60mg/kg body weight, dissolved in citric acid buffer, pH 4.5) was administered to eight animals to induce diabetes (Bell et al., 1983). Rats were randomly divided into ultrafine carbon black exposure groups (125 and 500 µg/ml) and control group (PBS). All protocols used in this experiment were approved by National Taiwan University’s animal care and use committee.
Bronchoalveolar lavage analysis.
Rats were sacrificed 24h after intratracheal instillation. BAL fluid was collected by washing the airway with a phosphate-buffered saline solution (PBS, pH = 7.4, 28 ml/kg body weight) five times. Lavage fluid was used to determine the total number of cells and cell differential counts. Macrophage, lymphocyte, neutrophils, eosinophils and basophiles were counted under light microscopy (200 cells/slide). The remaining lavage fluid was used for the
analysis of total protein and LDH activity. LDH activity was determined by autoanalyzer at National Taiwan University Hospital. Total protein was determined using total protein assay kit (BioRad Co.)
Determination of blood glucose, oxidative stress, inflammation and endothelial dysfunction.
A total of 15 ml whole blood was recovered from aorta. Immediately, 1 ml of whole blood was collected in citrate tube for blood glucose analysis using autoanalyzer (Glucometer 3, blood glucose meter; Miles Inc.) at National Taiwan University Hospital. 10ml whole blood was collected in ethylenediamine tetraacetic acid (EDTA) tubes. After centrifuged at 1200 rpm for 10 minutes, plasma aliquots were stored at -80℃ until analysis. The remaining 4 ml whole blood was collected in glass tube. Circulating inflammation markers were represented by cytokine IL-6 and TNF-α. Plasma levels of IL-6 and TNF-α were measured using ELISA kits (R&D Systems, U.S.A). Plasma ET-1 and NO were used to represent endothelial function. ET-1 was measured by ELISA (R&D Systems) with human ET-1 as standard (Bouthillier et al., 1998). Nitric oxide synthase assay kit with the Griess reagent (CALBIOCHEM Inc., U.S.A.) was used to determine plasma NO. The plasma concentration of NO was calculated as the sum of nitrite (NO2-) and
nitrate (NO3-) concentrations.
Statistical analysis.
SAS software package, version 8, was used for statistical analysis.
四、結果與討論
In vitro studies
Ultrafine carbon black (UfCB) induced a significant increase in DCF fluorescence at 50 and 150µg/ml as compared to control
(Figure 1, p < 0.05). Furthermore, the fluorescence intensity increased with the UfCB concentration. A549 cells exposed to 50µg/ml or 150µg/ml, and then incubated with DCFH for 30 min, exhibited a significant greater fluorescence than control (Figure 1, p<0.05). A dose-response relationship was observed. In cell-free system, ELF addition result in significant decrease in UfCB. After 4 hr treatment, ELF could reduce 90 % fluorescence induced by UfCB (Figure 1). Increasing oxidative DNA damage were observed with increased UfCB concentration, although it did not reach statistical significance (figure 2). Our results suggest that ELF can decrease total ROS induced by ultrafine carbon black, therefore ELF can protect A549 cells from oxidative damage. The components responsible for this antioxidative ability needs further study.
In vivo studies
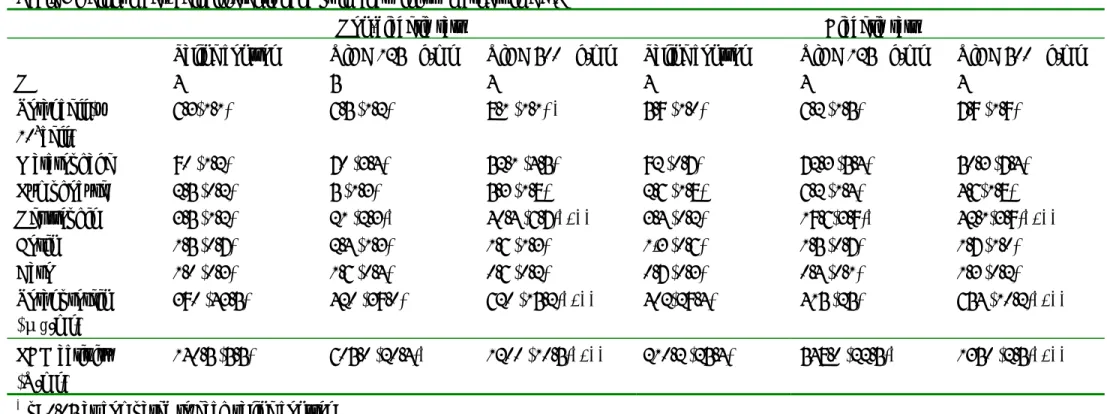
Characteristics of study animals were described in Table 2. Body weight of diabetic rats was lower than that of non-diabetic rats (510.5 g vs. 580.2 g). The mean plasma glucose level was 150.5 mg/dl in non-diabetic rats, and 350.5 mg/dl in diabetic rats (p < 0.05).
Diabetes had no effect on total cells, percentage of neutrophils, total protein and LDH activity in BAL (Table 3). In plasma analysis, diabetic rats demonstrated significantly greater cytokine IL-6 as compared with non-diabetic rats (p <0.05). Furthermore, diabetic rats had significantly increased level of plasma ET-1 (p < 0.05), and decreased level of plasma NO (p < 0.05). In non-diabetic rats, ufCB caused significant increases in total cells and proportion of neutrophils in bronchoalveolar lavage (Table 3, p < 0.05). Elevated total protein and LDH activity in bronchoalveolar
lavage were also observed after ufCB exposure (p<0.05). Plasma IL-6 and TNF-α, level also showed significant increase after ufCB exposure (Table 4, p < 0.05). Furthermore, ufCB exposure caused a significant reduction of plasma NO (p<0.05). However, there was no significant ufCB ueffect on plasma ET-1 in non-diabetic rats.
In STZ-diabetic rats, significant increases in pulmonary inflammation and injury markers were observed after ufCB exposure (Table 3, p<0.05). Plasma IL-6 and cytokine TNF-α significantly increased in diabetic rats after exposure to ufCB (Table 4, p < 0.05). In assessing endothelial function, we found a significant elevation of plasma ET-1 and a significant decrease in plasma NO after exposure to ufCB (p < 0.05).
We conclude that ufCB exposure may enhance the risk of cardiovascular diseases through the synergistic interaction between ufCB and diabetes in endothelium.
五、計畫成果自評 本計畫已完成奈米碳黑於呼吸道細胞 之氧化壓力傷害探討,並建立糖尿病大鼠 模式,探討奈米碳黑造成的發炎反應及內 皮細胞功能,研究成果達成計畫要求。 六、參考文獻
Andre M. Cantin, Gerald A. Fells, Richard C. Hubbard, and Ronald G. Crystal.1990 Antioxidant macromolecules in the epithelial lining fluid of the normal human lower respiratory tract. The Journal of Clinical
Investigation.86:962-971.
Amos AF, McCatry DJ, Zimmet P. 1997. The rising global burden of diabetes and its complications: estimates and
projections to the year 2010. Diabet Med. 14 (suppl 5):S1-S85.
Batalha JR, Saldiva PH, Clarke RW, Coull BA, Stearns RC, Lawrence J, Murthy CG, Koutrakis P, Godleski JJ. 2002 Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ Health Perspect. 110:1191-1197
Backes JM, Howard PA, Moriarty PM. 2004. Role of C-reactive protein in
cardiovascular disease.Ann Pharmacother 38:110-118.
Becher R, Hetland RB, Refsnes M, Dahl JE, Dahlman HJ, Schwarze PE. 2001 Rat lung inflammatory responses after in vivo and in vitro exposure to various stone particles. Inhal Toxicol. 13:789-805.
Beckman JA, Creager MA, Libby P. 2002. Diabetes and atherosclerosis:
epidemiology, pathophysiology, and management. JAMA. 15:
287:2570-2581.
Bell RH, Hye RJ.1983. Animal models of diabetes mellitus: physiology and pathology. J Surgical Res 35:433-460. Blackford JA Jr, Jones W, Dey RD,
Castranova V. 1997. Comparison of inducible nitric oxide synthase gene expression and lung inflammation following intratracheal instillation of silica, coal, carbonyl iron, or titanium dioxide in rats. J Toxicol Environ Health.51:203-218.
Blake GJ, Ridker PM. 2002. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 252:283-294. Bouthillier L, Vincent R, Goegan P,
Adamson IY, Bjarnason S, Stewart M, Guenette J, Potvin M, Kumarathasan P. 1998. Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1. Am J Pathol. 153:1873-1884.
Clarke R. W, Catalane, PJ, Koutrakis P, Krishna M, Sioiutas C, Paulauskis SJ. 1999. Urban air particulate inhalation alters pulmonary function and induces pulmonary inflammation in a rat model of chronic bronchitis. Inhal Toxicol 11: 637-656.
Catheart R., Schwiers E., Ames BN.(1983) Detection of picomole levels of hydroperoxides using fluorescent dichlorofluorescein assay. Anal. Biochem.134:111-6.
Clarke RW, Coull B, Reinisch U, Catalano P, Killingsworth CR, Koutrakis P,
Kavouras I, Murthy GG, Lawrence J, Lovett E, Wolfson JM, Verrier RL, Godleski JJ. 2000. Inhaled concentrated ambient particles are associated with hematologic and bronchoalveolar lavage changes in canines. Environ Health Perspect. 108:1179-87.
De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. 2000. Endothelial dysfunction in diabetes. Br J Pharmacol. 130:963-974.
Dincer Y, Akcay T, Alademir Z, Ilkova H.
2003. Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutat Res.525:129-130.
Ghio AJ, Devlin RB. 2001. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med.164:704-708.
Goldberg MS, Burnett RT, Bailar JC III, et al., 2001. The association between daily mortality and ambient air particle pollution in Montreal, Quebec. 2. Cause-specific mortality. Environ Res. 86:26-36.
Gordon T., Nadziejko C, Schlesinger R, Chen LC, 1998. Pulmonary and
cardiovascular effects of acute exposure to concentrated ambient particulate matter in rats. Toxicol. Letters. 96, 97: 285-288.
Guzik TJ, Korbut R, Adamek-Guzik T 2003. Nitric oxide and superoxide in
inflammation and immune regulation. J Physiol Pharmacol. 54:469-487.
Haak T, Jungmann E, Felber A, Hillmann U, Usadel KH. 1992. Increased plasma levels of endothelin in diabetic patients with hypertension. Am J Hypertens. 5:161-166.
Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. 1999. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc
Biol. 19:3071-3078.
Keston. A. S., and R. Brandt. 1965. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal. Biochem. 11 :1.
Lechleitner M, Koch T, Herold M, Dzien A, Hoppichler F. 2000. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J Intern Med. 248:67-76.
Lerman A, Edwards BS, Hallett JW,
Heublein DM, Sanberg SM, Burnett JC. 1991. Circulating and tissue endothelin immunoreactivity in advanced
atherosclerosis. N. Engl J Med 325: 997-1001.
Lei YC, Chan CC, Wang PY, Lee CT, Cheng TJ. 2004. Effects of dust storm particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Environ Res. 95:71-76.
Martin R. Wilson, Janet H. Lightbody, Ken Donalson, Jill Sales, Vicki Stone. (2002)Interaction between ultrafine particle and transition metals in vivo and in vitro. Toxicology and Applied Pharmacology 184:172-179.
Maritim AC, Sanders RA, Watkins JB. 2003. Diabetes, oxidative stress, and
antioxidants: a review. J Biochem Mol Toxicol. 17:24-38.
Michael JR, Markewitz BA. 1996.
Endothelins and the lung. Am J Respir Crit Care Med 154: 555-581.
Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. 2001. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 164:1665-1668. Nemmar A, Hoet PH, Vanquickenborne B,
Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. 2002. Passage of inhaled particles into the blood circulation in humans. Circulation. 105:411-414. Nishikawa T, Sasahara T, Kiritoshi S,
Sonoda K, Senokuchi T, Matsuo T, Kukidome D, Wake N, Matsumura T, Miyamura N, Sakakida M, Kishikawa H, Araki E. 2003. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular
complications in type 2 diabetes. Diabetes Care. 26:1507-1512. Packer R, Bergler-Klein J, Globits S,
Teufelsbauer H, Schuller M, Krauter A, Ogris E, Rodler S, Wutte M, Hartter E. 1993. Plasma big endothelin-1
concentrations in or congestive heart failure patients with or without systemic hypertension. Am J Cardiol 71:
1293-1299.
Peters A, Doring A, Wichmann HE, Koenig W. 1997. Increased plasma viscosity during the1985 air pollution episode: a link to mortality? Lancet
Peters A, Frohlich M, Doring A et al., 2001. Particulate air pollution is associated with an acute phase response in men: results from the MONICA-Augsbrug study. Eur Heart J 22:1198-1204. Pickup JC. 2004. Inflammation and activated
innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care
27:813-823.
Pope III CA, Dockery DW. 1999.
Epidemiology of particle effects. In: Air Pollution and Health (Holgate ST, Samet JM, Koren HS, Maynard RL, eds.). London: Academic Press 673-705. Pope III CA, Burnett RT, Thun MJ, Calle EE,
Krewski D, Ito K.2002.Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution, JAMA 287: 1132-1141. Pope III CA , Hansen ML, Long RW, Nielsen
KR, Eatough NL, Wilson WE, Eatough DJ. 2004. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 112:339-345.
Prahalad AK, Soukup JM, Inmon J, Willis R, Ghio AJ, Becker S, Gallagher JE. 1999. Ambient air particles: effects on cellular oxidant radical generation in relation to particulate elemental chemistry. Toxicol Appl Pharmacol. 158:81-91.
Rankin JA. 2004. Biological mediators of acute inflammation. AACN Clin Issues. 15:3-17.
Resnick HE, Howard BV. 2002. Diabetes and cardiovascular disease. Annu Rev Med. 53:245-267.
Ryu JK, Kim DJ, Lee T, Kang YS, Yoon SM, Suh JK. 2003. The role of free radical in the pathogenesis of impotence in
streptozotocin-induced diabetic rats. Yonsei Med J. 44:236-241,
Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. 2000. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994, N Engl J Med 343:
1742-1749.
Schalkwijk CG, Poland DC, van Dijk W, Kok A, Emeis JJ, Drager AM, Doni A, van Hinsbergh VW, Stehouwer CD. 1999. Plasma concentration of
C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 42:351-357
Schiffrin EL, Intengan HD, Thibault G, .Touyz RM. 1997. Clinical significance of endothelin in cardiovascular disease. Curr Opin Cardiol 12:354-367.
Schram MT, Chaturvedi N, Schalkwijk C, Giorgino F, Ebeling P, Fuller JH, Stehouwer CD; EURODIAB
Prospective Complications Study. 2003. Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB Prospective
Complications Study. Diabetes Care.26:2165-2173.
Schwartz J. 2001. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect 109:405-409.
Seaton A, MacNee W, Donaldon K, Godden D. 1995. Particulate air pollution and acute health effects. Lancet 345: 176-178.
Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, Watt M, Agius R, 1999. Stout R.Particulate air
pollution and the blood. Thorax. 54: 1027-1032.
Sioutas C, Kim S, Chang M. 1999. Development and evaluation of a
prototype ultra-fine particle concentrator, J Aerosol Med 30:1001-1017.
Sorensen M, Daneshvar B, Hansen M,
Dragsted LO, Hertel O, Knudsen L, Loft S. 2003. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. 111:161-166. Spranger J, Kroke A, Mohlig M, Hoffmann
K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. 2003. Inflammatory
cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 52:812-817.
Sun, Guobin, Kay Crissman, Joel Norwood, Judy Richards, Ralph Slade, and Gary E. Hatch. Oxidative interactions of synthetic
lung epithelial lining fluid with
metal-containing particulate matter. Am J
Physiol Lung Cell Mol Physiol 281:
L807–L815, 2001.
Tao F, Gonzalez-Flecha B, Kobzik L. 2003. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radical biology and medicine 35:327-340.
Zang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y. 2003.
Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 108:472-478.
Zanobetti A, Schwartz J, Gold DR. 2001. Are diabetes more susceptible to the health effects of airborne particles? Am J Respir Crit Care Med 164:831-833. Zanobetti A, Schwartz J. 2002.
Cardiovascular damage by airborne particles: are diabetes more susceptible? Epidemiology 13:588-592.
Ulrich MM, Alink GM, Kumarathasan P, Vincent R, Boere AJ, Cassee FR. 2002. Health effects and time course of particulate matter on the
cardiopulmonary system in rats with lung inflammation. J Toxicol Environ Health A 25;65:1571-1595.
Vincent R, Kumarathasan P, Goegan P, Bjarnason SG, Guenette J, Berube D, Adamson IY, Desjardins S, Burnett RT, Miller FJ, Battistini B. 2001. Inhalation
toxicology of urban ambient particulate matter: acute cardiovascular effects in rats. Res Rep Health Eff Inst. (104):5-54; discussion 55-62.
Vural P, Cevik A, Curgunlu A, Canbaz M. 2002. Effects of diabetes mellitus and acute hypertension on plasma nitric oxide and endothelin concentrations in rats. Clin Chim Acta. 320:43-47. Williams SB, Cusco JA, Roddy MA,
Johnstone MT, Creager MA. 1996. Impaired nitric oxide-mediated vasodilation in patients with
non-insulin-dependent diabetes mellitus. J Am Coll Cardiol.27:567-574.
Wilson MR, Lightbody JH, Donaldson K, Sales J, Stone V. 2002. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol Appl Pharmacol. 184:172-179.
Table 1 components and concentrations of ELFs
Figure 1
Effect of ultrafine carbon black on the oxidation of DCFH to DCF in (a) a cell free system, (b) a A549 cells system with or without ELF. * p<0.05 compared to control; **p<0.05 compared to 50µg/ml.
Constituents of synthetic lung epithelial ling fluid
components concentration Serum albumin, mg/ml 7.4 Phosphatidylcholine (egg), mg/ml 10 Lysozyme, mg/ml 2.5 Apotransferrin , mg/ml 0.2 Ascorbic acid ( AA), g/ml 50 Glutathione reduced
(GSH),g/ml
50 Uric acid (UA),g/ml 25 a-tocopherol (AT), g/ml 1 Catalase, U/ml 4 Glutathione peroxidase (GPx) U/ml 0.05 Superoxide dismutase ( SOD), U/ml 40
ELF system culture medium system
Fl uo re sc en ce u n it s 0 100 200 300 400 500 600 control 50µg/ml ufCB 150µg/ml ufCB (b) * * ** *
ELF system culture medium system
Fl uor e scen c e uni ts 0 100 200 300 400 control 50µg/ml ufCB 150µg/ml ufCB (c) * * ** *
ELF system culture medium system
Y Dat a 0 100 200 300 400 500 600 control 50µg/ml ufCB 150µg/ml ufCB * * * **
Figure 2
Effect of ultrafine carbon black on single strand breaks assessed by the comet assay.
% of tail length %of tail intensity tail moment 0
50 100 150 200
control (culture medium) 50µg/ml ufCB 150µg/ml ufCB
(d)
Table 2 Basic characteristics of experimental animals
Non-diabetic rats (n=13) STZ-diabetic rats (n=12)
Body weight (g) 510.5±8.3 580.2±29.7*
Blood glucose (mg/dL) 150.5±9.7 350.5±9.7*
* Mean + standard deviation, * p<0.05 as compared to non-diabetic rats Table 3 Cell number, cell differential and pulmonary injury markers in BAL
Non-diabetic rats Diabetic rats
Saline control UfCB 125 µg/ml UfCB 500 µg/ml Saline control UfCB 125 µg/ml UfCB 500 µg/ml
N 4 5 4 4 4 4 Total cell (x 104cell) 6.3(1.1) 6.5 (1.2) 8.1 (1.1) * 5.9 (1.0) 6.2 (1.5) 7.9 (1.9) Marcrophage % 90 (1.2) 70 (3.4) 52.1 (4.5) 92 (0.7) 72.3 (5.4) 50.3 (7.4) Lymphocyte % 2.5 (0.2) 5 (1.3) 5.3 (1.8) 2.6 (1.8) 6.2 (1.4) 4.6(1.8) Neutrophil % 3.5 (1.2) 21 (2.3)* 40.4 (6.7)*, ** 3.4 (0.2) 19.6(3.9)* 42.1(3.9)*, ** Eosin % 1.5 (0.7) 2.4 (1.3) 1.6 (1.3) 1,3 (0.6) 1.5 (0.7) 1.7 (1.0) Baso % 1.0 (0.3) 1.6 (0.4) 0.6 (0.2) 0.7 (0.3) 0.4 (0.1) 1.3 (0.2) Total protein (μg/ml) 390 (43.5) 420 (39.0) 620 (15.2)*, ** 402(29.4) 415 (25) 654 (10.2)*, ** LDH activity (U/ml) 140.5 (5.5) 605.0 (20.4)* 1200 (10.5)*, ** 210.2 (25.4) 548.0 (22.5)* 1350 (2.5)*, **
* p<0.05 as compared to each saline control
Table 4
Systemic inflammatory and endothelial dysfunction markers in plasma
Non-diabetic rats Diabetic rats
Saline control 125 µg/ml 500 µg/ml Saline control 125 µg/ml 500 µg/ml
N 4 5 4 4 4 4 Systemic inflmmation Plasma IL-6 (pg/ml) 30.5 (8.1) 39.8 (4.5) 50.2 (2.7) *, ** 41.2 (5.2) 50.8 (4.7) 65.0 (7.5) * Plasma TNF-α (pg/ml) 4.6 (2.5) 4.3 (3.2) 6.8 (1.1) 5.1 (1.2) 4.9 (1.5) 8.2 (0.7) *, ** Endothelial dysfunction Plasma ET-1 (pg/ml) Plasma NO (µM) 1.3 (0.5) 89.4 (4.7) 1.9 (0.4) 90.5 (1.8) 2.0 (0.8) 85.8 (5.0) 1.9 (0.3) 80.5 (2.0) 2.1 (0.7) 75.2 (3.9) 2.9 (0.1)* 72.2 (1.5)*
* p<0.05 as compared to each saline control