Volume 190, number 5 CHEMICAL PHYSICS LETTERS 13 March 1992
Ultraviolet laser-induced fluorescence of the
C2H
radical
Y e n - C h u H s u , P e i - R e n Wang, M i n g - C h i e h Yang, D. P a p o u s e k , Y i t - T s o n g C h e n a n d W h e i - Y i C h i a n g
Institute of Atomic and Molecular Sciences, Academia Sinica, P.O. Box 23-166, Taipei 10764, Taiwan, ROC and Department of Chemistry, National Taiwan University, Taipei, Taiwan, ROC
Received 18 November 1991
In the spectral range 34500-40000 cm- 1, two molecular species were detected by means of laser-induced fluorescence in the 193 nm photolysis of acetylene: one is C2(a 3Hu), the other is most likely C2H(X). The identification of C2H is based upon the rotational constants (B"= 1.4397-1.4534 cm-t), the deuterium isotope effect on the vibronic bands, and the wavelength-re- solved fluorescence.
1. Introduction
The photochemistry of acetylene has been exten- sively investigated. Several laboratories have ob- served emissions from C2 (A ~FIu, d 3I-Is, C q-Is) and CH (A 2A ) produced by multiphoton dissociation of acetylene [ 1-3 ]. The presence of CEH in the 193 nm photolysis of acetylene has been proved by the time- of-flight mass spectrometric technique [ 4 ] and time- resolved Fourier transform infrared emission [5]. However, no C2 H band has been identified in the visible and ultraviolet ( U V ) spectral region. It has been reported [6,7] that the continuous emission spectrum between 400 and 600 nm is most likely as- sociated with electronically excited C2H.
Although much experimental (refs. [8-16], and references therein) and theoretical (refs. [ 17-22 ], and references therein) work has been done on the microwave and infrared spectra of the C2H radical, some problems remain unsolved. For example, the frequency ~ of the CH stretching mode [ 14,16 ] and the A - X energy separation [ 13,17-19] are not yet definitively established. Major difficulties arise from large vibronic coupling between the ~, and ,X states [ 1 l, 13,14 ]; the energy of the A 21-1 state is about 3650 c m - l above that of the X 2• + state. According to ab initio calculations [19,20], when the C - C bond is elongated from the equilibrium position of the state, complicated vibronic interactions between the
and X states are expected due to the Renner-Teller effect and the avoided curve crossing.
Little is known about the visible or UV spectrum of the ethynyl radical. Its UV spectrum has been studied only by matrix isolation [23] and multi- photon ionization [24]. No definite assignment of the B(2 2E÷ ) state has yet been made [23,25 ]. Here, we report the laser-induced fluorescence (LIF) spec- tra of C2H and C 2 ( e , - a ) observed in the 193 nm photodissociation of acetylene.
2. Experiment
An ArF laser (Lambda Physik LPX 110i) was used to photolyze the precursor, acetylene. The power density of the photolysis light source was kept as small as possible to suppress some multiphoton processes of acetylene. With varied time delay ( 2 - 1 0 ~ts), a tunable UV laser was used to probe the photofrag- ments by means of the LIF technique. The tunable UV radiation of 34500-40000 cm-~ was produced by frequency doubling the output of a commercial dye laser pumped by an excimer laser ( L a m b d a Phy- sik E M G 202). The induced fluorescence signal in the wavelength range of 400-600 nm was collected to record the excitation spectrum of the monitored species. In our high-resolution (0.12 c m - J ) experi- ments, line frequencies were calibrated against the
Volume 190, number 5 CHEMICAL PHYSICS LETTERS 13 March 1992 absorption spectrum of iodine with an estimated ac-
curacy of + 0.02 c m - 1. The excitation spectrum due to the acetylene ~,--,X band [26] near 40000 c m - can be identified by comparing the spectrum ob- tained with/without the photolysis laser.
To record the wavelength-resolved emission, the induced fluorescence was dispersed by a 0.3 m monochromator (JY HR360) and detected by an optical multichannel analyzer (OMA PAR 1461 ); the gate of the OMA was chosen to be 80 ns wide and was opened at the rising edge of the pulse (20 ns) of the probe laser to minimize the relaxations occurring in the excited state of the emitter. The best resolu- tion of the dispersed emission obtained in this work is 12 c m - 1. Because the emission intensity decreased substantially at frequencies above the excitation fre- quency, the dispersed fluorescence spectrum in the higher frequency region could be recorded at only 50 cm-~ resolution. The absolute frequency of the dis- persed fluorescence was referred to the emission lines of an iron-neon hollow-cathode lamp with estimated accuracy + 2.0 c m - 1.
A small amount of acetylene (13-33 Pa) in hy-
drogen ( M G 99.9%) or argon ( M G 99.99%) was constantly flowed through the observation zone to prevent further reactions of C2H. The system was studied at a total pressure 0.04-2.7 kPa, which was monitored by a capacitance manometer (MKS 0-1.3 kPa or 0-13.3 kPa). Acetylene ( M G 99.6%) was prepurified by passage successively through two dry- ice/acetone traps. To study deuterium isotope ef- fects, C2D2 (Cambridge Isotope Laboratories,
> 98%) was used without further purification.
3 . R e s u l t s a n d d i s c u s s i o n
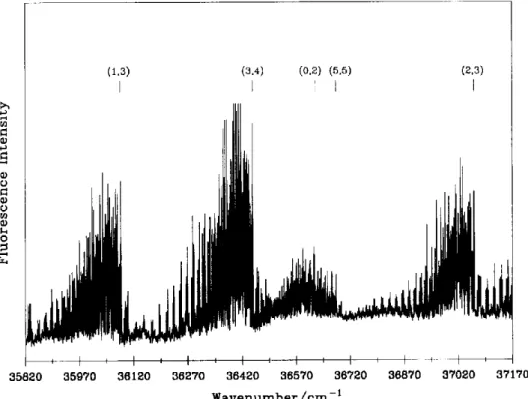
We have identified two molecular species by the LIF technique in the photolysis products of acety- lene. We found that C2 (a 31-Iu) molecules were dom- inant in the high-pressure regime by increasing the pressure above 80 Pa or lengthening the time inter- val (4-20 ~ts) between the photolysis and probe las- ers. This result indicates that most observed C2(a) molecules are not the primary product, in agreement with the previous report [4]. The observed LIF spectrum of C2 in fig. 1 is assigned as the Fox-Herz-
~q O O 0
2
(1,3) (3,4) (0,2) (5,5) (Z,3) I L I b Ij j
I i I i I i I i I i I i I i 35820 35970 36120 36270 36420 36570 36720 36870 37020 87170 W a v e n u m b e r / c m - tFig. 1. LIF excitation spectrum of C2 e , - a transition observed in a mixture of C2H2 (80 Pa) in H2 (2.6 kPa). Values in the parentheses represent the vibrational assignments (v', v" ).
Volume 190, number 5 CHEMICAL PHYSICS LETTERS 13 March 1992 berg system (e 3FIg~-a 3Hu) [27]. When deuterated
a c e t y l e n e C202 was photolyzed instead of C2H2, a n
identical spectrum was obtained, consistent with this assignment.
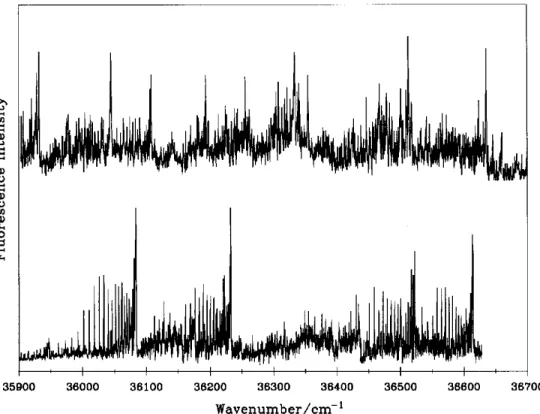
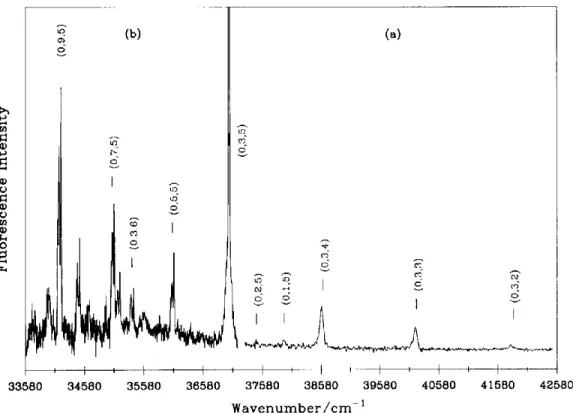
The LIF spectrum of the other molecular species was detectable only at smaller pressure (27-53 Pa) and shorter delay ( 1-8 ~ts). About fifteen bands have been observed in the spectrum interval 34500-40000 c m - ~ with the deuterium isotopic effect. An excerpt of the spectrum is shown in fig. 2, indicating that the spectral carrier is a molecular species containing hy- drogen atom (s). Transition of parallel type (i.e. I I - FI and (I)-~) have been observed. We have also stud- ied the emission spectrum at the bandhead of s o m e of the observed transitions. The result due to the band at 37010 cm-~ is shown in fig. 3; two vibrational fre- quencies, u2=448-502 cm -~ and u3=1568-1623 c m - ~ were deduced under the assumption of an an- harmonic oscillator-rigid rotor. All these results sup- port the assignment that the spectral carrier of fig. 2 is a polyatomic species with hydrogen atom (s).
Table 1 lists molecular species which might be
present in the photolysis process of acetylene at 193 nm and their rotational parameters previously re- ported [ 8,28-33 ]. After excluding the assignments CH and C2, we simulated the spectra Of CEH3 (vinyl radical), H2CC (vinylidene) and C4H2 (l,3-buta- diyne) using the rotational constants in table 1 and
the i f - B " value estimated from the observed P
branch. However, no simulated rotational contours were similar to the spectrum in fig. 4. Moreover, al- ternating intensities of rotational lines are expected for the molecules H E C C o r C 4 H 2 .
One distinct feature seen in fig. 4 is that, with in- creasing J, the splitting of the doublets in the P branch increases, indicating that at least one of the states has either A- or K-type doubling. As the 37010 c m - I
band is a parallel-type transition, a 2 H - : H transition is thus assigned. To describe the rotational levels in
a 2I-I vibronic state, the effective Hamiltonian of
Brown et al. [ 34 ] was transformed into the basis sets of Hund's case (b). Rotational assignments in the 37010 cm -~ band are indicated in fig. 4. Values of parameters obtained by a standard nonlinear least-
0) O O M O , I , f , I , I , I , , I , 35900 36000 36100 36200 36300 36400 36500 36600 36700 W a v e n u m b e r / e m -I
Volume 190, number 5 CHEMICAL PHYSICS LETTERS 13 March 1992 t~ (1) 0 0 r~ O) o
2
o I o I 3 3 5 8 0 3 4 5 8 0 (b) t o o t ' I 3 5 5 8 0 3 6 5 8 0 (~) o o o o 37580 38580 39580 40580 41580 42580 W a v e n u m b e r / c m -1Fig. 3. Wavelength-resolved emission resulting from the excitation at 37018 cm-~, near the head of the 37010 cm-~ band. The spectral resolution in the region (a) is 50 cm- ~ and that of the region (b) is 12 cm- ~. Values in parentheses represent the vibrational assignments
(vh v2, v3).
Table 1
Rotational constants ") of possible species involved in the photodissociation of acetylene
CH b) C2(aaHu) c) C2H d) H2CC(~3B2)e) C2H3 f) C4H2 g)
B 14.192330 1.623964 1.4568249 1.24 1.083026 0.14641
C 1.09 0.948643
A 9.57 7.90934
a) In units of cm-1. b) Ref. [28]. ¢) Ref. [29]. d)Ref. [7 ]. ~) Calculated from the molecular geometry reported in ref. [30]. r) Ref. [31]. g)Ref. [32]. s q u a r e s fit to t h e o b s e r v e d l i n e f r e q u e n c i e s a r e g i v e n in t a b l e 2. Large d e v i a t i o n s at J " / > 12.5 in o n e o f t h e A - d o u b l i n g c o m p o n e n t s are p r o b a b l y d u e to local p e r t u r b a t i o n s . R o t a t i o n a l p a r a m e t e r s B " ( f o r t h e 36075 c m -1 b a n d , B " = 1 . 4 3 9 7 ( 1 3 ) c m -~, B ' = 1 . 2 1 3 2 ( 1 2 ) c m - t ; for t h e 3 6 2 2 4 c m - l b a n d , B " = 1 . 4 5 1 6 ( 4 ) c m - ] , B ' = 1 . 2 2 9 4 ( 4 ) c m - ~ ; for t h e 3 7 9 4 6 c m -L b a n d , B " = 1 . 4 5 4 3 ( 9 ) c m - l , B ' = 1 . 2 4 3 5 ( 9 ) c m - t ; f o r t h e 38108 c m - l b a n d , B " = 1 . 4 4 3 4 ( 4 ) c m -~, B ' = 1 . 2 3 4 8 ( 7 ) c m - t ) ob- t a i n e d f r o m analysis o f t h e o t h e r f o u r b a n d s are also c o n s i s t e n t w i t h t h e p r e v i o u s l y r e p o r t e d v a l u e s for t h e e t h y n y l r a d i c a l [ 8 , 1 0 , 1 1 , 1 3 , 1 4 ], i n d i c a t i n g t h a t t h e spectral c a r r i e r is C2H. D e t a i l e d r o t a t i o n a l analysis o f t h e o b s e r v e d b a n d s will be p u b l i s h e d in a f o r t h - c o m i n g p a p e r . T h e s m a l l e f f e c t i v e s p i n - o r b i t c o n s t a n t , A" = - ( 2 . 1 9 + 0 . 1 8 ) c m -~, o f t h e 3 7 0 1 0 c m -~ b a n d
ID 0 0 0 17.5
I
I
I
I
16.5 15.5 135 125I I
I I
I I
I I
II
135L i
:'1
P1 115 P~ 12.5 11.5 105 36890 36902 36914 36926 W a v e n u m b e r / c m - ' 36938 36950 O) ,+J 0 0 02
105 9.5 8.5 6.5 4.5n
n
n
n
I
7 5 5,5 3.5A
n
n
n
n
I
, I 36956 36968 , I , I , 36980 36992 W a v e n u m b e r / c m - t is.sA ~ ~ 5 A i~5 ^ 9^5,]s I 11 I[IIIIIIIIIIIII~R 1 2.5 1 . ~IP1
14.5~ ~ 1~5 .Ix 10~5 ^ 6A5k~.6I I I I lllilll[llllll~R 2
a ~ 5 Q I 2.5 0.5Q2
I 3 7 0 1 6 I 3 7 0 0 4 Fig. 4. L I F e x c i t a t i o n s p e c t r u m o f C2H o f the 3 7 0 1 0 c m - 1 b a n d m e a s u r e d w i t h 0 . 1 2 c m - 1 r e s o l u t i o n .Volume 190, number 5 CHEMICAL PHYSICS LETTERS 13 March 1992
Table 2
Molecular constants ofthe 37010 cm -j band a)
Lower state Upper state
B 1.44944(93) 1.2433(11)
D× 104 -2.160(27) -2.335(34)
A -2.19(18) -1.97(18)
qX102 --1.49(19) --0.94(22)
qDX 105 --2.46(54) --0.7 b)
a) Values are in units of cm -l, numbers in parentheses denote one standard deviation in terms of the last digit.
b~ Constrained value.
4. Concluding remarks
I n the range 3 4 5 0 0 - 4 0 0 0 0 c m - ~ o f excitation en- ergy, L I F spectra o f two types were i d e n t i f i e d in the p r o d u c t s o f the 193 n m p h o t o d i s s o c i a t i o n o f acety- lene. O n e is due to C2 (a ~ e ) ; the o t h e r is t e n t a t i v e l y assigned as the transition from a high vibrational level o f C2H ( X ) to the high-lying 2I-I v i b r o n i c state. The l a t t e r assignment is s u p p o r t e d b y the d e t e r m i n e d ro- t a t i o n a l constants, the o b s e r v e d d e u t e r i u m isotope effect a n d the wavelength-resolved emission.
indicates that its lower state has a b o u t 90% ,'~-state c h a r a c t e r [ 13,22]. Based u p o n the wavelength-re- solved fluorescence o f the 37010 c m - 1 b a n d , we as- sign the lower state o f the 37010 c m -1 b a n d to be ,X (0, 3 ~, 5) or ,X (0, 5 I, 3) b y a s s u m i n g t h a t x33= - 14 c m - l a n d x23= - 31 c m - i for the v i b r a t i o n a l levels o f the ,X state [9,15 ]. O n e t e n t a t i v e a s s i g n m e n t is m a d e in fig. 3. T h i s result places the u p p e r state o f the 37010 c m -~ b a n d at ~ 4 6 3 9 0 o r 43960 c m -~. T h a t only lines with even AVE have p r o m i n e n t in- tensities in fig. 3 i n d i c a t e s t h a t the g e o m e t r i e s o f its u p p e r a n d lower electronic states are linear, consis- tent with the Zl-l-2H assignment.
A b initio calculations [21,25 ] have shown that the vertical excitation energies r e q u i r e d for t r a n s i t i o n s from the ,X 2E+ state to the 2 2~-~+ a n d 2 21"1 states are n e a r 6.8 a n d 7.3 eV. It has also b e e n shown that as the C - C b o n d increases to 0.141 nm, the energies o f the 3 2A' a n d 2 ZA" states, which are c o r r e l a t e d to the 2 2E+ a n d 2 2I-1, states respectively, decrease b y 1 eV or more. M o r e o v e r , 2 2I-I can be even b e l o w 2 2E+ [25] ( b u t no o p t i m i z e d g e o m e t r y has been given [ 25 ] ). T a k i n g into a c c o u n t the o b s e r v e d small value o f B ' , we i n t e r p r e t o u r s p e c t r u m as a t r a n s i t i o n from a v i b r a t i o n a l l y excited state o f the ,X state to the 2 Ell (3 2A' ) state, which has a large C - C b o n d length ( ~ 0.135 n m ) . Nevertheless, it has been pre- d i c t e d t h a t the g e o m e t r y o f 3 2A' is b e n t [25 ], which c a n n o t explain o u r results. In o r d e r to resolve the na- ture o f the o b s e r v e d transitions, m o r e e x p e r i m e n t a l w o r k a n d ab i n i t i o calculations on the high-lying electronic states o f the ethynyl radical are necessary.
Acknowledgement
We gratefully acknowledge the s u p p o r t o f A c a d e - m i a Sinica a n d the N a t i o n a l Science Council, Re- p u b l i c o f China. We also t h a n k Professor T.A. M i l l e r for his valuable c o m m e n t s on the m a n u s c r i p t .
References
[ 1 ] J.R. McDonald, A.P. Baronavski and V.M. Donelly, Chem. Phys. 33 (1978) 161.
[2] H. Okabe, R.J. Cody and J.E. Alien Jr., Chem. Phys. 92 (1985) 67.
[3]Y.-C. Hsu, M.-S. Lin and C.-P. Hsu, J. Chem. Phys. 94 (1991) 7832.
[4] A.M. Wodtke and Y.T. Lee, J. Phys. Chem. 89 (1985) 4744. [5] T.R. Fletcher and S.R. Leone, J. Chem. Phys. 90 (1989)
871.
[6] H. Okabe, J. Chem. Phys. 62 (1975) 2782.
[ 7 ] Y. Saito, T. Hikida, T. Ichimura and Y. Mori, J. Chem. Phys. 80 (1984) 31.
[ 8 ] K.V.L.N. Sastry, P. Helminger, A. Charo, E. Herbst and F.C. DeLucia, Astrophys. J. 251 ( 1981 ) L119.
[ 9 ] M.E. Jacox and W.B. Olson, J. Chem. Phys. 86 ( 1987 ) 3134. [ 10] J.M. Brown and K.M. Evenson, J. Mol. Spectry. 131 (1988)
161.
[ 11 ] H. Kanamori and E. Hirota, J. Chem. Phys. 89 (1988) 3962. [ 12 ] M. Vervloet and M. Herman, Chem. Phys. Letters 144
(1988) 48.
[ 13] W.-B. Yan, C.B. Dane, D. Zeitz, J.L. Hall and R.F. Curl, J. Mol. Spectry. 123 (1987)486.
[ 14] J.W. Stephens, W.-B. Yan, M.L. Richnow, H. Solka and R.F. Curl, J. Mol. Struct. 190 (1988) 41.
[ 15 ] K.M. Ervin and W.C. Lineberger, J. Phys. Chem. 95 ( 1991 ) 1167.
[ 16] W-B. Yan, H.E. Warner and T. Amano, J. Chem. Phys. 94 (1991) 1712.
[ 17 ] G. Fogarasi, J.E. Boggs and P. Pulay, Mol. Phys. 50 ( 1983 ) 139.
Volume 190, number 5 CHEMICAL PHYSICS LETTERS 13 March 1992 [ 18 ] W.P. Kraemer, B.O. Roos, P.R. Bunker and P. Jensen, J.
Mol. Speetry. 120 (1986) 236.
[ 19 ] H. Thiimmel, M. Perle, S.D. Peyerimhoffand R.J. Buenker, Z. Physik D 13 (1989) 307.
[20] M. Perle, S.D. Peyerimhoff and R.J. Buenker, Mol. Phys. 71 (1990) 693.
[21 ] A.G. Koures and L.B. Harding, J. Phys. Chem. 95 ( 1991 ) 1035.
[22] M. Perle, W. Reuter and S.D. Peyerimhoff, J. Mol. Spectry. 148 (1991) 201.
[23] K.W. Chang and W.R.M. Graham, J. Chem. Phys. 76 (1982) 5238.
[24] T.A. Cool and P.M. Goodwin, J. Chem. Phys. 94 (1991) 6978.
[25] S.-K. Shih, S.D. Peyerimhoff and R.J. Buenker, J. Mol. Spectry. 74 (1979) 124.
[26] J.K.G. Watson, M. Herman, J.C. Van Craen and R. Colin, J. Mol. Spectry. 95 (1982) 101.
[27]J.L. Hardwick and D.H. Winicur, J. Mol. Spectry. 115 (1986) 175.
[28] P.F. Bernath, C.R. Brazier, T. Olsen, R. Hailey, W.T.M.L. Fernando, C. Woods and J.L. Hardwick, J. Mol. Speetry. 147 (1991) 16.
[29]T. Suzuki, S. Saito and E. Hirota, J. Mol. Speetry. 113 (1985) 399.
[30] K.M. Ervin, J. Ho and W.C. Lineberger, J. Chem. Phys. 91 (1989) 5974.
[31 ] H. Kanamori, Y. Endo and E. Hirota, J. Chem. Phys. 92 (1990) 197.
[32] J.L. Hardwick and D.A. Ramsay, Chem. Phys. Letters 48 (1977) 399.
[33] C. Amiot, J. Chauville and J.-P. Maillard, J. Mol. Spectry. 75 (1979) 19.
[34] J.M. Brown, E.A. Colbourn, J.K.G. Watson and F.D. Wayne, J. Mol. Spectry. 74 (1979) 294.