國立臺灣大學公共衛生學院預防醫學研究所 博士論文
Institute of Preventive Medicine National Taiwan University
PhD Thesis
同胱胺酸與血管病之危險性
Homocysteine and the Risk of Vascular Diseases
孫 瑜 Yu Sun
指導教授:簡國龍 副教授
Advisor: Associate Professor Kuo-Liong Chien
中華民國 98 年 1 月
January, 2009
誌謝
感謝當年 陳為堅教授, 陳秀熙教授, 季瑋珠教授,及所有預醫 所的老師,接受我進入臺大公衛學院的殿堂。感謝 賴美淑教授的指 導,為了完成 賴老師交待的作業,先”逼出”了兩篇研究,並順利發 表。
更感謝恩師 簡國龍副教授,除了在”臨床試驗”課程的教導,讓 我得以完成研究並發表,更在最後這兩年準備畢業的過程中,給了我 很多的幫助與指導。簡老師的辛苦教導,永誌難忘。此外,我也感謝 所有的口試委員,給了我很多寶貴意見,讓本文得以順利完成。日後 倘若我在學術的領域上,能有任何的成就,都要歸功這些年來我所遇 到的這麼多好老師。
最後,我要特別感謝我的先生呂建榮醫師,沒有他這些年來給我 的精神鼓勵,工作上的幫忙,及家庭的支持,我不可能完成學業。一 路走來,每每看到兩個可愛的寶貝女兒呂佳樺、呂明臻,所有的事情 都變得甜美。
感謝 諸菩薩!
謹以此書
獻給我最親愛的
父親 孫天清 先生
母親 孫曾雪 女士
中文摘要
關鍵詞: 同胱胺酸;大腦白質病變;中風;心血管疾病;失智症 背景:
同胱胺酸是否是血管病的獨立危險因子,我們提出一些可能的假說:同胱胺酸有可 能造成血流動力學的改變而減緩流速,進而造成血管動脈硬化;可能造成腦部大腦 白質病變,進而引發中風及失智症;同胱胺酸是血管病的危險因子;高血清同胱胺 酸與失智症有關,如果降低血清同胱胺酸,可能可以改善智力,或阻止智力惡化。
研究方法:
針對以上的假設,我們執行以下計劃:1.觀察性研究:觀察不同濃度的同胱胺酸 對頸動脈與椎動脈的血流動力影響;2.病例對照研究:有大腦白質病變者其同胱胺 酸濃度是否與無病變者不同;3.長期追蹤研究:同胱胺酸的濃度是否為未來發生腦 中風及心臟病的預測因子;4.隨機雙盲研究:維他命療法對降同胱胺酸及智力改善 或減緩智力退化是否有臨床效用。
結果:
同胱胺酸並不影響大血管之血流動力學。同胱胺酸為大腦白質病變之危險因子,
每增加 1 μmol/L 同胱胺酸,其發生白質病變之相對危險值為 1.15 (95% CI, 1.01-1.31)。在長期世代追蹤研究,平均追蹤時間 11.95 年,同胱胺酸高的族群,
其發生腦中風的危險並無顯著增加,但發生心血管疾病之危險性及死亡率有顯著 增加。我們定出臨床上最適當的切點,發現同胱胺酸大於 9.47 μmol/L 者,發生 心血管病之危險為小於此值的人的 2.3 倍 (95% CI,1.24-4.18),大於 11.84 μmol/L 者,死亡之危險為 2.4 倍(95% CI,1.76-3.32)。降同胱胺酸維他命療法之
隨機雙盲實驗,治療半年後,維他命組,血清中葉酸及 B12 濃度明顯高於用安慰 劑組,且同胱胺酸濃度在治療組也明顯低於對照組,然而此二組智能之變化並沒 有因用維他命治療而有不同。
結論:
同胱胺酸並不影響血管的血流動力,但與微小血管產生的大腦白質病變有關。長 期追蹤無症狀之成人,同胱胺酸並不會增加發生腦中風的危險,但卻是心血管病 及死亡之危險因子。以降低同胱胺酸之維他命療法,對阿茲海默症患者之智能改 善,沒有明顯的幫助。
Abstract
Key words: homocysteine; cerebral white matter lesion; stroke; coronary heart disease; dementia
Background
The relationship between elevated plasma homocysteine (Hcy) and vascular disease is stronger in retrospective than in prospective studies. We proposed the following 4 hypotheses: 1. Hcy may influence the hemodynamic flow of cerebral arteries and then may further induce atherosclerotic change; 2. Hcy may induce microangiopathy and lead to cerebral white matter change which may be related to future stroke and dementia;
3. Baseline Hcy may be related to future vascular event; 4. Hcy-lowering therapy with vitamin supplementation might be benefit for persons with dementia.
Material and methods
We conducted a cross-sectional study to explore the relationship between Hcy and the hemodynamic status of carotid and vertebral artery; a case-control study for Hcy and cerebral white matter lesions; a cohort study for Hcy and long-term vascular events; an experimental randomized control trial study for Hcy-lowering therapy on dementia.
Results
Hcy was not associated with the hemodynamic change on the extracranial cerebral arteries. However, Hcy is an independent risk factor for cerebral white matter change (multivariate RR 1.15, 95% CI 1.01-1.31). In the prospective cohort study with median 11.95 years of follow-up, participants with Hcy more than 9.47 µmol/L had a 2.3-fold risk for cardiovascular events (95% CI, 1.24-4.18, p=0.008), and participants with Hcy more than 11.84 µmol/L had a 2.4 fold risk for death (95% CI, 1.76-3.32, p<0.0001).
Multivitamin supplements significantly elevated the concentration of vitamin B12
(p<0.0001) and folic acid (p<0.0001) and lowered the plasma homocysteine
concentration (p=0.004) after 26 weeks’ treatment. However, no significant differences between the vitamin and placebo groups in the scores of cognition and activities of daily living were found.
Conclusions
Hcy was not associated with the hemodynamic change on the large extracranial cerebral arteries. The effects of Hcy on the brain may be related to cerebral microangiopathy.
Homocysteine was significantly related to the cardiovascular events and all-cause death, with optimal cutpoint values as 9.47µmol/L and 11.84µmol/L respectively. Oral supplements by over-the-counter multi-vitamins containing B6, B12, and folic acid decreased Hcy concentration in patients with mild to moderate Alzheimer’s dementia.
However, there were no statistically significant beneficial effects on cognition and function for daily living.
目錄
誌謝 ... 2
中文摘要 ... 4
Abstract ... 6
Chapter 1. Introduction and literature review ... 16
1.1 Historic aspects of homocysteine and vascular disease ... 16
1.2 Biochemistry, metabolism and determinants of serum homocysteine ... 17
1.2.1 Source of homocysteine ... 17
1.2.2 Methionine cycle (Figure 1) ... 17
1.2.3 Determinants of serum homocysteine ... 17
1.3 Vascular pathology and pathophysiology of hyperhomocysteinemia ... 18
1.3.1 Vascular pathology of hyperhomocysteinemia ... 18
1.3.2 Plausible mechanism of vascular damage by hyperhomocysteinemia .. 18
1.4 Homocysteine as a risk factor for vascular diseases... 19
1.4.1 Homocysteine, hemodynamics and atherosclerosis ... 19
1.4.2 Homocysteine, microangiopathy and cerebral white matter lesions (WML) ... 19
1.4.3 The association between total serum homocysteine and clinical vascular events ... 20
1.5 The points favor or not favor on a causal relationship between Hcy and vascular diseases or cognitive impairment ... 22
1.5.1 The points in favor of a causal relationship between Hcy and vascular diseases ... 22
1.5.2 The points that cast doubt on a causal relationship between Hcy and
vascular diseases ... 23
1.6 Homocysteine as a risk factor for dementia ... 24
Chapter 2. Hypotheses and objectives ... 25
2.1 Hypotheses ... 25
2.2 Objectives ... 25
Chapter 3. Subjects and Methods (Figure 2) ... 26
3.1 Impacts of homocysteine on hemodynamic status ... 26
3.1.1 Participants ... 26
3.1.2 Ultrasound procedure and hemodynamic measurements ... 26
3.1.3 Laboratory analysis... 27
3.1.4 Statistical analysis ... 27
3.2 Homocysteine and cerebral white matter lesions (WML) on MRI ... 28
3.2.1 Participants ... 28
3.2.2 MRI protocol ... 29
3.2.3 Statistical analysis ... 29
3.3 Homocysteine and long-term vascular events (Chin-Shan cohort) ... 30
3.3.1 Participants and study design ... 30
3.3.2 Ascertainment of events ... 30
3.3.3 Measurements of serum homocysteine and other biochemical variables ... 31
3.3.4 Statistical methods ... 31
3.4 Homocysteine lowering clinical trial on Alzheimer’s disease ... 33
3.4.1 Participants and study design ... 33
3.4.2 Study regimens ... 34
3.4.3 Assessment and outcome measures ... 35
3.4.4 Outcome measures ... 35
3.4.5 Safety evaluations ... 36
3.4.6 Data analysis ... 37
Chapter 4. Results ... 39
4.1 Homocysteine and the hemodynamic status of the extracranial large arteries (Table I-1 to Table I-7) ... 39
4.2 Homocysteine and microangiopathy related cerebral WML (Table II-1 to Table II-2) ... 40
4.3 Homocysteine and long-term clinical vascular events (Chin-Shan cohort) (Table III-1 to Table III-4) ... 40
4.4 Homocysteine lowering clinical trials on dementia (Table IV-1 to Table IV-4) 42 4.4.1 Efficacy of homocysteine –lowering vitamin therapy ... 42
4.4.2 End points ... 43
4.4.3 Safety ... 44
Chapter 5. Discussion ... 46
5.1 Homocysteine and cerebral hemodynamic status ... 46
5.2 Homocysteine and cerebral white matter lesions (WML) ... 50
5.3 Homocysteine and clinical vascular events ... 52
5.4 Homocysteine-lowering vitamin therapy on dementia ... 55
Chapter 6. Summary, Conclusions and Future direction ... 59
6.1 Summary of the findings in these serial studies ... 59
6.2 Strength and limitations ... 61
6.3 Clinical implications ... 65
6.4 Conclusions ... 66 6.5 Future research directions ... 68
References ... 70
Figures
Figure 1. Metabolic pathway of homocysteine ... 92 Figure 2. Study design of four serial studies ... 93
Figures for homocysteine and cerebral hemodynamic status ... 94
Figure I-1. Duplex image (including B-mode and Doppler) of the common carotid artery ... 94 Figure I-2. Duplex image (including B-mode and Doppler) of the vertebral artery ... 95
Figures for homocysteine and cerebral white matter lesions ... 96
Figure II-1. T2-weighted brain MR image with hyperintensity signals (pointed by white arrow) on the periventrcular (left) and subcortical area (right) ... 96
Figures for homocysteine and long-term vascular events ... 97
Figure Ⅲ-1. Relative risk for stroke during a median follow-up of 11.95 years, according to quartile of homocysteine concentration at baseline (1994-1995) in the Chin-Shan Community Cardiovascular Study ... 97 Figure Ⅲ-2. Relative risk for CHD during a median follow-up of 11.95 years, according to quartile of homocysteine concentration at baseline (1994-1995) in the Chin-Shan Community Cardiovascular Study ... 98 Figure Ⅲ-3. Relative risk for all-cause death during a median follow-up of 11.95 years, according to quartile of homocysteine concentration at baseline (1994-1995) in the Chin-Shan Community Cardiovascular Study ... 99 Figure Ⅲ-4. Receiver-Operating Characteristic Curves for coronary heart disease (CHD) events during a median follow-up of 11.95 years ... 100
Figure Ⅲ-5. Receiver-Operating Characteristic Curves for death events during a median follow-up of 11.95 years ... 101
Figures for homocysteine-lowering therapy ... 102
Figure Ⅳ-1. Patients enrollment and completion throughout the study period .. 102 Figure Ⅳ-2. Odds ratios for the effects on cognition and daily living scores of 26 weeks of combined multivitamins or placebo in patients with mild or moderate Alzheimer’s dementia ... 103 Figure Ⅳ-3. Mean changes form baseline in cognition scale in the
homocysteine-increase and homocysteine-decrease groups with 26 weeks of combined multivitamins or placebo in patients with mild or moderate Alzheimer’s dementia ... 104
Tables
Tables for homocysteine and cerebral hemodynamic status ... 105
Table Ⅰ-1. Characteristics of the study population according to homocysteine quartiles ... 105 Table Ⅰ-2. Hemodynamic parameter values of the carotid artery according to homocysteine quartiles ... 106 Table Ⅰ-3. Hemodynamic parameter values of the vertebral artery according to homocysteine quartiles ... 107 Table Ⅰ-4. Adjusted mean values of flow parameters of carotid artery across homocysteine quartiles ... 108 Table Ⅰ-5. Adjusted mean values of flow parameters of vertebral artery across homocysteine quartiles ... 109 Table Ⅰ-6. Relations of plasma homocysteine to the flow parameter of carotid artery ... 110 Table Ⅰ-7. Relations of plasma homocysteine to the flow parameter of vertebral artery ... 111
Tables for homocysteine and cerebral white matter lesions ... 112
Table Ⅱ-1. Demographic, laboratory data and homocysteine levels in subjects with normal brain MRI and patient with white matter lesions (WML) on MRI ... 112 Table Ⅱ-2. Effects of homocysteine on the risk of white matter lesions on the brain MRI adjusting for potential confounders ... 113
Tables for homocysteine and long-term vascular events ... 114
Table Ⅲ-1 .Characteristics of the study population according to homocysteine quartiles ... 114 Table Ⅲ-2. Incidence cases, person-years, incidence rates, and relative risk (RR) for stroke, CHD and all-cause death outcomes during a median follow-up of 11.95 years, according to quartile of homocysteine concentration at baseline (1994-1995) in the Chin-Shan Community Cardiovascular Study. ... 115 TableⅢ-3. Subgroup analyses of relative risk (RR) for ischemic stroke,
hemorrhagic stroke, and ischemic versus hemorrhagic stroke; RR for
cardiovascular (CV) death, non-cardiovascular (non-CV) death, and cardiac versus non-cardiac death in fully adjusted model ... 116 Table Ⅲ-4. Sensitivity, specificity and best Youden's index of the cutoff values, and the hazard ratio for the risk of stroke, cardiovascular events and all-cause of death under the cutoff value of homocysteine ... 117
Tables for homocysteine lowering therapy ... 118
Table Ⅳ-1. Demographic, baseline Hcy, B12, folic acid and cognitive function of the study population. ... 118 Table Ⅳ-2. Serum concentrations from baseline to 26 weeks with combined multivitamins or placebo in patients with mild to moderate Alzheimer's dementia.
Values are median interquartile range. ... 119 Table Ⅳ-3. Changes from baseline to 26 weeks in cognition and daily living function with combined multivitamins or placebo in patients with mild to moderate Alzheimer's dementia (intent-to-treat population). ... 120
Appendix ... 122
Chapter 1. Introduction and literature review
This research is specifically devoted to homocysteine (Hcy) and its relationship to vascular disease and dementia1, 2. Hcy plays a central role in folate and methionine metabolism, is now regarded by many as a potentially major risk factor for
cardiovascular and cerebrovascular diseases3, 4. Hcy is also reported as a possible risk factor for Alzheimer’s disease2. However, observational studies about the relationship between Hcy and vascular diseases are not consistent with the prospective studies1. Furthermore, the clinical benefit for the Hcy-lowering therapy on the prevention of vascular disease or Alzheimer’s dementia is still unclear 5-7. This research aimed to explore the role of Hcy on the brain vessels regarding cerebral hemodynamic status;
cerebral microangiopathy related white matter lesions; long term vascular events in baseline healthy subjects; as well as the Hcy-lowering therapy on cognitive function in patients with dementia.
1.1 Historic aspects of homocysteine and vascular disease
Recognition of an association between the sulfur-containing amino acid Hcy and vascular disease had its origin in the 1960s 8. It followed the identification of a new inborn error of metabolism in which a large quantity of Hcy was excreted in the urine8 and shown to be associated with precocious vascular disease 9. It was soon established that the disorder was due to cystathionine β-synthase deficiency 10. The identification of other cases soon followed and in 1964 there was the first documentation of the
pathological findings in homocysteinuria and the description of widespread of vascular changes and thrombosis 9. In1969, it was first suggested by McCully that among the complex metabolic changes occurring in cystathionine β-synthase deficiency and the factor mediating the vascular changes was a greatly elevated concentration of
homocysteine 11. McCully noted that hyperhomocysteinemia arising from different
metabolic defects was associated with premature arteriosclerosis.
1.2 Biochemistry, metabolism and determinants of serum homocysteine 1.2.1 Source of homocysteine
Homocysteine is derived primarily from methione in dietary protein. Foods contain only trace amounts of homocysteine, which maintained at low concentration in both animal and plant cells. But the abundance of methione, the proximal precursor of homocysteine, varies widely according to the source of food proteins. Ingestion of a meal rich in
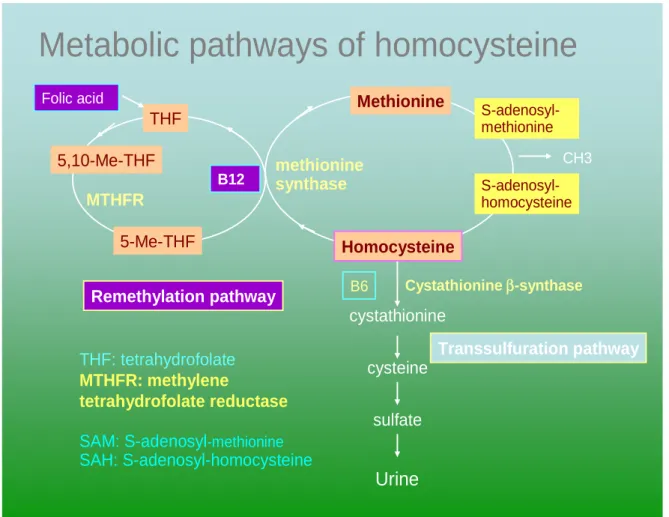
protein elevate plasma homocysteine 12. 1.2.2 Methionine cycle (Figure 1)
Homocysteine is a metabolite of the methione degradation cycle. Methionine is
metabolized via-S-adenosyl methionine and S-adenocyl-homocysteine to homocysteine in the course of producing methyl groups for use in synthetic process. On a normal diet about 50% of the homocysteine formed is metabolized via the transsulfuration pathway.
The first step involves the enzyme cystathionine β-synthase for which pyridoxine (B6) is the co-factor and deficiencies of this enzyme result in the usual form of
homocysteinuria. The remaining 50% of formed homocysteine is remethylated to methinone and requires 5, 10-methyltetrahydrofolate as substrate, and
methylcobalamine as a co-factor. Remethylation by trimethylglycine may also occur via a separate pathway. The methionine cycle is complete when homocysteine in
remethylated back to methionine 13.
1.2.3 Determinants of serum homocysteine
There are many variables that influence serum Hcy concentration. Age and sex are two of the stronger determinants of fasting Hcy levels 14. Serum Hcy increases throughout life in both sex and greater in men than in women. Life style and diet are also correlated with Hcy. Smokers have higher levels of Hcy than non-smokers 15. Hcy levels are
inversely correlated with vitamin intake 16. Folate and cobalamin deficiency are common cause of moderate to severe hyperhomocysteinemia. Several diseases also influence the Hcy levels. Renal failure is the clinical status most often responsible for elevation of Hcy 14. Hcy is moderately elevated in hypothyroidism and low in
hyperthyroidism 17. This may be related to the influence of thyroid status on riboflavin or folate function, glomerular infiltration rate, or creatinine synthesis. A variety of drugs affect Hcy levels. They act via different mechanisms, including inhibition of vitamin (folate, cobalamin or vitamin B6) function, by affecting Hcy production and interfering with renal function 14. Genetic defects in the enzymes that metabolize Hcy can
contribute to mild, moderate and severe hyperhomocystenemia, depending on the nature of the gene product and the level of residual enzyme activity 14. The most common enzyme defect associated with moderately raised Hcy is due to a single, common, polymorphic variant of the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene.
1.3 Vascular pathology and pathophysiology of hyperhomocysteinemia 1.3.1 Vascular pathology of hyperhomocysteinemia
The findings of vascular damage, characterized by fibrin deposition, focal necorsis of artery wall, swelling of endothelial cells, and microthrombi suggest a common pathophysiological atherogenic process in hereditary hyperhomocysteinemia 9. The development of arteriosclerotic plaques in homozygous homocysteinria closely parallels the pathology of human atherosclerosis.
1.3.2 Plausible mechanism of vascular damage by hyperhomocysteinemia In vitro evidence exists for effects of Hcy on atherogenesis and thrombogenesis.
Experimental studies have shown that homocysteine may be harmful to vascular smooth muscles cells and promote proliferation of vascular smooth muscle cells. Hcy may also
have other possibly detrimental effects 18-24. However, most of the studies showing these effects have been undertaken with supra-physiological concentrations of
nonphysiological forms of Hcy 25. Thus, the results of many studies 19-24 may not be generalisable to humans with mild and moderate hyperhomocysteinuria.
1.4 Homocysteine as a risk factor for vascular diseases 1.4.1 Homocysteine, hemodynamics and atherosclerosis
Hemodynamic status of slow flow and low shear stress related vascular remodeling is one of the pathogenesis of atherosclerosis 26-28. Hemodynamic data obtained by
ultrasonography include blood flow velocity, flow resistance and flow volume 29, 30. Hcy has been reported to be higher in those with slow coronary artery flow than in those with normal flow velocity 31. Hcy is an important factor for atherosclerosis in the large cerebral arteries 32. In 1,041 Framingham residents who had Hcy measurement and carotid sonography. The adjusted odds ratio (OR) for stenosis ≥ 25% was 2.0 (95%
confidence interval, CI, 1.4-2.9) for subjects with Hcy levels in the highest compared to the lowest quartile 32. In the Atherosclerosis Risk in Communities (ARIC) Study,
subjects with thickened intima-medial carotid walls (≥ 90th percentile) were more likely to have elevated Hcy levels compared to those without thickened walls (<70th percentile)
33. Hcy may possibly slow the blood flow velocity, and further induce atherosclerosis with reducing the brain flow volume. However, the reports investigating the relationship between serum Hcy and the cerebral hemodynamic status were limited.
1.4.2 Homocysteine, microangiopathy and cerebral white matter lesions (WML) Sclerosis of small cerebral arteries and arterioles is responsible for the diffuse
periventricular white matter abnormalities and the central lacunar lesions evidenced in computed or magnetic resonance tomography 34. The severity of cerebral white matter
lesions (WML) increases with age and the presence of arterial hypertension 35-37.
Although the clinical significance of these lesions remains to be fully understood, WML have been associated with dementia, depression, stroke and mortality 38-41. Patients with Alzheimer’s disease (AD), vascular dementia, and depression have more severe WML than control 38, 39.
It is well known that hyperhomocysteinemia is an independent risk factor for vascular disease. The pathophysiologic mechanism by which Hcy induces angiopathy remains unclear, although various hypotheses have been proposed 42-44. A prospective study performed in Japanese showed significant association between Hcy and risk of lacunar infarcts 45. This finding indicated that Hcy may be related to small vessel disease. Silent brain lacunar infarcts and WML are thought have a small vessel disease origin and are frequently seen in neurologically asymptomatic elderly people 35, 36, 46 and are common among persons with cognitive impairment and dementia 38, 39. Studies have determined the association between Hcy and vascular lesions as well as Hcy and
dementia 2, 47-51. In view of aforementioned report, we propose a hypothesis that Hcy may induce the risk of cerebral arteriole angiopathy and further induce WML and lacunar infarcts; and then further cause cognitive impairment. A population-based study from Rotterdam demonstrated that Hcy is associated with cerebral WML 52. However, studies about the association between Hcy and WML are limited among Asia
population.
1.4.3 The association between total serum homocysteine and clinical vascular events
Epidemiological studies, using cross-sectional, case-control, and cohort designs, have examined the association between Hcy and cerebrovascular disease. Many case-control
and cohort studies have identified a strong, independent and dose-related association between moderately elevated Hcy and atherosclerotic vascular disease, including stroke
1. Brattstrom et al., first reported a case-control study in 1984 that moderate
hyperhomocysteinemia was a possible risk factor for atherosclerotic cerebrovascular diseases 53. These investigators measured non-protein-bounded Hcy following methionine loading in 19 patients with TIA or minor stroke. Sixteen of these patients had Doppler or angiographic evidence of internal carotid artery stenosis or occlusion. In 1992 Brattstrom et al., in a large series of stroke patients, found that plasma Hcy
concentrations were not only elevated in carotid artery disease and lacunar stroke but also in hemorrhagic and embolic stroke 47. Coull and colleagues found similar
elevations of Hcy in patients with acute stroke and TIA. Plasma Hcy concentration was moderately but significantly higher in patients than in control (P<0.0001)48. In a
meta-analysis employing 27 studies relating Hcy to arteriosclerotic vascular in relation to the effects of folic acid on lowering Hcy concentration, Boushey et al., determined the effects of Hcy on the risk of three categories of vascular disease (coronary,
cerebrovascular and peripheral artery diseases)54. Elevations of Hcy were considered an independent graded risk factor for arteriosclerotic vascular disease: a 5 µmole/L Hcy increment elevates risk by as much as a cholesterol increase of 0.5 mmole/L (20 mg/dL).
The OR for cerebrovascular disease is 1.5 (95% CI, 1.3 to 1.9) 54.
However, not all reports have been consistent. Several prospective cohort studies have failed to demonstrate a positive association between Hcy and stroke 55, 56. A Finnish study failed to show a significant association between hyperhomocysteinemia and stroke 55. The relation of serum total Hcy with incidence of atherosclerotic disease was investigated among 7424 men and women aged 40-64 years free of atherosclerotic disease at baseline in 1977. During the 9-year follow-up, 134 male ad 131 female cases
with either myocardial infarction or stroke were identified. The mean serum Hcy concentration of male cases and controls was 9.99 µmole/L and 9.24 µmole/L at baseline and that of female cases and controls was 9.58 µmole/L and 9.24 µmole/L respectively. There was also no significant association between Hcy and atherosclerotic disease, myocardial infarction or stroke in logistic regression analyses. The odds ratios varied from 1.00 to 1.26 for Hcy. The results of this prospective population-based study do not support the hypotheses that serum Hcy is a risk factors for atherosclerotic disease.
Furthermore, two large randomized placebo-controlled trials of Hcy-lowering therapy, Vitamins to Prevent Stroke Study (VITATOPS) for patients with patients with a recent transient ischemic attack or stroke, as well as the Vitamins to Prevent Stroke (VISP) study for patients with a first ever non-disabling stroke, failed to show the clinical benefit on stroke prevention 57, 58.
1.5 The points favor or not favor on a causal relationship between Hcy and vascular diseases or cognitive impairment
In view of the literatures, we can summarize some following findings and conclusions about this issue.
1.5.1 The points in favor of a causal relationship between Hcy and vascular diseases
(1) An association between elevated Hcy and atherosclerotic vascular disease is
biologically plausible; experimental studies have shown elevated Hcy to be atherogenic and thrombogenic 19-23.
(2) Systematic review of epidemiological studies 59, 60 and meta-analysis study 4 have revealed a reasonably strong, independent, dose-related relationship between higher plasma concentration Hcy and the occurrence of cerebral, coronary, and peripheral vascular disease.
(3) High Hcy has been associated with dementia 49-51, 61. The influence of elevated Hcy on the intracranial small vessels disease was thought to be the cause of cognitive impairment 62.
(4) Reducing plasma Hcy by means of multivitamin therapy produces favorable effects on surrogate markers of vascular disease, such as endothelial function 63-65, and carotid artery intima thickness 66.
1.5.2 The points that cast doubt on a causal relationship between Hcy and vascular diseases
(1) Most experimental studies that have shown hyperhomocysteinemia to be atherogenic and thrombogenic have been undertaken with supraphysiological concentrations of Hcy 19-25.
(2) There have been inconsistencies in the results of epidemiological studies obtained by different methods; stronger associations have been found in retrospective
cross-sectional, or case-control studies, and small or no associations have been found in prospective cohort studies 55, 60.
(3) The temporal relationship between the onset of elevated Hcy and the onset of white matter lesions, cognitive impairment, stroke and other vascular events is unclear 52, 67, 68. (4) There is no reliable evidence homocysteine lowering therapy can prevent vascular events 5, 6, 57, 58, 69. Two large randomized placebo-controlled trials of Hcy-lowering therapy, Vitamins to Prevent Stroke Study (VITATOPS) for patients with a recent transient ischemic attack or stroke, as well as the Vitamins to Prevent Stroke (VISP) study for patients with a first ever non-disabling stroke, failed to show the clinical benefit on stroke prevention 57, 58.
Importantly, it is necessary to reassess the role of Hcy on the vascular diseases.
1.6 Homocysteine as a risk factor for dementia
Subjects with cardiovascular risk factors and a history of stroke have an increased risk of both vascular dementia and Alzheimer’s disease 70-72. Plasma total Hcy has recently emerged as a vascular risk factor. And vascular factor of hypoperfusion is related to AD
73-75, hyperhomocysteinemia may play a role in developing AD. Besides, it is generally known that deficiency of certain vitamins, particularly vitamin B12, can cause cognitive impairment, which can be reversed by correcting the deficiency76-79. Methionine
synthesis from homocysteine requires folic acid as a methyl donor, and methyl cobalamin (vitamin B12) as the intermediate methyl acceptor and donor. Vitamin B6 participates in the alternate homocysteine catabolic pathway leading to the production of cysteine. Thus vitamin deficiency can induce hyperhomocysteinemia 80, 81. There is evidence that plasma homocysteine (Hcy) concentration may be a modifiable risk factor for cognitive decline 2. Hcy concentration has been reported to be higher in persons with Alzheimer’s dementia 82-84. The results of several studies showed that Hcy was inversely associated with performance of cognitive function 85-87.
There is great interest in cheap and less harmful over-the-counter vitamin supplements that may retard the worsening of cognition in the elderly.
The inverse relation between plasma Hcy levels and blood concentrations of folic acid and vitamin B12 makes it difficult to distinguish the independent effects of each on cognitive function. There is no report of Hcy-lowering therapy for mild to moderate Alzheimer’s dementia.
Chapter 2. Hypotheses and objectives
2.1 Hypotheses
(1) Hypotheses for the effects of Hcy on cerebrovascular disease
(a) Hcy may influence the hemodynamic flow of cerebral arteries with reducing the flow velocities and then further induce atherosclerotic change.
(b) Hcy may induce microangiopathy and resulted cerebral white matter change which are essential for future stroke and dementia.
(c) The impacts of elevated plasma Hcy are significant on vascular diseases
(2) Plasma Hcy levels are higher in patients with AD. Hcy induced vascular factors that may underlie AD. Therefore homocysteine lowering therapy with vitamin
supplementation might be benefit for persons with AD.
2.2 Objectives
Our aims are as follows:
(1) To explore the influence of Hcy on the hemodynamics of the extracranial cerebral arteries – carotid and vertebral arteries;
(2) To evaluate the association between Hcy and the microangiopathy related cerebral white matter lesions;
(3) To evaluate the prediction of Hcy on vascular events among ethnic Chinese healthy people;
(4) To evaluate the benefit of Hcy-lowering therapy on cognitive performance in patients with AD.
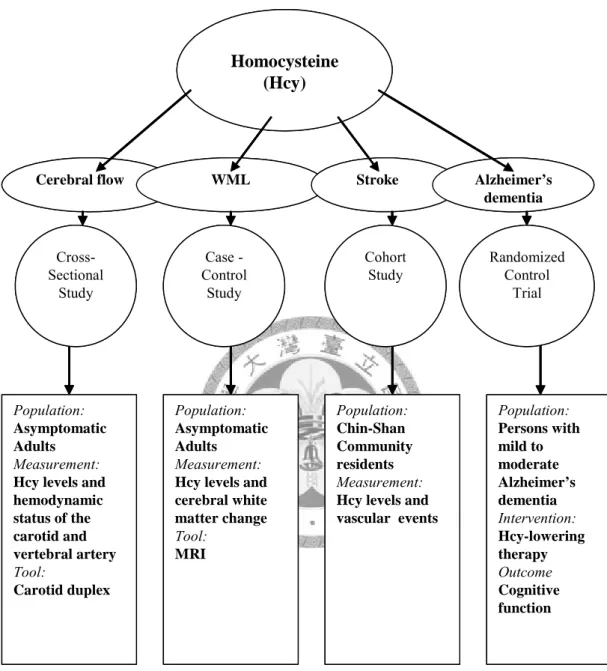
Chapter 3. Subjects and Methods (Figure 2)
3.1 Impacts of homocysteine on hemodynamic status 3.1.1 Participants
The study participants were invited from the population presenting to En Chu Kong Hospital (Taipei, Taiwan) for a health physical check-up between 1999 and 2007. We recruited 542 participants (mean age ± SD, 55.2 ± 14.8 years, 56% males), who were free from history of stroke, transient ischemic attack, coronary heart disease, or intermittent claudication. All participants underwent detailed studies that included clinical questionnaires, physical examination that included measurements of height, body weight, body mass index, and blood pressure. Duplex ultrasonography on the carotid artery, vertebral artery and blood laboratory tests were performed.
3.1.2 Ultrasound procedure and hemodynamic measurements
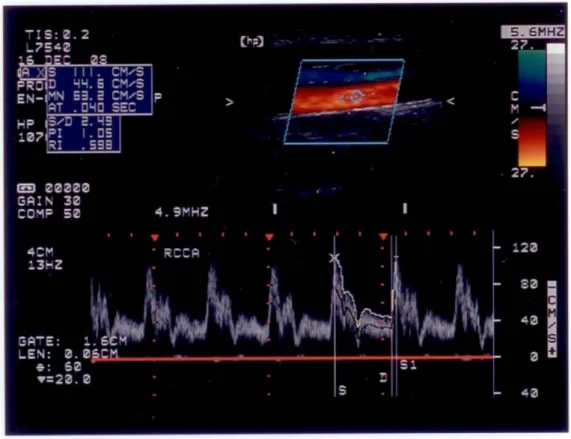
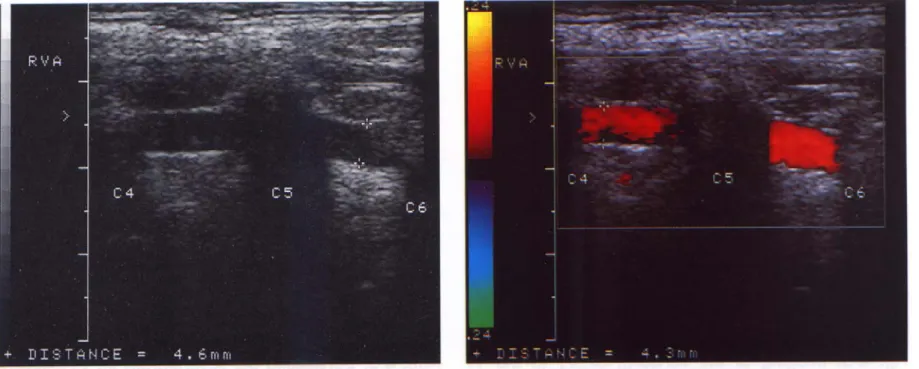
Ultrasonography was performed with a Hewlett-Packard 5500 system equipped with a high-resolution broadband width linear array transducer (4-10 MHz) 88. Participants were examined in the supine position. An experienced technologist who was kept unaware of the patient’ clinical data made all ultrasound measurements. Images were obtained bilaterally from the proximal common carotid artery (CCA) to distal CCA, including bifurcation, internal carotid artery (ICA) and external carotid artery (ECA), as well as the vertebral artery (VA) obtained from C2 to C6 segments (Figure I-1, I-2).
We measured the flow velocity and resistance of the CCA, ICA, ECA and VA as the flow parameters 89. We also calculated the flow volume of the VA. Regarding the flow velocity, we measured the peak systolic velocity, end-diastolic velocity, time average flow velocity, mean flow velocity. The following formula is used to calculate the mean flow velocity: (mean flow velocity = [peak systolic velocity + 2 end-diastolic velocity]/3) 90. As for the resistance of the vessel, resistance index and pulsatility index
were measured. Resistance index is calculated with the formula: (resistance index = (peak systolic velocity - end-diastolic velocity)/ peak systolic velocity) 91. Pulsatility index described the shape of the waveforms. The formula calculating pulsatility index is:
[pulsatility index = (peak systolic velocity - end-diastolic velocity )/ mean flow velocity)] 92. Pulsatility index and resistance index are believed as presumptive
measures of downstream vascular resistance 90. We measured diameter to calculate the flow volume. Color-coded flow image was used to measure the diameter. Flow volume is the product of flow velocity and the area in which this velocity occurs (Flow volume [cm3/s] = flow velocity [cm/s] × area [cm2]) 90.
3.1.3 Laboratory analysis
Blood samples were collected to determine the levels of Hcy, total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, blood glucose,
hemoglobin, platelets, blood urea nitrogen, creatinine, and uric acid after an overnight fasting with standard techniques. Venous blood samples for Hcy, collected into tubes containing ethylenediaminetetraacetic acid, were centrifuged within 30 minutes at 2000 rpm for 10 minutes, and then separately stored at -70°C until analysis. Serum Hcy concentrations were measured by fluorescence polarization immunoassay (Abbott IMx System) 93, 94, which were correlated well to high performance liquid chromatography.
The coefficients of variation were within 5.2% 95. 3.1.4 Statistical analysis
Populations were categorized according to Hcy quartiles. Continuous variables were presented as the mean (SD) or the median, while the categorical data were presented in contingency tables. All the flow parameters of the examined vessels in the statistical analyses were the mean values from bilateral measurements, including diameter, peak systolic velocity, end-diastolic velocity, time average flow velocity, mean flow velocity,
resistance index, pulsatility index and total flow volume. ANOVA and the χ2 test were used to test the differences of the vascular risk factors and CCA and VA flow parameters between Hcy quartiles.
Multiple linear regression models were used to examine the relationship between Hcy and the aforementioned flow parameters of the examined vessels. We estimated the means of the flow parameters with adjustment for age and sex and additionally for current smoking, systolic blood pressure, body mass index, serum creatinine, serum uric acid and high density lipoprotein. In above analyses, we modeled Hcy concentrations as quartiles to avoid the assumption of linearity and to reduce the effects of outliers. To test for linear trends across Hcy quartiles, we used median Hcy concentration for each category in the multivariate model. In addition, to estimate the effects of Hcy, we calculated the odds ratio (OR) and corresponding 95% confidence interval (CI) for the change of flow parameters of the CCA and VA according to one-SD increase of Hcy.
All statistical tests were 2-tailed and the P values <0.05 were considered
statistically significant. Analyses were performed with SAS software (version 9.1; SAS Institute).
3.2 Homocysteine and cerebral white matter lesions (WML) on MRI 3.2.1 Participants
This study population was drawn from subjects presenting to En Chu Kong Hospital (Taipei, Taiwan) for a routine physical check-up between 1999 and 2007.
Detailed physical examinations and questionnaires were performed and administered.
MRI was performed on all the study subjects. The inclusion criteria for case subjects were as follows: healthy adults with no history of vascular disease including
cerebrovascular disease, transient ischemic attack, and myocardial infarction; WML was
noted on the MRI check-up. A diagnosis of WML was made by MRI examination and by agreement between independent experienced neurologist and neuroradiologist. As for the control subjects, we selected healthy individuals, who were free from a recent or past history of cerebrovascular disease or of myocardial infarction, presenting at our hospital for a health examination, and were with normal brain MRI. Both case and control group were prospectively enrolled during the same period. Baseline
demographic data and a history of conventional vascular risk factors were obtained from participants from both groups. Diagnosis of brain MRI study was made without
knowledge of Hcy levels and presence of risk factors.
3.2.2 MRI protocol
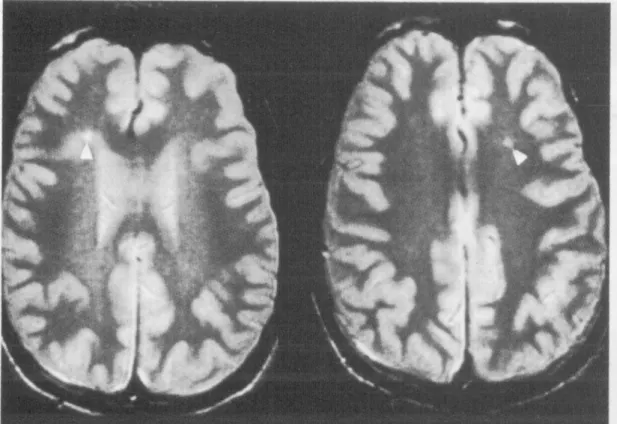
WML were considered present if visible as hyperintense on FLAIR and T2 weighted images on the subcortical or periventricular area (Figure II-1), without prominent hypointensity on T1 weighted scans.
All examinations were performed using 1.5-T magnet system (Eclipse, Picker International, Inc., Highland Hts, Ohio). The image protocol consisted of T2-weighted spin echo for coronal slice (TR/TE = 3,600/112 msec), T1-weighted spin echo for sagittal slice (TR/TE=500/12.1 msec), FLAIR (TR/TE=6,000/96 msec, inversion time 2,100) for axial slice, diffusion weighted image and MR angiography.
3.2.3 Statistical analysis
To estimate the magnitude of the association between Hcy and WML, we used OR and 95% CI. For the crude analysis of baseline characteristic, we used the χ2 test for
categorical data, and the two sample t-test for continuous data. For the multivariate analysis, we used logistic regression to adjust for possible confounders, i.e., age, sex,
hypertension and diabetes mellitus. Logistic regression models were also used to estimate the OR of WML for quartiles of Hcy levels, to assess the relation between Hcy and other relevant risk factors. The relationship between Hcy levels and the presence of WML was also analyzed using multivariate regression models.
3.3 Homocysteine and long-term vascular events (Chin-Shan cohort) 3.3.1 Participants and study design
The participants were enrolled in the Chin-Shan Community Cardiovascular Study, a prospective community-based study of risk factors and cardiovascular consequences.3, 96,
97 In brief, the study was started in 1990 with an initial cohort 3602 participants, who were recruited on the basis of official registrations. This study was approved by the institutional review boards of the National Taiwan University. The study collected information regarding medical history, the results of physical examination and
laboratory tests, and an assessment of health status that included evidence of stroke and cardiovascular disease since baseline assessment and the follow-up period. Among the participants, 2117 subjects had homocysteine measurement during 1994 to 1995, in whom 2009 were free from history of stroke, cardiovascular disease and cancer. We collected detailed information about lifestyle factors including alcohol intake, smoking, and regular exercise, as well as data regarding socioeconomic status and family history of stroke and coronary heart disease (CHD). With regard to the follow-up schedule, we gathered information about stroke, cardiovascular events and deaths through monthly collections of official death certificate documents, by annual questionnaires, and by house-to-house visits.
3.3.2 Ascertainment of events
The study outcomes were stroke, CHD and all-cause death. Stroke was defined as a
sudden neurological deficit of vascular origin that lasted longer than 24 h and was proved by evidence from an imaging study 98. Transient ischemic attacks were not included in this definition. Incident CHD cases were defined as non-fatal myocardial infarction, fatal CHD, and hospitalization for percutaneous coronary intervention and coronary artery bypass surgery. Fatal CHD was considered to have occurred if fatal myocardial infarction was confirmed by hospital records, if CHD was listed as the cause of death on the death certificate or was the underlying and most plausible cause of death
99. Deaths from any cause were identified from official certificate documents and further
verified by house-to-house visits.
3.3.3 Measurements of serum homocysteine and other biochemical variables The procedures of blood sampling have been reported elsewhere 98, 100, 101. In brief, all venous blood samples drawn after a 12-h overnight fast were immediately refrigerated and transported within 6 h to the National Taiwan University Hospital. Serum samples were then stored at -70 ºC until analysis. Serum Hcy were collected into tubes
containing ethylenediaminetetraacetic acid and were measured by fluorescence
polarization immunoassay (Abbott IMx System) 93, 94, which correlated well with high performance liquid chromatography. The coefficients of variation were within 5.2% 95. 3.3.4 Statistical methods
Participants were classified by quartiles of Hcy concentration. Continuous variables were presented as mean (SD) or median values, and categorical data were presented in contingency tables. ANOVA and the chi-square test were used to test differences between quartiles. Relationships between baseline Hcy concentrations and blood pressure, fasting glucose, body mass index and lipid profiles were evaluated with age- and sex-adjusted Spearman partial correlation coefficients.
Incidence rates for stroke, CHD and all-cause death were calculated for each Hcy
quartile by dividing the number of cases by the number of person-years of follow-up.
We estimated the relative risk (RR) and 95% confidence interval (CI) by the
multivariate Cox proportional hazards models. We specified 4 models for estimating the RRs of events in higher Hcy quartiles relative to the lowest quartile. In model 1, we estimated the univariate RR of Hcy with the first quartile as the reference. In model 2, we adjusted for age and sex variables. In model 3, we additionally adjusted for body mass index, educational level, occupation, alcohol intake, smoking, and regular exercise.
In model 4, we adjusted for the presence/absence of hypertension and diabetes at baseline, family history of stroke and coronary heart disease, continuous variables of total cholesterol, triglyceride, HDL-C and LDL-C concentrations along with
adjustments for the variables in model 3. We modeled Hcy concentrations as quartiles to avoid the assumption of linearity and to reduce the effects of outliers, and we used median Hcy concentrations for the categories to test for linear trends across quartiles. In secondary analyses, we constructed receiver-operating-characteristic (ROC) curves to generate the optimal cutoff point with highest Youden’s index for the occurrence of stroke, CHD and death. We then calculated hazard ratios for stroke, CHD and death for high and low Hcy level using aforementioned cutpoints. The multivariate-adjusted hazard ratios were presented as the model 4.
To test the feasibility of the cutpoints of Hcy, ROC curves were further plotted again for full adjusted model (age, sex, body mass index, educational level, occupation, alcohol intake, smoking, regular exercise, hypertension, diabetes and family history of stroke and coronary heart disease) with Hcy and for that without Hcy. The
discrimination of models with and without events were assessed by comparing the c statistics for CHD and death respectively, where Hcy level was treated as binary variable with aforementioned cutpoints. In addition, the increased discriminative value
of Hcy was further examined with the method described by Pencina et al.102. Two statistics, including, net reclassification improvement, and, integrated discrimination improvement, were presented with the priori meaningful risk categories set as follows:
the 10-year estimated low, median and high risk rate were defined at 5%, 15% and 25%
for stroke; 10%, 25% 30% for CHD ; and 5%, 10% 20% for death 103.
All statistical test were 2-tailed with type I error of 0.05, and P values <0.05 were considered statistically significant. Analyses were performed with SAS software (version 9.1; SAS Institute).
3.4 Homocysteine lowering clinical trial on Alzheimer’s disease 3.4.1 Participants and study design
This is a 26-week, double-blind, placebo-controlled, randomized clinical trial conducted in En Chu Kong Hospital, Taiwan from July 2003 to March 2006. The inclusion criteria were history of cognitive decline that was gradual in onset and progressive over a period of more than 6 months; clinical diagnosis of mild to moderate AD based on criteria set forth by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) 104; a score of 10-26 on the Mini-Mental State Examination (MMSE) 105, 106; and a screening score of 1-2 on Clinical Dementia Rating Scale (CDR) 107. Exclusion criteria were the following: history of epilepsy; clinically significant hepatic, renal, pulmonary, metabolic or endocrine disturbances, or significant cardiovascular disease that would impede the subject's ability to complete the trial; vascular dementia or evidence of cerebrovascular disease; low serum levels of folic acid (<5 ng/ml) and vitamin B12 (<180 pg/ml); use of any vitamin supplements and all the agents for the treatment of dementia (approved, experimental, or over the counter) except for
acetylcholine esterase inhibitors (AChEIs); cognitive impairment due to acute cerebral
trauma, hypoxic cerebral damage, infection, primary or metastatic cerebral neoplasm, significant endocrine or metabolic disease, or mental retardation. Computer tomography (CT) or Magnetic radioimaging (MRI) was performed on all study participants to rule out structural brain lesion (i.e. stroke, tumor, chronic subdural hematoma, etc.)
For ethical considerations, all of the 89 enrolled participants were also prescribed with AChEIs, the FDA approved treatment for AD patients. Except for one taking rivastigmin (® Exelone), all of the other patients were concurrently taking donepezil (® Aricept) in addition to our study regimens. All of the patients were then randomly allocated to receive the add-on therapy of either multi-vitamin or placebo according to a 1:1 ratio for six months. All of the study personnel and participants were blinded to treatment assignments and randomization was performed using a computer-generated randomization list. Efficacy measurements for the double-blinded phase were performed at baseline and week 26.
The study was conducted in accordance with Declaration of Helsinki and was approved by the hospital’s Institution Review Board. Written informed consent was given by each patient or patient’s representative or by the caregiver participating in this study.
3.4.2 Study regimens
The study regimens used mecobalamin preparation (trade name Methycobal) capsule (Eisai Co.) containing vitamin B12 0.5 mg with an active methyl base, and another over-the-counter vitamin named Pramet®FA (Abbott Laboratories Services Corp.) containing folic acid 1 mg, pyridoxine HCl 5 mg, iron ferrous 60 mg, nicotinamide 10 mg, calcium carbonate 250 mg, riboflavin 2 mg, thiamine mononitrate 3 mg, calcium pantothenate 1 mg, ascorbic acid 100 mcg, iodine 100 mcg, copper 150 mcg, vitamin B12 3 mcg, vitamin A 4000 I.U., and vitamin D3 400 I.U. In Taiwan, Pramet®FA tablet
was originally used as a kind of nutritional supplement for pregnant women. The placebo of Pramet®FA and Methycobal were made by the Genovate company (the manufacturer for Abbott Corp. in Taiwan) and Eisai Pharmaceutics Company respectively and were identical in size and color to vitamin supplements.
3.4.3 Assessment and outcome measures
Patients were assessed at the beginning with detailed history taking, blood tests,
neuro-imaging studies, and neuropsychological tests. Screening cognitive tests included the scale of CDR and MMSE. Baseline blood tests included standard fasting venous blood sample for blood chemistry (liver and renal function profile, fasting sugar), complete blood count (CBC), thyroid function (T3, TSH), syphilis blood test (VDRL, TPHA), and serum levels of B12, folic acid, and homocysteine.
The study patients were enrolled and received baseline cognitive measures, including Cognitive Abilities Screening Instrument (CASI, Chinese version, C-2.0, Liu)
108-110, Alzheimer’s Disease Assessment Scale (ADAS-Cog/11, Chinese version, Liu)
111-113, the Simplified Barthel Activities of Daily Living Index (ADL), and the Instrumental Activities of Daily Living Scale (IADL). The patients were then
interviewed by the same neurologist, who was blinded to the patients’ assigned study medication, at the outpatient department at weeks 2, 6, 10, 14, 18, 22, and 26 for physical and neurological examination. Any subjective or objective findings, as well as any favorite effects or adverse events (AEs) after treatment were recorded. At week 26, serum levels of B12, folic acid, and Hcy were re-measured. Cognitive performance and daily living function were assessed by the scores of MMSE, CASI, ADAS-Cog/11, ADL, and IADL.
3.4.4 Outcome measures
The primary efficacy outcomes were the change from baseline to week 26 in
ADAS-Cog 113 for the cognition evaluation, which includes an assessment of 11 items on the cognitive subscale of the ADAS (spoken language, comprehension, recall of test instruction, word-finding difficulty, following commands, naming, constructions, ideational praxis, orientation, word recall, word recognition). Cognitive assessments were carried out at screening baseline and at week 26 by the same independent rater who was blinded to the patients’ assigned study medication and other study information, such as adverse events.
Secondary efficacy outcomes included assessment of cognition changes from baseline to week 26 on activities by using the score of the CASI and MMSE. The CASI complete form (CASI-C) provides quantitative assessment (scoring from 0 to 100) of attention, concentration, orientation, short-term memory, long-term memory, language abilities, visual construction (copying two intersecting pentagons), list-generating fluency, abstraction, and judgment. These instruments were based on information collected independently from the subject and caregiver. Another secondary outcome measurement was an evaluation of the change from baseline to week 26 of activities of daily living function. The measurements included the ADL and the IADL. The ADL indices were measured using a 10-item for evaluating daily function (toilet, grooming, feeding, physical ambulation, dressing, bathing), while the IADL scale (based on the report of M. P. Lawton and E. M. Brody) 114 used an 8-item evaluation of the ability to use the telephone, shopping, food preparation, housekeeping, laundry, mode of
transportation, responsibility for own medications, ability to handle finances. Secondary outcomes also included the change of plasma Hcy, serum vitamin B12, and folic acid levels.
3.4.5 Safety evaluations
Safety and tolerability were evaluated by comparing treatment groups with respect to
physical examination findings, changes in vital signs, laboratory test abnormalities, concomitant medication use, and compliance with study medication, as well as the monitoring of adverse events (AEs) throughout the study. An AE was defined as any undesirable effect experienced by a patient during the trial, whether or not related to treatment. A serious adverse event (SAE) was defined as any AE that was life threatening or resulted in death, hospitalization, prolongation of hospitalization, or significant disability.
3.4.6 Data analysis
To compare the effects of multi-vitamin therapy and placebo on the cognitive function assessed using the ADAS-cog scale between the time of enrollment and week 26, according to an intention-to-treat strategy, the initial assumption was that there were 2 points of differences in the ADAS-cog scale between these 2 groups, with 4 of common standard deviation. We estimated the sample size required for a two-tailed significance level of 0.05 and a power of 0.90. On this basis, 43 patients were needed in each group.
Assuming the drop-out rate was 20%, the ideal number of total enrolled participants should be up to 108.
We first compared characteristic variables between the placebo and treatment groups, including baseline plasma levels of homocysteine, vitamin B12, and folic acid, as well as cognitive screening tests using the chi-square test for categorical data and the Student’s t test for continuous data. The changes in plasma concentrations of vitamin B12, folic acid, and homocysteine were then compared, from baseline to 6 months, using non-parametric Wilcoxon two sample tests because the values of the variables were not normally distributed.
Paired t–test was used to evaluate the efficacy of the intervention on cognition (MMSE, CASI, ADAS- Cog) and daily living function (IADL and ADL) by comparing
the changes from baseline to 6 months between the placebo and treatment groups. All were based on an intention-to-treat analysis. A multiple linear model was performed to assess the association between the change of homocysteine and change of cognition.
The ANCOVA test was used to compare the efficacy of intervention adjusting the effect of baseline cognition status, age, and sex variables. Linear regression was used to assess the association between the score change and the Hcy change after adjustment for baseline Hcy values, age, and sex. The number of adverse events between the placebo and treatment groups was also compared by using the chi-square test and Fish exact test if the expected number less than 5.
Chapter 4. Results
4.1 Homocysteine and the hemodynamic status of the extracranial large arteries (Table I-1 to Table I-7)
The median (interquartile range) and mean (± SD) of tHcy for the 542 study participants were 9.1 (7.4 to 11.3) and 9.9 μmol/L (± 4.9) respectively. The median (interquartile range) of tHcy was 7.1 (6.4 to 9.2) μmol/L in women compared with 10.1 (8.4 to 12.1) μmol/L in men (P <0.0001). The respective ranges for tHcy (μmol/L) quartiles were:
first, 1.91-7.36; second, 7.37-9.07; third, 9.08-11.32; and fourth, 11.33-58.7. Baseline characteristics of the study population by tHcy quartiles were listed in Table I-1. Higher tHcy levels were associated with older age, current smoking, systolic blood pressure, body mass index, serum creatinine, and serum uric acid. With regards to flow
parameters across tHcy quartiles, participants with higher tHcy levels were more likely to have slow flow velocity (systolic and diastolic flow velocity) in the CCA, ICA and VA (Table I-2, I-3). The resistance (including resistance index and pulsatility index) was increased in the CCA and decreased in the VA as Hcy increase (Table I-2, I-3). The diameter and flow volume of the vertebral artery were similar across tHcy quartiles.
After adjusting for demographics and risk factors, the least-squares mean values of the carotid artery and the VA flow parameters across quartiles of tHcy are shown in Table I-4, I-5. There was no significant difference on the flow velocity, resistance, VA diameter and flow volume between different levels of tHcy. When tHcy considered as a continuous variable, the tHcy levels were not significantly related to the aforementioned hemodynamic status of the CCA, ICA and VA (Table I-6, I-7). The results were
unchanged if we calculated the parameters of the examined vessels from left and right side separately (data not shown).
4.2 Homocysteine and microangiopathy related cerebral WML (Table II-1 to Table II-2)
We conducted a case control study in a total of 258 asymptomatic adults (mean age 51 years) which were classified into case group with microangiopathy related cerebral white matter lesions noted on the MRI and control group with normal brain MRI study.
The mean serum Hcy levels was 9.1 µmol/L in the 65 participants of case group and 8.9 µmol/L in control group (p=0.83). The demographic and laboratory data between theses two groups were shown in Table II-1. However, after fully adjustment of the
confounding variables such as age, sex, blood pressure and other vascular factors, Hcy remained an independent risk factor for cerebral white matter lesions. Every 1 µmol/L increase of Hcy, the relative risk for white matter change was 1.15 (95% CI 1.01-1.31, P=0.03) (Table II-2).
4.3 Homocysteine and long-term clinical vascular events (Chin-Shan cohort) (Table III-1 to Table III-4)
Baseline characteristics of the participants are shown in Table III-1. Participants in the highest Hcy quartile were less likely to be female and be in professional business job, compared with those in the lowest quartile. They were likely to smoke, and drink alcohol and had a higher prevalence of taking regular exercise and had hypertension at baseline. A higher Hcy concentration was associated with an old age, higher systolic blood pressure, higher triglyceride and lower HDL-C concentrations.
Over a median 11.95 years’ follow-up among the 2009 participants, we
documented 114 cases of stroke (including 92 ischemic and 22 hemorrhagic strokes), 95 cases of CHD and 380 deaths (including 50 cardiovascular mortality cases). The
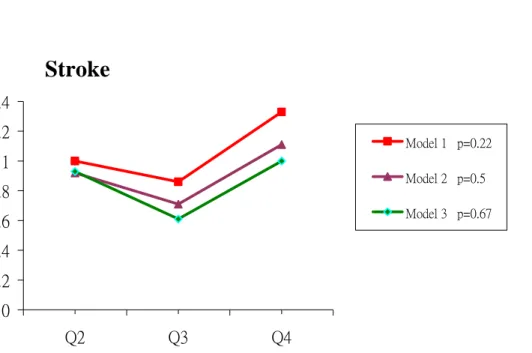
incidence rates for each event increased appreciably with Hcy quartile for stroke, CHD and all-cause death (Table III-2). The RRs for individuals in the highest quartile of Hcy, compared with those in the lowest quartile, were 4.40 (95% CI, 2.43-7.94; P for trend
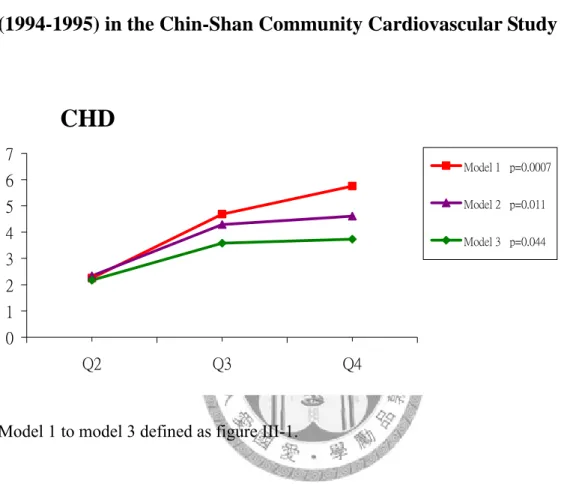
<0.0001) for stroke, 13.90 (95% CI, 5.00-38.65; P for trend <0.0001) for CHD and 5.59 (95% CI, 3.99-7.84, P for trend <0.0001). After fully adjustment for risk factors (model 4), the RRs for individuals in the highest quartile of Hcy compared with those in the lowest quartile were 1.0 (95% CI, 0.49-2.45; P for trend, 0.67) for stroke, 3.73 (95% CI, 1.22-11.40; P for trend, 0.04) for CHD and 1.35 (95% CI, 0.90-2.03, P for trend, 0.001) for all-cause of death. A significant linear trend for an increased RRs of CHD and death with each quartile of Hcy levels was found (Table III-2, Figure III-1, III-2, III-3).
We then performed subgroup analyses in the type of stroke and death. We analyzed the association of Hcy level with subtype of stroke including ischemic and hemorrhagic stroke, and the association of Hcy with cardiovascular mortality and non-cardiovascular death. High Hcy was not significantly associated with stroke including ischemia and hemorrhage: the multivariate RRs across Hcy quartiles were similar as shown in Table III-3. For the risk of death, a significant linear trend on the cardiovascular death
(P=0.0002) as well as on the non-cardiovascular death (P=0.017) with risk increased as serum Hcy elevation. We found that Hcy effect on the cardiovascular death was more prominent (P=0.002) (Table III-3).
Secondary analyses
To determine the optimal cutpoint of Hcy in predicting the main events, we performed a secondary analysis by constructing a receiver-operating characteristic (ROC) curve for the events. The optimal cutpoints with highest Youden’s index (sum of sensitivity and specificity -1) were 9.7, 9.47 and 11.84 for stroke, CHD and death respectively. We then used these cutpoints of Hcy in multivariate Cox proportional
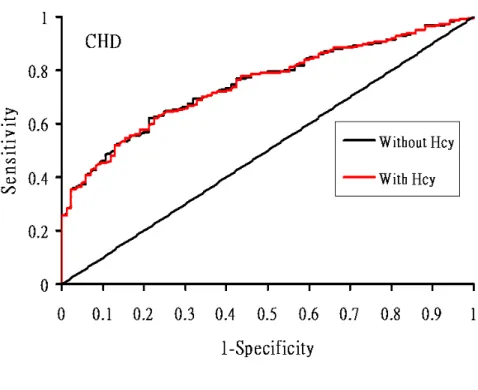
hazard model. The RRs were 0.94 (95% CI, 0.57-1.54, P <0.79) for stroke, 2.3 (95% CI, 1.24-4.18, P=0.008) for CHD and 2.4 (95% CI, 1.76-3.32, P <0.0001) for death (Table III-4). The c statistic was 0.803 in stroke. However, we found that the c statistic
remained the same value after adding Hcy in multivariate model. For predicting CHD, the c statistic of the fully adjusted model without Hcy was 0.749, and it increased to 0.763 after adding Hcy. The c statistic also increased from 0.839 to 0.845 when Hcy added to this full model for the prediction of death. The discriminative ability with additional use of the threshold of Hcy was not significant in prediction of stroke: the net reclassification improvement was estimated at 0.0016 (P=0.36) and the integrated discrimination improvement (IDI) was estimated as 0.000028. (P=0.45) for the risk of stroke. For CHD events, the integrated discrimination improvement increased to 0.01 (p=0.008) for CHD. For predicting death, the integrated discrimination improvement was 0.028 (p=0.00002). The ROC curve of fully model (variables including age, sex, BMI, education, job, smoking, drinking, exercise habit, hypertension, diabetes mellitus, family history of vascular event, serum fasting glucose, total cholesterol, triglyceride, HDL, LDL) with and without Hcy for predicting CHD was shown in Figure III-4 while ROC for predicting death was shown in Figure III-5.
4.4 Homocysteine lowering clinical trials on dementia (Table IV-1 to Table IV-4)
4.4.1 Efficacy of homocysteine –lowering vitamin therapy
Of the 154 patients who were screened, 65 were excluded because they did not want to enter the trial or they had been taking vitamin supplements or they did not completely meet the inclusion criteria. The remaining 89 patients (mean age: 75 ± 7.3 years) with mild-to-moderate AD were randomized, with 45 assigned to the vitamin group and 44 to
the placebo group. Seven participants in the vitamin group and 10 in the placebo group were lost to follow-up. Five participants in the vitamin group and 4 in the placebo group withdrew due to adverse events. Of the total 63 study participants left, 54 completed the tests of cognition (27 in each group) and were included in the final analysis (Figure IV-1). The characteristics of the two groups at baseline are shown in Table IV-1.
The characteristics including sex, age, baseline level of homocysteine, B12, folic acid, MMSE, CASI results were not different between the two groups. The mean baseline MMSE score was around 18 in both groups.
4.4.2 End points
As compared to baseline levels, the Hcy level was reduced while B12 and folic acid were increased after the intervention. The mean plasma homocysteine concentration was 3.02 μmole per liter lower in the vitamin group than in placebo group (p=0.008). The values of plasma concentrations of Hcy, folic acid, and vitamin B12 were not normally
distributed at baseline and at six months. The changes of plasma concentration of Hcy, folic acid, and vitamin B12 concentrations of each individual were checked from baseline to week 26 and were compared between the placebo and vitamin groups using non-parametric Wilcoxon two-sample tests. They were significantly different between these 2 groups (Table IV-2). The median changes were a 9.9 ng/mL increase for folic acid and a 237 pg/mL increase for B12 in the vitamin group, while the median changes were decreased for folic acid and B12 in the placebo group.
The intervention effects on cognition and daily function was assessed by reviewing the change of scores on the MMSE, CASI, ADAS-cog, ADL, and IADL for each
individual participant from baseline to 6 months. The increase of score in the tests of MMSE, CASI or ADL indicates improvement of cognition and daily function. However, higher score of the tests of ADAS-cog and IADL suggested deterioration of cognitive