Ms. Ref. No.: THEOCULARSURFACE-D-13-00028
Title: Risk of Skin Cancer in Patients with Pterygium: a Nation-Wide Population-Based Cohort Study (Version 2)
Short title: Risk of Skin Cancer in Patients with Pterygium
Han-Chieh Yu,1,2 Cheng-Li Lin,3,4 Zoe Tzu-Yi Chen,5 Fung-Rong Hu,2 Fung-Chang Sung,3,4* I-Jong Wang,2,6*
1. Department of Ophthalmology, New Taipei City Hospital, New Taipei, Taiwan 2. Department of Ophthalmology, National Taiwan University Hospital, Taipei,
Taiwan
3. Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
4. Department of Public Health, China Medical University, Taichung, Taiwan 5. Department of Ophthalmology, Taipei City Hospital Zhongxing Branch, Taipei,
Taiwan
6. Graduate Institute of Clinical Medical Science, China Medical University, Taichung, Taiwan
*Author to whom correspondence should be addressed: Fung-Chang Sung, PhD, MPH, Department of Public Health, China Medical University, 91 Hsueh-Shih Road, Taichung City 40402, Taiwan, Phone: +886-4- 22053366 ext 6220; Fax: +886-4- 22053366 ext 6122; email: fcsung@mail.cmu.edu.tw;
I-Jong Wang, MD, PhD, Department of Ophthalmology, National Taiwan University Hospital, Taipei 100, Taiwan; Phone: +886-2-2312-3456 ext. 2131; Fax: +886-2-2341- 2875; email: ijong@ms8.hinet.net
Financial Disclosure(s)
The authors have no proprietary or commercial interest in any of the materials discussed in this article.
Abstract
This study aimed to investigate the association between pterygium and skin cancer linking to ultraviolet (UV) radiation using claims data in 1997-2010, obtained from the Taiwan National Health Insurance Research Database. The study included 19701 patients with pterygium and 78804 sex and age frequency matched comparison subjects.. Multivariate Cox regression analyses were performed to assess the relationship between pterygium and risk of skin cancer by the end of 2010. The incidence rates of malignant melanoma (MM) and nonmelanoma skin cancer (NMSC) in two cohorts and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) of the diseases were measured. Results showed that the incidences of MM and NMSC were both higher in the pterygium cohort than in the comparison cohort (5.5 vs. 3.2 and 32.3 vs. 15.0 per 100,000 person years, respectively). After adjusting for age, sex, UV index, occupation, and the other comorbidities, pterygium remained a significant predictor of NMSC (hazard ratio [HR], 1.64; 95% confidence interval [CI], 1.11-2.42), but not MM (HR, 1.46; 95% CI, 0.59-3.65). We conclude that pterygium patients are associated with an increased risk of NMSC, but not significant for MM.
Introduction
Pterygium is a proliferative fibrovasculartissue overgrowth arising from bulbar conjunctiva and growing onto the cornea, and has been associated with outdoor jobs.1 Patients aged 20-40 years are at the highest risk of the disease in Singapore2 (latitude 1∘N), while the risk rises to a peak at 54.8 years of age in Hong Kong3 (latitude 22∘N). Epidemiological studies revealed a positive correlation between pterygium and higher ultraviolet (UV) radiation exposure in lower-latitude regions.1
R
ay-tracing analysis revealed that incident light from temporal limbus focus on the nasal limbus, the usual location of the pterygia, with a 20-fold concentration of intensity.4UV radiation is considered the main environmental cause of skin cancers and the associated risk has been increasing over recent years.5 Malignant melanoma (MM) and nonmelanoma skin cancer (NMSC) are the two major groups of skin cancer. NMSC includes heterogenous types of skin cancers, which are mainly composed of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).6Though is less common, MM accounts for three-fourths of all skin cancer deaths.7 Importantly, intermittent and intense sun exposure increases the risk of MM among white population.8 SCC is also significantly associated with cumulative lifelong sun exposure, whereas for BCC, recreational sun exposure during childhood and intense or intermittent exposure might be associated its occurrence.9 Previous studies have found increasing incidence of NMSC in regions of lower latitude, though for MM the results are inconsistent.10
Pterygium and skin cancers are both well-known UV-related human diseases. To the best of our knowledge, no study has examined whether patients with pterygium are at a higher risk of skin cancers. We designed a population-based cohort study on the association between pterygium and skin cancers, utilizing reimbursement database of the Taiwan National Health Insurance (NHI).
Methods Data sources
The Taiwan NHI programme is a single-payer universal insurance system with more than 99% of 23.74 million citizens covered by the end of 2009.11 This insurance programme provides comprehensive medical services, with claims data containing information on diagnoses and medications for ambulatory services and inpatient care. The National Health Research Institutes (NHRI), Department of Health, has established and updated the National Health Insurance Research Database (NHIRD) for public uses. This study used claims data for 1,000,000 insured people randomly selected from all registered population. We used the International Classification of
Disease, Ninth Revision (ICD-9-CM) to define status of diseases, with the Ethics Review Board of China Medical University (CMU-REC-101-012).
Study participants
From the 1997-2010 claims data, we identified patients aged 20 years and older with newly diagnosed pterygium (ICD-9-CM code 372.40-44) as the pterygium cohort. We used the date of pterygium diagnosis as the patient’s index date. Patients with cancer (ICD-9-CM code 140-208), missing information on age or sex were excluded. For each pterygium case, four insured persons without history of pterygium and/or cancer were identified as the comparison cohort, frequency matched by age, sex, and index year. We used mean UV index at places of residence, published by the Taiwan Environmental Protection Administration, to represent UV exposure of the subjects that is compatible with previous methods.12 We calculated the mean UV index of all the administrative areas where subjects resided from 2001 to 2010, the years for which the daily UV index was available. Subjects who live in the offshore islands were excluded due to lack of UV index data. The registered occupations of insured population include public servants, teachers, employees of public or private enterprise, self-employed, farmers, fishers, seamen, veterans, low- income, and religious people. We selected farmers, fishers, and seamen as outdoor occupations.
Criteria and definition
The confirmation of MM (ICD-9-CM code 172) and NMSC (ICD-9-CM code 173) was based on the registry of Catastrophic Illness Patient Database (CIPD), a sub-data set of the NHI research database. Each subject was monitored from the index date until being diagnosed with the cancers, or censored because of loss to follow-up, death, withdrawal from insurance, or the end of 2010. We identified events of MM and NMSC for each cohort. Comorbidities included in our study were senile cataract13 (ICD-9-CM code 366.1), age-related macular degeneration (AMD) (ICD-9-CM code 362.42, 362.43, 362.50, 362.51, 362.52, and 362.57), diabetes mellitus14 (ICD-9-CM code 250), rheumatic disease15 (ICD-9-CM code 446.5, 710.0–710.4, 714.0–714.2, 714.8, 725), parkinsonism16 (ICD-9-CM code 332), solar retinopathy (ICD-9-CM code 363.31), contact dermatitis due to solar radiation (ICD-9-CM code 692.7), photokeratitis (ICD-9-CM code 370.24), pinguecula (ICD-9-CM code 372.51), pingueculitis (ICD-9-CM code 372.34), cancer of lip (ICD-9-CM code 140), cancer of eye (ICD-9-CM code 190.0, 190.3-190.6), toxic effect of arsenic and its compounds17 (ICD-9-CM code 985.1), AIDS/HIV35 (ICD-9-CM code 042-044), organ transplant recipients18 (ICD-9-CM code v42.0-v42.1, v42.6-v42.8), and non-Hodgkin's lymphoma19 (ICD-9-CM code 200, 202-203).
Statistical analysis
Distributions in sociodemographic factors, including age, sex, UV index at places of residence, occupation, and comorbidities were compared between the 2 cohorts.
We categorized ages (≤ 55 and >55 years), UV indexes at places of residence (<6 [low to moderate], and ≥ 6 [high to very high]), and occupation (outdoor jobs vs. non- outdoor). We used Chi-square test to examine categorical variables and t-test to examine the continuous variables. Incidence densities of MM and NMSC by sociodemographic status and comorbidities were calculated. The pterygium to comparison cohort incidence rate ratio (IRR) and 95% confidence interval (CI) were calculated using Poisson regression. Cox proportion hazards regression was used to estimate hazard ratios (HRs) and 95% CIs to assess the risk of cancers. Given that senile cataract, diabetes, and parkinsonism were more prevalent among the study subjects, stratified analysis was performed to evaluate the interaction pattern among these three comorbidities. The disease-free rates were estimated using the Kaplan- Meier method, and the difference between the curves of the two cohorts was examined using the log-rank test. We used the SAS software (version 9.1 for Windows; SAS Institute Inc., Cary, NC) for all statistical analyses and the results were considered significant when two-tailed P-values were less than 0.05. The Kaplan- Meier survival curve was plotted using the R software (version 2.14.1; R Development Core Team, Vienna, Austria).
Results
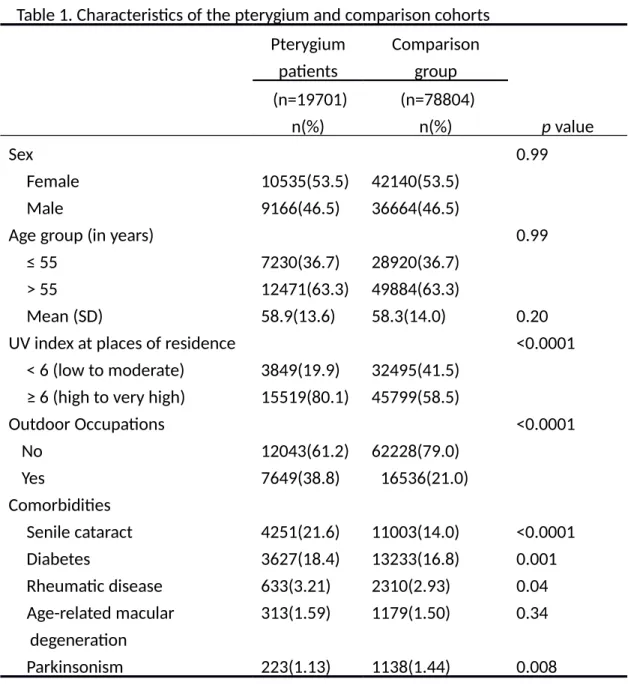
Eligible study subjects consisted of 19701 patients in the pterygium cohort and 78804 persons in the non-pterygium comparison cohort, with similar sex and age distributions (Table 1). The mean age was slightly higher for the pterygium cohort (58.9 vs. 58.3 years old). Pterygium patients were more likely than the comparison cohort to live in areas with higher UV index (80.1% vs. 58.5%, p<0.0001), work outdoor jobs (38.8% vs. 21.0%, p<0.0001), and have comorbidities such as senile cataract (21.6% vs. 14.0%, p<0.0001), diabetes (18.4% vs. 16.8%, p = 0.001) and rheumatic disease (3.21% vs. 2.93%, p = 0.04). However, the comparison cohort was more prevalent with parkinsonism (1.44% vs. 1.13%, p = 0.008). There were only few cases of solar retinopathy, contact dermatitis due to solar radiation, photokeratitis, pinguecula, pingueculitis, cancer of lip, cancer of eye, toxic effect of arsenic and its compounds, AIDS/HIV, organ transplant recipients, and non-Hodgkin's lymphoma.
We therefore did not included in the multivariate analysis. Results of Kaplan-Meier analysis in Figure 1 show that the pterygium cohort had higher incidence of MM and NMSC than the comparison cohort had, with the difference was significant for NMSC, but not for MM.
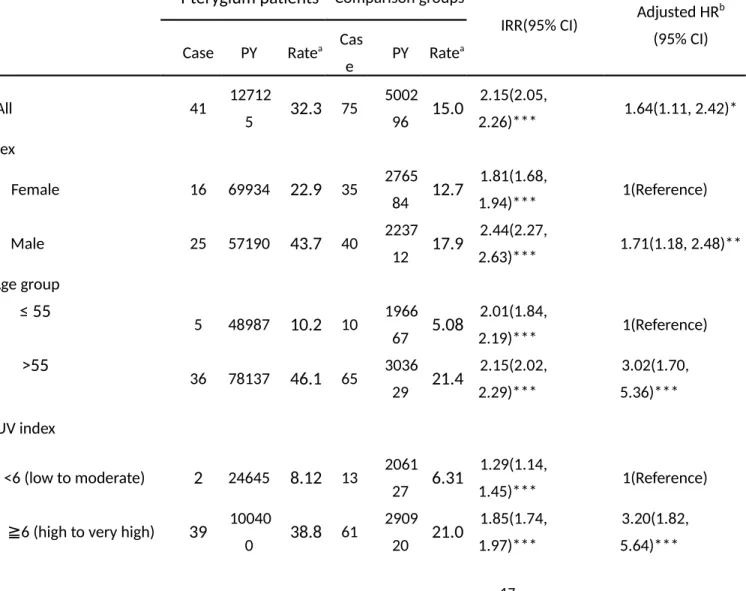
There were few MM cases identified, 7 in the pterygium cohort and 16 in the
comparison cohort. The incidence was 1.72-fold (95% CI = 1.62-1.83) higher in the pterygium cohort (5.50 vs. 3.20 per 100,000 person-years), but the adjusted HR was not significant (Table 2). The sex-specific incidence rate of MM was higher in men than in women in both cohorts, with an adjusted HR of 3.71 (95% CI = 1.46-9.47) for men comparing with women. The age-specific incidence rate of MM increased with age in both cohorts with a higher IRR for the younger group. However, the adjusted risk of MM was significantly higher for the older group compared with the younger group (HR = 3.65, 95% CI = 1.02-12.8). Pterygium patients with outdoor jobs had no significant risk of MM in the Cox model analysis. Rheumatic disease, diabetes and senile cataract had no -significant association with MM as well for the pterygium cohort.
Table 3 demonstrates the incidences of NMSC in both cohorts, pterygium-to- non-pterygium rate ratios, and HR of NMSC by sociodemographic status and comorbidities. The incidence of NMSC was 2.15-fold greater in the pterygium cohort than non-pterygium cohort (32.3 vs. 15.0 per 100,000 person-years), with an adjusted HR of 1.64 (95% CI = 1.11-2.42). The sex-specific IRR of NMSC in the pterygium cohort was higher in men than in women (IRR = 2.44, 95% CI = 2.27-2.63 vs. IRR =1 .81, 95% CI = 1.68-1.94), with an adjusted HR of 1.71 (95% CI = 1.18-2.48) for men compared with women. The age-specific incidence rate of NMSC increased with age in both cohorts. The adjusted HR of NMSC was 3.02 (95% CI = 1.70-5.36) in the older group. The IRR of NMSC was higher for those living in areas of higher UV index, with an adjusted HR of 3.20 (95% CI = 1.82-5.64) for higher UV exposure.
Pterygium patients that worked outdoors comparing to the non-outdoor workers were at a higher risk of having NMSC, but not significant. The incidence rates of NMSC increased in those with comorbidities in both cohorts. Among the comorbidities, the HRs of NMSC were significant for those with senile cataract (adjusted HR = 1.92, 95% CI = 1.25-2.94) and parkinsonism (adjusted HR = 3.97, 95%
CI = 1.83-8.60).
Stratified analysis measuring the interaction of senile cataract, diabetes and parkinsonism failed to show any contribution to the risk of MM (data not shown).
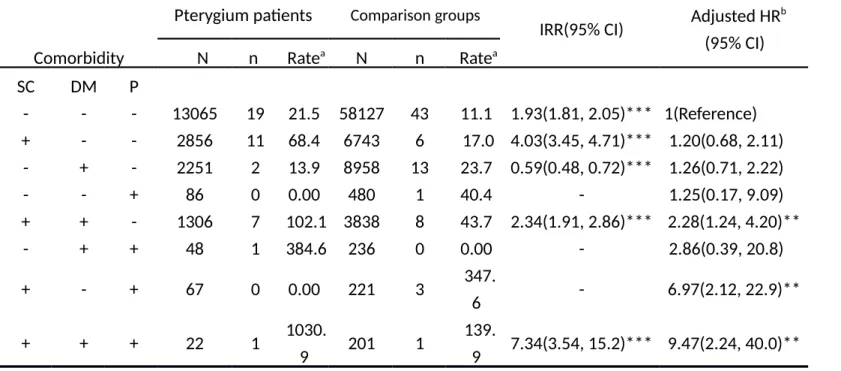
Table 4 shows that among the subjects without any of these comorbidities, the risk of NMSC was 1.93-fold higher in the pterygium cohort than in the comparison cohort (21.5 vs. 11.1 per 100,000 person-years). The multivariate Cox model analysis showed that these comorbidities increased the risk with a high adjusted HR of 9.47 (95% CI = 2.24-40.0) when subjects had all three comorbidities, but only one case of the cancer in each cohort.
Discussion
Our study demonstrated that the risk of NMSC was higher in patients with pterygium than in the comparison cohort. After adjusting for age, sex, UV index at places of residence, occupation and comorbidities, the risk of NMSC remained significant in the pterygium cohort. To the best of our knowledge, this is the first population-based cohort study to find a positive association between pterygium and NMSC, although the association was found to be moderate.
The patients with pterygium in our cohort tended to live in areas with higher UV index and worked outdoors. These results are compatible with previous reports. The prevalence and risk of pterygium are higher in those who worked outdoors and exposed to high UV radiation.1 Threlfall et al20 found that pterygium was strongly associated with sun exposure in a case-control study. However, the association between outdoor occupation and the risk of skin cancer was not significant in our hazard analysis. Due to limited classification in the registry, we could only select farmers, fishers, and seamen as outdoor workers, which represent only a part of population with high occupational UV exposure. In addition to the ambient UV radiation levels, measurements of personal outdoor activity and anatomical distribution are required for an accurate estimation of lifetime UV exposure, but this information is unavailable in the claims data.
The risk of MM and NMSC were higher in the male subjects of both cohorts.
Males are known to have higher incidences of BCC,21 SCC,22 and MM.23 It is worth noting that living in regions of higher UV index leaded to higher risk of developing NMSC but not MM in our cohorts. In a descending order of incidence, superficial spreading melanoma (SSM), nodular melanoma (NM), acral lentiginous melanoma (ALM), and lentigo maligna melanoma (LMM) are the four principle types of melanoma in white populations. In contrary, ALM comprises more than 50% of the melanoma cases in Taiwan.24 ALM usually occurs on areas that are not exposed to the sun such as palms, soles, and subungual sites, is generally not a result of UV radiation. This may explain the lack of association between high UV index and MM in our study.
Our study revealed a significant association between senile cataract and NMSC . No published study has ever evaluated this relationship. Since UV radiation is the common etiology of these two diseases, the positive association between senile cataract and NMSC is possible an indirect association due to the relation between UV and cataract.26We also found that parkinsonism was associated with higher risk of NMSC. Olsen et al25 have observed a 25% increased risk of NMSC in patients with PD in a large cohort study.
In the analysis of interaction between comorbidities, we noted that pterygium patients with coexisting senile cataract, DM and parkinsonism had 9.47-fold higher
hazard of NMSC than those without these comorbidities. However, this result should be interpreted with caution because there was only one case of NMSC in each cohort.
The findings of this study faced the following limitations. First, the disease definition was based solely on the diagnosis in the claims. However, the misdiagnosis of cancer is not likely. Under the Taiwan NHI system, applying for certification of catastrophic illness with cancer requires histological and/or cytological confirmation.
Second, the ICD-9-CM coding of MM mixes all subtypes of the cancer. Grouping ALM with other UV-related subtypes would introduce random misclassification bias. The estimated HR of MM associated with pterygium would be towards the null. Third, different types of NMSC cannot be determined based on the ICD-9-CM coding in the claims registry. Finally, we could only approximate the lifetime UV exposure with UV index of the residential areas. Despite the limitation, this is the first large cohort study for investigating relationship between pterygium and skin cancers using nationwide UV index data. Our results of analysis on UV index concurred with the findings of previous studies.12
In conclusion, this retrospective population-based cohort study demonstrated that patients with pterygium are 1.64-fold more likely to develop NMSC than those without pterygium, after adjusting for age, sex, UV exposure in the living area, occupation, and other comorbidities. Pterygium patients should be reminded to keep a high index of suspicion with skin lesions.
Acknowledgments
The study was supported partly by the National Sciences Council, Executive Yuan, Taiwan (Grant Numbers NSC 100-2621-M-039-001), China Medical University Hospital (Grant Number 1MS1), and Taiwan Department of Health Clinical Trial and Research Center for Excellence (Grant Numbers DOH102-TD-B-111-004 and
DOH102-TD-C111-005).
Authors' role
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported. Contributions of authors:
Involved in conception and design of study (H.C.Y., I.J.W.); data collection (H.C.Y., C.L.L.); analysis and interpretation of data (C.L.L., F.R.H., F.C.S.); provision of materials, patients, or resources (H.C.Y., C.L.L.); statistical expertise (C.L.L., F.C.S.);
literature search (H.C.Y., I.J.W.); administrative, technical, or logistical support (H.C.Y., F.R.H., I.J.W.); preparation of manuscript (H.C.Y., C.L.L., F.C.S., I.J.W.); review and final approval of the article (H.C.Y., C.L.L., F.R.H., F.C.S., I.J.W.).
References
1. Taylor HR. Aetiology of climatic droplet keratopathy and pterygium. Br J Ophthalmol 1980;64(3):154-63.
2. Saw SM, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol 1999;6(3):219-28.
3. Karukonda SR, Thompson HW, Beuerman RW, et al. Cell cycle kinetics in pterygium at three latitudes. Br J Ophthalmol 1995;79(4):313-7.
4. Coroneo MT, Muller-Stolzenburg NW, Ho A. Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic Surg 1991;22(12):705-11.
5. Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 2010;146(3):283-7.
6. Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet 2010;375(9715):673-85.
7. Hall HI, Miller DR, Rogers JD, Bewerse B. Update on the incidence and mortality from melanoma in the United States. J Am Acad Dermatol 1999;40(1):35-42.
8. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer 1997;73(2):198-203.
9. Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol 1995;131(2):157-63.
10. Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B 2001;63(1-3):8-18.
11. Chen TM. Taiwan's Natinal Health Insurance System: High Value for the Dollar. In: Okma KGH, ed. Six countries, six reform models : the healthcare reform experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan : healthcare reforms "under the radar screen". Hackensack, N.J.: World Scientific, 2010.
12. Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations--US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol 2005;141(4):477-81.
13. Lim R, Mitchell P, Cumming RG. Cataract associations with pinguecula and pterygium: the Blue Mountains Eye Study. Am J Ophthalmol 1998;126(5):717-9.
14. Chuang TY, Lewis DA, Spandau DF. Decreased incidence of nonmelanoma skin cancer in patients with type 2 diabetes mellitus using insulin: a pilot study. Br J Dermatol 2005;153(3):552-7.
15. Ji J, Liu X, Sundquist K, et al. Cancer risk in patients hospitalized with polymyalgia rheumatica and giant cell arteritis: a follow-up study in Sweden. Rheumatology (Oxford) 2010;49(6):1158-63.
16. Bertoni JM, Arlette JP, Fernandez HH, et al. Increased melanoma risk in Parkinson disease: a prospective clinicopathological study. Arch Neurol 2010;67(3):347-52.
17. Lin W, Wang SL, Wu HJ, et al. Associations between arsenic in drinking water and pterygium in southwestern Taiwan. Environ Health Perspect 2008;116(7):952-5.
18. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370(9581):59-67.
19. Hu S, Federman DG, Ma F, Kirsner RS. Skin cancer and non-Hodgkin's lymphoma: examining the link. Dermatol Surg 2005;31(1):76- 82.
20. Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol 1999;128(3):280-7.
21. Lovatt TJ, Lear JT, Bastrilles J, et al. Associations between ultraviolet radiation, basal cell carcinoma site and histology, host characteristics, and rate of development of further tumors. J Am Acad Dermatol 2005;52(3 Pt 1):468-73.
22. Chuang TY, Popescu NA, Su WP, Chute CG. Squamous cell carcinoma. A population-based incidence study in Rochester, Minn. Arch Dermatol 1990;126(2):185-8.
23. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). Bethesda, MD:
National Cancer Institute, 2012.
24. Chang JW. Cutaneous melanoma: Taiwan experience and literature review. Chang Gung Med J 2010;33(6):602-12.
25. Olsen JH, Friis S, Frederiksen K, et al. Atypical cancer pattern in patients with Parkinson's disease. Br J Cancer 2005;92(1):201-5.
26. Saadat, Farvardin-Jahromi M. Occupational sunlight exposure, polymorphism of glutathione S-transferase M1, and senile cataract risk.
Occup Environ Med 2006;63:503-4.
gure legend
Figure 1. Probability free of melanoma (A), and nonmelanoma skin cancer (B) for pterygium and comparison cohorts.
Table 1. Characteristics of the pterygium and comparison cohorts Pterygium
patients
Comparison group (n=19701)
n(%)
(n=78804)
n(%) p value
Sex 0.99
Female 10535(53.5) 42140(53.5)
Male 9166(46.5) 36664(46.5)
Age group (in years) 0.99
≤ 55 7230(36.7) 28920(36.7)
> 55 12471(63.3) 49884(63.3)
Mean (SD) 58.9(13.6) 58.3(14.0) 0.20
UV index at places of residence <0.0001
< 6 (low to moderate) 3849(19.9) 32495(41.5)
≥ 6 (high to very high) 15519(80.1) 45799(58.5)
Outdoor Occupations <0.0001
No 12043(61.2) 62228(79.0)
Yes 7649(38.8) 16536(21.0)
Comorbidities
Senile cataract 4251(21.6) 11003(14.0) <0.0001
Diabetes 3627(18.4) 13233(16.8) 0.001
Rheumatic disease 633(3.21) 2310(2.93) 0.04
Age-related macular degeneration
313(1.59) 1179(1.50) 0.34
Parkinsonism 223(1.13) 1138(1.44) 0.008
Table 2. Cox model measured adjusted hazard ratios of melanoma for pterygium and comparison cohorts
Pterygium patients Comparison groups
IRR(95% CI) Adjusted HRb (95% CI) Case PY Ratea Case PY Rat
ea
All 7 127185 5.50 16 50053
2
3.2
0 1.72(1.62, 1.83) 1.46(0.59, 3.65) Sex
Female 2 69968 2.86 4 27669
5
1.4
5 1.98(1.82, 2.15) 1.00(Reference)
Male 5 57217 8.74 12 22383
7
5.3
6 1.63(1.50, 1.77) 3.71(1.46, 9.47) Age group
≤ 55 2 49992 4.00 1 19670
3
0.5
1 8.03(7.29, 8.85) 1.00(Reference)
>55
5 78193 6.39 15 30382 8
4.9
4 1.30(1.20, 1.40) 3.62(1.02, 12.8) UV index
<6 (low to moderate) 1 24640 4.06 4 20616 3
1.9
4 2.09(1.87, 2.34) 1.00(Reference)
≧6 (high to very high) 6 100466 5.97 12 29111 3
4.1
2 1.45( .35, 1.56) 1.75(0.61, 5.05)
Outdoor Occupations
No 6 76369 7.86 8 39211
3
2.0
4 3.85(3.62, 4.10) 1.00(Reference)
Yes 1 50753 1.97 8 10813
3
7.4
0 0.27(0.23, 0.32) 1.26(0.51, 3.13) Comorbidities
Senile cataract
No 6 103767 5.78 12 44520
1
2.7
0 2.15(2.02, 2.28) 1.00(Reference)
Yes 1 23419 4.27 4 55331 7.2
3 0.59(0.50, 0.70) 1.17(0.42, 3.29) Diabetes
No 5 105578 4.74 12 42546
6
2.8
2 1.68(1.57, 1.79) 1.00(Reference)
Yes 2 21608 9.26 4 75066 5.3
3 1.74(1.52, 1.99) 1.48(0.57, 3.84) Rheumatic disease
No 6 124005 4.84 16 48919
5
3.2
7 1.48(1.39, 1.57) 1.00(Reference)
Yes 1 3181 31.4 0 11337 0.0
0 - 1.74(0.23, 13.1)
Age-related
macular degeneration
No 7 125593 5.57 16 49529
8
3.2
3 1.73(1.63, 1.83) -
Yes 0 1592 - 0 5234 - - -
Parkinsonism
No 7 125963 5.56 16 49535
4
3.2
3 1.72(1.62, 1.83) -
Yes 0 1223 - 0 5178 - - -
PY = person-years; CI = confidence interval; HR = hazard ratio; IRR = incidence rate ratio.
aRate: incidence rate, per 100,000 person-years.
bAdjusted HR: multivariate analysis including age, sex, UV index at places of residence, occupation, senile cataract, diabetes, and rheumatic disease.
*p<0.05, **p<0.01, ***p<0.001.
Table 3. Cox model measured adjusted hazard ratios of nonmelanoma for pterygium and comparison cohorts
Pterygium patients Comparison groups
IRR(95% CI) Adjusted HRb (95% CI) Case PY Ratea Cas
e PY Ratea
All 41 12712
5 32.3 75 5002
96 15.0 2.15(2.05,
2.26)*** 1.64(1.11, 2.42)*
Sex
Female 16 69934 22.9 35 2765
84 12.7 1.81(1.68,
1.94)*** 1(Reference)
Male 25 57190 43.7 40 2237
12 17.9 2.44(2.27,
2.63)*** 1.71(1.18, 2.48)**
Age group
≤ 55 5 48987 10.2 10 1966
67 5.08 2.01(1.84,
2.19)*** 1(Reference)
>55
36 78137 46.1 65 3036
29 21.4 2.15(2.02, 2.29)***
3.02(1.70, 5.36)***
UV index
<6 (low to moderate) 2 24645 8.12 13 2061
27 6.31 1.29(1.14,
1.45)*** 1(Reference)
≧6 (high to very high) 39 10040
0 38.8 61 2909
20 21.0 1.85(1.74, 1.97)***
3.20(1.82, 5.64)***
Outdoor Occupations
No 18 76356 23.6 48 3919
57 12.3 1.93(1.81,
2.05)*** 1(Reference)
Yes 23 50705 45.4 27 1080
53 25.0 1.82(1.65,
2.00)*** 1.17(0.79, 1.74) Comorbidities
Senile cataract
No 22 10374
8 21.2 57 4450
03 12.8 1.66(1.56,
1.76)*** 1(Reference)
Yes 19 23376 81.3 18 5529
3 32.6 2.50(2.22,
2.81)*** 1.92(1.25, 2.94)**
Diabetes
No 30 10553
7 28.4 53 4252
79 12.5 2.28(2.16,
2.41)*** 1(Reference)
Yes 11 21587 51.0 22 7501
7 29.3 1.74(1.54,
1.96)*** 1.48(0.97, 2.25) Rheumatic disease
No 38 12395
2 30.7 75 4889
59 15.3 2.00(1.90,
2.11)*** 1(Reference)
Yes 3 3173 94.6 0 1133
7 0.00 - 0.91(0.29, 2.87)
Age-related
macular degeneration
No 39 12553
8 31.1 73 4950
63 14.8 2.11(2.00,
2.22)*** 1(Reference)
Yes 2 1587 126.
0 2 5233 38.2 3.29(2.29,
4.75)*** 1.74(0.62, 4.83) Parkinsonism
No 39 12590
7 31.0 70 4951
32 14.1 2.19(3.08,
2.31)*** 1(Reference)
Yes 2 1218 164.
2 5 5164 96.8 1.70(1.11, 2.58)* 3.97(1.83, 8.60)***
PY = person-years; CI = confidence interval; HR = hazard ratio; IRR = incidence rate ratio.
aRate, incidence rate, per 100,000 person-years.
bAdjusted HR, multivariate analysis including age, sex, UV index at places of residence, occupation, senile cataract, diabetes, and rheumatic disease.
*p<0.05, **p<0.01, ***p<0.001.
Table 4. Risk of nonmelanoma skin cancer in patients with pterygium combined with senile cataract, diabetes, or parkinsonism
Pterygium patients Comparison groups
IRR(95% CI) Adjusted HRb (95% CI)
Comorbidity N n Ratea N n Ratea
SC DM P
- - - 13065 19 21.5 58127 43 11.1 1.93(1.81, 2.05)*** 1(Reference) + - - 2856 11 68.4 6743 6 17.0 4.03(3.45, 4.71)*** 1.20(0.68, 2.11)
- + - 2251 2 13.9 8958 13 23.7 0.59(0.48, 0.72)*** 1.26(0.71, 2.22)
- - + 86 0 0.00 480 1 40.4 - 1.25(0.17, 9.09)
+ + - 1306 7 102.1 3838 8 43.7 2.34(1.91, 2.86)*** 2.28(1.24, 4.20)**
- + + 48 1 384.6 236 0 0.00 - 2.86(0.39, 20.8)
+ - + 67 0 0.00 221 3 347.
6 - 6.97(2.12, 22.9)**
+ + + 22 1 1030.
9 201 1 139.
9 7.34(3.54, 15.2)*** 9.47(2.24, 40.0)**
N, sample size; n, events of nonmelanoma.
IRR = incidence rate ratio; CI = confidence interval; HR = hazard ratio; SC = senile cataract; DM = diabetes mellitus; P = parkinsonism.
aRate, incidence rate, per 100,000 person-years.
bAdjusted HR (95% CI), Cox model measured hazard ratios and 95% confidence intervals in multivariate analysis including age, sex, UV index at places of residence, occupation and comorbidities (rheumatic disease and age-related macular degeneration)
*p<0.05, **p<0.01, ***p<0.001