國立成功大學「邁向頂尖大學計畫」
延攬優秀人才工作報告表
NCKU’s “Aim for the Top University Project”
Work Report Form for Distinguished Scholars
□續聘
continuation of employment■離職
resignation100 年 7 月 13 日更新 受聘者姓名
Name of the Employee
許晉源
■男 女Male Female
聘 期 Period of Employment
from 106 年(y) 01 月(m) 01 日(d) to 106 年(y) 07 月(m) 31 日(d) 研究或教學或科技研發與
管理計畫名稱 The project title of research,
teaching, technology development and management
表皮生長因子誘導血管生成素樣
蛋白 4 表現對頭頸癌細胞轉移
所扮演的角色
計畫主持人
(申請單位主管)
Project Investigator (Head of Department/Center)
陳炳焜
補助延聘編號
Grant Number
HUA 106 – 3 - 22 - 044
一、研究、教學、科技研發與管理工作全程經過概述。(由受聘人填寫)Please summarize the entire research, teaching, or science and technology R&D and management work process (To be completed by the employee)
Background and significance
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world (1). Although there has been much research on the treatment of HNSCC, survival rates have been slowly improving over the last 30 years (1, 2). Over 50% of newly diagnosed patients do not achieve complete remission, and nearly 10% are recurrent cases with metastasis to distant organs (3). Therefore, studies that provide a deeper understanding of HNSCC are required to develop effective therapeutic strategies.
Previous studies show that more than 95% of HNSCCs have increased epidermal growth factor receptor (EGFR) mRNA levels compared with normal tissues (4, 5). As EGFR is overexpressed in many human tumor types and is associated with poor prognosis and decreased survival (6), activation of the EGFR signaling pathway or expression of EGFR family members could be a major factor in tumor metastasis (7, 8). Angiopoietin-like protein 4 (ANGPTL4) is a secretary protein that is cleaved into an N-terminal coiled-coil fragment (N-ANGPTL4) and a C-terminal fibrinogen-like domain (C-ANGPTL4) that modulates the disposition of circulating triglycerides (9). Although the role of ANGPTL4 in lipid metabolism has been well characterized, its role in cancer progression is poorly understood. Although recent studies suggest that ANGPTL4 is involved in cancer progression, the precise role of ANGPTL4 in angiogenesis and cancer progression is still debated. For example, ANGPTL4 has been reported to have proangiogenic effects and antiangiogenic effects in different models (10-12). Moreover, ANGPTL4 mediates TGFβ–induced lung metastasis of breast cancer (13) but inhibits metastasis of melanoma cells (14). These conflicting data support the need of additional research to address the role of ANGPTL4 in cancer progression. In our previous studies we found that EGF-induced angiopoietin-like 4 (ANGPTL4) was involved in tumor metastasis (15). Notably, the mechanisms of EGF-induced ANGPTL4 and the functional roles of ANGPTL4 in HNSCC metastasis remain unclear. Previous studies showed that ANGPTL4 induction by prostaglandin E2 (PGE2) under hypoxic conditions promotes colorectal cancer progression (16). ANGPTL4 also promotes oral squamous cell carcinoma metastasis by stimulating cell invasion (17). In addition, ANGPTL4 induced by TGFβ via the Smad signalling pathway promotes breast cancer metastasis (13). It is unclear from previous studies whether ANGPTL4 expression is associated with PGE2-enhanced HNSCC metastasis. Thus, ANGPTL4 inhibition provides a new strategy for the treatment of EGFR-mediated HNSCC metastasis.
PGE2-induced ANGPTL4 expression regulates HNSCC invasion
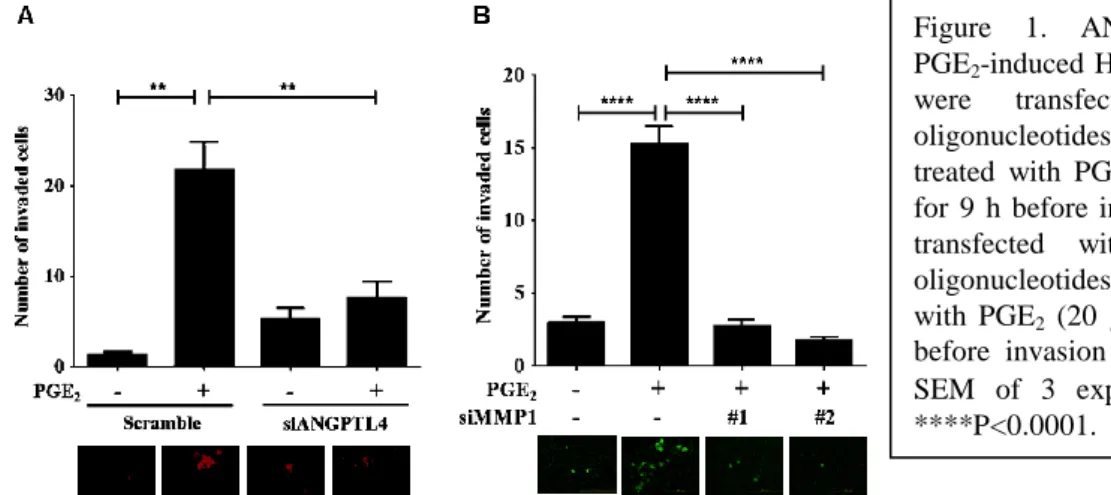
In our previous studies we found that EGF-induced ANGPTL4 was involved in tumor metastasis (15). However the mechanisms of EGF-induced ANGPTL4 and the functional roles of ANGPTL4 in HNSCC metastasis remain unclear. In addition, the expression of COX-2 was also induced in cells treated with EGF (18). Furthermore, we found that either inhibition of COX-2 activity using celecoxib or knockdown of COX-2 in cells inhibited EGF-induced ANGPTL4 expression PGE2 significantly induced the expression of ANGPTL4 mRNA and protein in time-dependent manners. However, the functional roles of ANGPTL4 regulated by COX-2 in HNSCC metastasis was unclear. Therefore, we performed invasion and transendothelial invasion assays to examine whether ANGPTL4 was involved in PGE2-induced FaDu cells invasion. Inhibition of ANGPTL4 gene expression using ANGPTL4 siRNA, dramatically repressed PGE2-stimulated cell transendothelial invasion (Fig. 1A). Furthermore, transendothelial invasion assays also confirmed the function of PGE2-induced MMP1 expression in the regulation of HNSCC cells to invade vessels (Fig. 1B). These results suggest that induction of ANGPTL4 and MMP1 are essential for PGE2-induced HNSCC invasion.
PGE2-induced ANGPTL4 expression promotes the expression of MMPs and EMT markers
To clarify the mechanisms involved in the regulation of tumor invasion by PGE2-induced ANGPTL4 expression, we examined MMPs in cells treated with PGE2. Down-regulation of ANGPTL4 significantly inhibited EGF-induced MMP-1, MMP-2, MMP-3 and MMP-9 mRNA expression (Fig. 2A). These results reveal that the induction of ANGPTL4 expression is essential for PGE2-induced MMP-1, MMP-2, MMP-3 and MMP-9 expression. We next examined the effect of PGE2-induced ANGPTL4 on the expression of EMT markers such as fibronectin, snail, slug, twist, vimentin, E-cadherin and N-cadherin. The results showed that PGE2-induced the expression of fibronectin, snail, slug, twist, vimentin, E-cadherin and N-cadherin were inhibited in ANGPTL4 knockdown cells (Fig.
2B-I). These results suggest that PGE2-induced the MMPs and EMT markers expression were dependent on the induction of ANGPTL4.
Figure 1. ANGPTL4 and MMP1 regulates PGE2-induced HNSCC cell invasion. A) FaDu cells were transfected with ANGPTL4 siRNA oligonucleotides (siANGPTL4) for 24 h, then treated with PGE2 (20 μM) in serum-free medium for 9 h before invasion assays. C) FaDu cells were transfected with 2 different MMP1 siRNA oligonucleotides (siMMP1) for 24 h, then treated with PGE2 (20 μM) in serum-free medium for 9 h before invasion assays. Values represent means ± SEM of 3 experiments. **P<0.01, ***P<0.001,
****P<0.0001.
PGE2-induced ANGPTL4 expression promotes tumor invasion in vivo
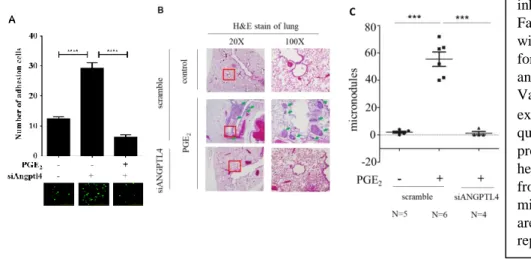
Distant metastasis relies on tumor cell attachment to blood vessels (19). We further studied the possibility that metastatic processes enhanced by PGE2-induced ANGPTL4 expression rely on the interaction between tumor and endothelial cells. The interaction between tumor and HMEC-1 cells was enhanced in tumor cells pretreated with EGF (Fig. 3A), and PGE2-induced cell-cell interaction was inhibited in ANGPTL4 knockdown cells (Fig. 3A). To further evaluate the effect of ANGPTL4 on PGE2-induced metastasis in vivo, the distant dissemination (e.g., pulmonary colonization) of tumor cells was studied in mice. Briefly, parental and siANGPTL4 cells were pretreated with PGE2 for 9 h and then injected into the tail vein of mice. No obvious lung nodules were detected when ANGPTL4 was depleted in the HNSCC cells, whereas the PGE2-treated parental tumor cells developed significant nodules (Fig. 3B and C). Hematoxylin and eosin (H&E) staining revealed that the lungs of mice receiving the PGE2-treated parental tumor cells contained significantly more and larger micrometastatic colonies than those receiving the siANGPTL4 cells (Fig, 3B and C). These results suggest that ANGPTL4 is essential for PGE2-primed HNSCC metastasis.
Hypoxia-inducible factors (HIFs) mediate adaptive physiological responses to hypoxia (20). In human cancers that are accessible for O2 electrode measurements, intratumoral hypoxia is common and severe hypoxia is associated with increased risk of mortality (21). HIF activity in regions of intratumoral hypoxia mediates angiogenesis, epithelial-mesenchymal transition, stem-cell maintenance, invasion, metastasis, and resistance to radiation therapy and chemotherapy (21). HIFs play key roles in many crucial aspects of cancer biology including angiogenesis (22-24), stem cell maintenance (25-27), metabolic reprogramming (28, 29), autocrine growth factor signaling (30, 31), epithelial-mesenchymal transition (32-34), invasion (35, 36), metastasis (37-39), and resistance to radiation therapy (40) and chemotherapy (41). HIF-1 activates transcription of gene encoding secreted ANGPTL4 proteins that promote extravasation of cancer cells into the parenchyma at metastatic sites such as the lung (39). HIF-1 also actively suppresses mitochondrial oxidative metabolism by increasing the expression of pyruvate dehydrogenase kinase 1 (PDK1), which phosphorylates and inactivates pyruvate dehydrogenase, the enzyme that converts pyruvate to acetyl-CoA for entry into the TCA cycle (42). Thus, it is unclear whether ANGPTL4 and PDK1 expression is associated with EGF-enhanced HNSCC metastasis.
EGF-induced PDK1 expression promotes tumor invasion in vivo
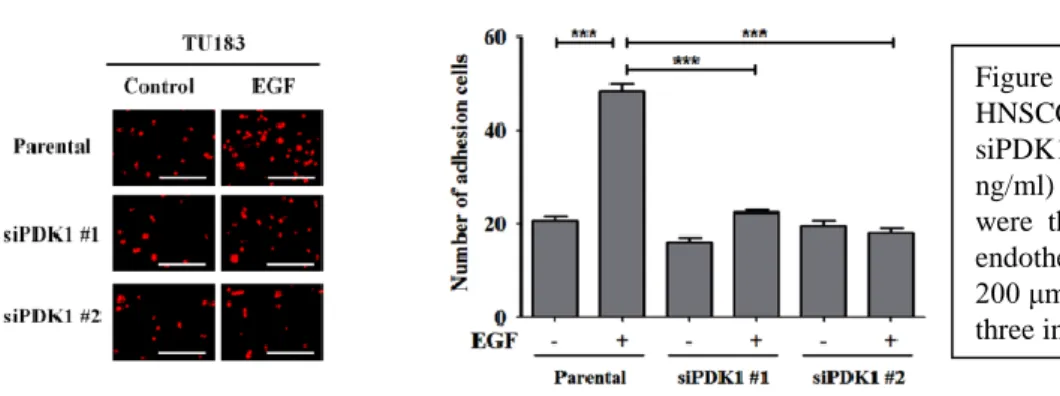
Distant metastasis relies on tumor cell attachment to blood vessels (19). We further studied the possibility that metastatic processes enhanced by EGF-induced PDK1 expression rely on the interaction between tumor and endothelial cells. The interaction between tumor and HMEC-1 cells was enhanced in tumor cells pretreated with EGF (Fig. 4A and Fig. 5), and EGF-induced cell-cell interaction was inhibited in PDK1 knockdown cells (Fig. 4A and Fig. 5). To further evaluate the effect of PDK1 on EGF-induced metastasis in vivo, the distant dissemination (e.g., pulmonary colonization) of tumor cells was studied in mice. Briefly, parental and siPDK1 cells were pretreated with EGF for 3 h and then injected into the tail vein of mice. No obvious lung nodules were detected when PDK1 was depleted in the HNSCC cells, whereas the EGF-treated parental tumor cells developed significant nodules (Fig. 4B and C). Hematoxylin and eosin (H&E) staining revealed that the lungs of mice receiving the EGF-treated parental tumor cells contained significantly more and larger micrometastatic colonies than those receiving the siPDK1 cells (Fig, 4B and C). These results suggest that PDK1 is essential for EGF-primed HNSCC metastasis.
Figure 3. EGF-primed HNSCC metastasis is inhibited in PDK1 knockdown cells. A) FaDu and siANGPTL4 cells were pretreated with PGE2 (20 μM) in serum-free medium for 9 h. Cells were then labeled with DiO and cultured with endothelial cells for 3 h.
Values represent means ± SEM of 3 experiments. B, C) Colonies in lungs were quantified at 2 months and the average is presented. Quantitation of the number of hematoxylin and eosin–stained lung nodules from SCID mice was performed under a microscope. A magnified view of the boxed area is shown below each image. Values represent means ± SEM. ***P , 0.001.
Conlusion
In this study, we reveal for the first time that PGE2-induced ANGPTL4 expression resulting in HNSCC metastasis. Furthermore, EGF-induced PDK1 expression enhances HNSCC metastasis via activation of the fibronectin signaling pathway. Thus, ANGPTL4 or PDK1 inhibition represents a new strategy for the treatment of EGFR-mediated HNSCC metastasis. But the correlation between ANGPTL4 and PDK1 still need to be improved.
References:
1. Leemans, C. R., Braakhuis, B. J., and Brakenhoff, R. H. (2011) The molecular biology of head and neck cancer. Nat Rev Cancer 11, 9-22
2. Warnakulasuriya, S. (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45, 309-316
3. van Houten, V. M., van den Brekel, M. W., Denkers, F., Colnot, D. R., Westerga, J., van Diest, P. J., Snow, G. B., and Brakenhoff, R. H. (2000) Molecular diagnosis of head and neck cancer. Recent Results Cancer Res 157, 90-106
4. Grandis, J. R., and Tweardy, D. J. (1993) Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 53, 3579-3584
5. Ishitoya, J., Toriyama, M., Oguchi, N., Kitamura, K., Ohshima, M., Asano, K., and Yamamoto, T. (1989) Gene amplification and overexpression of EGF receptor in squamous cell carcinomas of the head and neck. Br J Cancer 59, 559-562
6. Salomon, D. S., Brandt, R., Ciardiello, F., and Normanno, N. (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19, 183-232
7. Huang, M., Anand, S., Murphy, E. A., Desgrosellier, J. S., Stupack, D. G., Shattil, S. J., Schlaepfer, D. D., and Cheresh, D.
A. EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene 31, 2783-2793
8. Normanno, N., Tejpar, S., Morgillo, F., De Luca, A., Van Cutsem, E., and Ciardiello, F. (2009) Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 6, 519-527
9. Lee, E. C., Desai, U., Gololobov, G., Hong, S., Feng, X., Yu, X. C., Gay, J., Wilganowski, N., Gao, C., Du, L. L., Chen, J., Hu, Y., Zhao, S., Kirkpatrick, L., Schneider, M., Zambrowicz, B. P., Landes, G., Powell, D. R., and Sonnenburg, W. K.
(2009) Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J Biol Chem 284, 13735-13745
10. Ma, T., Jham, B. C., Hu, J., Friedman, E. R., Basile, J. R., Molinolo, A., Sodhi, A., and Montaner, S. (2010) Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi's sarcoma. Proc Natl Acad Sci U S A 107, 14363-14368
11. Le Jan, S., Amy, C., Cazes, A., Monnot, C., Lamande, N., Favier, J., Philippe, J., Sibony, M., Gasc, J. M., Corvol, P., and Germain, S. (2003) Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol 162, 1521-1528
Figure 4. EGF-primed HNSCC metastasis is inhibited in PDK1 knockdown cells. (A) FaDu and siPDK1 cells were pre-treated with EGF (50 ng/ml) in serum-free medium for 3 h. Cells were then labeled with DiI and cultured with endothelial cells for 3 h.
Scale bar represents 200 μm. Values represent the means ± S.E.M. of three experiments. (B) and (C) Colonies in the lungs were quantified at 2 months, and the average is presented. Quantitation of the number of H&E-stained lung nodules from the SCID mice was performed under a microscope. A magnified view of the boxed area is shown below each image. Values represent the means ± S.E.M. **P < 0.01, ***P < 0.001.
Figure 5. PDK1 is essential for EGF-induced HNSCC-endothelial cell interaction. TU183 and siPDK1 cells were pre-treated with EGF (50 ng/ml) in serum-free medium for 3 h. The cells were then labeled with DiI and cultured with endothelial cells for 3 h. Scale bar represents 200 μm. Values represent the means ± S.E.M. of three independent experiments. ***P < 0.001.
16. Kim, S. H., Park, Y. Y., Kim, S. W., Lee, J. S., Wang, D., and DuBois, R. N. (2011) ANGPTL4 induction by prostaglandin E2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res 71, 7010-7020
17. Tanaka, J., Irie, T., Yamamoto, G., Yasuhara, R., Isobe, T., Hokazono, C., Tachikawa, T., Kohno, Y., and Mishima, K.
(2015) ANGPTL4 regulates the metastatic potential of oral squamous cell carcinoma. J Oral Pathol Med 44, 126-133 18. Hsu, J. Y., Chang, K. Y., Chen, S. H., Lee, C. T., Chang, S. T., Cheng, H. C., Chang, W. C., and Chen, B. K. (2015)
Epidermal growth factor-induced cyclooxygenase-2 enhances head and neck squamous cell carcinoma metastasis through fibronectin up-regulation. Oncotarget 6, 1723-1739
19. Albini, A., Tosetti, F., Li, V. W., Noonan, D. M., and Li, W. W. (2012) Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol 9, 498-509
20. Semenza, G. L. (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24, 97-106 21. Semenza, G. L. (2012) Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends
Pharmacol Sci 33, 207-214
22. Liao, D., and Johnson, R. S. (2007) Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev 26, 281-290 23. Lee, K., Qian, D. Z., Rey, S., Wei, H., Liu, J. O., and Semenza, G. L. (2009) Anthracycline chemotherapy inhibits HIF-1
transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci U S A 106, 2353-2358
24. Lee, K., Zhang, H., Qian, D. Z., Rey, S., Liu, J. O., and Semenza, G. L. (2009) Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A 106, 17910-17915
25. Barnhart, B. C., and Simon, M. C. (2007) Metastasis and stem cell pathways. Cancer Metastasis Rev 26, 261-271
26. Suda, T., Takubo, K., and Semenza, G. L. (2011) Metabolic regulation of hematopoietic stem cells in the hypoxic niche.
Cell Stem Cell 9, 298-310
27. Wang, Y., Liu, Y., Malek, S. N., Zheng, P., and Liu, Y. (2011) Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 8, 399-411
28. Zhang, H., Gao, P., Fukuda, R., Kumar, G., Krishnamachary, B., Zeller, K. I., Dang, C. V., and Semenza, G. L. (2007) HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407-420
29. Luo, W., Hu, H., Chang, R., Zhong, J., Knabel, M., O'Meally, R., Cole, R. N., Pandey, A., and Semenza, G. L. (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732-744
30. Franovic, A., Gunaratnam, L., Smith, K., Robert, I., Patten, D., and Lee, S. (2007) Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A 104, 13092-13097
31. Lau, C. K., Yang, Z. F., Ho, D. W., Ng, M. N., Yeoh, G. C., Poon, R. T., and Fan, S. T. (2009) An Akt/hypoxia-inducible factor-1alpha/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin Cancer Res 15, 3462-3471
32. Esteban, M. A., Tran, M. G., Harten, S. K., Hill, P., Castellanos, M. C., Chandra, A., Raval, R., O'Brien T, S., and Maxwell, P. H. (2006) Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res 66, 3567-3575
33. Krishnamachary, B., Zagzag, D., Nagasawa, H., Rainey, K., Okuyama, H., Baek, J. H., and Semenza, G. L. (2006) Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res 66, 2725-2731
34. Mak, P., Leav, I., Pursell, B., Bae, D., Yang, X., Taglienti, C. A., Gouvin, L. M., Sharma, V. M., and Mercurio, A. M.
(2010) ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 17, 319-332
35. Sullivan, R., and Graham, C. H. (2007) Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev 26, 319-331
36. Krishnamachary, B., and Semenza, G. L. (2007) Analysis of hypoxia-inducible factor 1alpha expression and its effects on invasion and metastasis. Methods Enzymol 435, 347-354
37. Erler, J. T., Bennewith, K. L., Nicolau, M., Dornhofer, N., Kong, C., Le, Q. T., Chi, J. T., Jeffrey, S. S., and Giaccia, A. J.
(2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222-1226
38. Wong, C. C., Gilkes, D. M., Zhang, H., Chen, J., Wei, H., Chaturvedi, P., Fraley, S. I., Wong, C. M., Khoo, U. S., Ng, I. O., Wirtz, D., and Semenza, G. L. (2011) Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A 108, 16369-16374
39. Zhang, H., Wong, C. C., Wei, H., Gilkes, D. M., Korangath, P., Chaturvedi, P., Schito, L., Chen, J., Krishnamachary, B., Winnard, P. T., Jr., Raman, V., Zhen, L., Mitzner, W. A., Sukumar, S., and Semenza, G. L. (2012) HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs.
Oncogene 31, 1757-1770
40. Moeller, B. J., Richardson, R. A., and Dewhirst, M. W. (2007) Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev 26, 241-248
41. Rohwer, N., and Cramer, T. (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 14, 191-201
42. Semenza, G. L. (2011) Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol 76, 347-353
二、 研究或教學或科技研發與管理成效評估( 由計畫主持人或單位主管填寫 )
Please evaluate the performance of research, teaching or science and technology R&D and management Work: (To be completed by Project Investigator or Head of Department/Center)
(1)是否達到延攬預期目標?
Has the expected goal of recruitment been achieved?
達到延攬預期目標。
(2)研究或教學或科技研發與管理的方法、專業知識及進度如何?
What are the methods, professional knowledge, and progress of the research, teaching, or R&D and management work?
受延攬人在延攬期間,有效協助計畫進行,並執行後續的相關實驗,使得我們對於癌細胞如何轉移的過
程又有更進一步的了解。。
(3)受延攬人之研究或教學或科技研發與管理成果對該計畫(或貴單位)助益如何?
How have the research, teaching, or R&D and management results of the employed person given benefit to the project (or your unit)?
受延攬人在延攬期間,使計畫能夠順利進行,對本計畫有顯著的助益,更進一步對於我們實驗室接下去
要探討代謝反應與癌細胞轉移有更深入的了解。
(4)受延攬人於補助期間對貴單位或國內相關學術科技領域助益如何?
How has the employed person, during his or her term of employment, benefited your unit or the relevant domestic academic field?
受延攬人所研究的丙酮酸脫氫激酶 1 在表皮生長因子促進頭頸鱗狀癌轉移中扮演的角色,是目前國內
相關學術領域中尚未探討的領域,對於未來代謝改變與癌症轉移相關學術領域的研究有顯著的助益。
(5)具體工作績效或研究或教學或科技研發與管理成果:
Please describe the specific work performance, or the results of research, teaching, or R&D and management work:
受延攬人在延攬期間,研究丙酮酸脫氫激酶 1 在表皮生長因子促進頭頸鱗狀癌轉移中扮演的角色,並且發表
在 FASEB journal (2016 impact factor : 5.498)。
(6) 是否續聘受聘人?
Will you continue hiring the employed person?■ 續聘 Yes □不續聘 No
※ 此報告表篇幅以三~四頁為原則。
This report form should be limited to 3-4 pages in principle.※ 此表格可上延攬優秀人才成果報告繳交說明網頁下載。
This report form can be downloaded in http://scholar.lib.ncku.edu.tw/explain/