Lay Gaik Teoh
Department of Materials Science and Engineering, National Cheng Kung University, Tainan, Taiwan, Republic of China
Jiann Shieh
National Nano Device Laboratories, Hsinchu, Taiwan, Republic of China Wei Hao Lai and Min Hsiung Hona)
Department of Materials Science and Engineering, National Cheng Kung University, Tainan, Taiwan, Republic of China
(Received 17 December 2004; accepted 2 June 2004)
The effects of mesoporous structure on grain growth were investigated in this study.
The synthesis was accomplished using block copolymer as the organic template and tungsten chloride as the inorganic precursor. Thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy, x-ray diffractometry (XRD), transmission electron microscopy, and N2adsorption/desorption isotherms were used to characterize the microstructures obtained for different temperatures. TGA and XRD analyses demonstrate that copolymers were expelled at 150–250 °C, and mesoporous structure was stable up to 350 °C. The pore diameter and the surface area evaluated from the Barrett-Joyner-Halenda model and Brunauer–Emmett–Teller method indicated that the average pore diameter is 4.11 nm and specific surface area is 191.5 m2/g for 250 °C calcination. Arrhenius equation used to calculate the activation energy for grain growth demonstrates that the activation energy for grain growth was about 38.1 kJ/mol before mesostructure collapse and 11.3 kJ/mol after collapse. These results show evidence of two different mechanisms governing the process of grain growth. The presence of the pore can be related to the obstacle for grain growth.
I. INTRODUCTION
With the large specific surface area and narrow pore- size distribution, mesoporous tungsten oxide acting as a semiconducting ceramic material is currently attracting attention in various applications such as gas sensing, electrochromic, and optical devices.1–4Generally, meso- porous materials, prepared by self-assembling surfactants as organic templates in sol-gel process,5–8 have poor crystallinity, which is disadvantageous for applications.
Most of the mesoporous structure-related work focused on the preparation of silica, but the references concerning the characteristic of mesoporous transition metal oxide are deficient. Until recently, there are a few reported examples of mesoporous WO3, but the relation between the calcined temperature and mesoporous structure
including specific surface area, pore size, and crystalli- zation, especially the activation energy, for grain growth has yet been discussed.
The properties of nanostructured materials have been found to depend on the characteristic length associated with them. For example, the sensitivity of semiconduct- ing gas sensor can be improved as the grain size is re- duced to the length of space charge layer.9In the sol-gel process, however, grain growth usually accompanies heat treatment, which is an unavoidable process for de- hydroxylation. Grain growth is the result of atom diffu- sion in grain boundary. To inhibit grain growth, the mechanisms for the reduction of grain boundary mobility and thermodynamic driving force by impurity pinning and metastable systems, respectively, have been pro- posed.10,11 In this study, we adjusted the calcined tem- perature to investigate the effect of mesoporous structure on the grain growth of nanocrystallite. According to the activation energy calculation and the microstructure analyses, the results revealed that the mesoporous
a)Address all correspondence to this author.
e-mail address: mhhon@mail.ncku.edu.tw DOI: 10.1557/JMR.2004.0362
structure may provide another approach to suppress the grain growth.
II. EXPERIMENTAL
Mesoporous WO3 was prepared at room temperature in the following way: 0.5 g amphiphilic triblock copoly- mer (designated EO100PO64EO100; Pluronic, Mount Ol- ive, NJ, F127) was dissolved in 10 ml ethanol and mixed with an inorganic precursor, 2g WCl6, for 1 h under vigorous stirring. The solution exhibited a yellow color immediately after the addition of WCl6 to the ethanol- block copolymer solution and successively became green and then blue as tungsten was reduced. According to the mechanism proposed by Yang et al.,12,13WCl6first re- acts with ethanol by alcoholysis reaction to produce W(OEt)xCl6−x; then this species can associate with the poly(ethylene oxide) (PEO), through the coordination bonds between metal ions and oxygen atoms of copoly- mer, to form the mesoporous structure by hydrolysis with the moisture in air. The resulting sol solution was aged at 60 °C in air for 14 days, and calcined at temperatures between 200 and 450 °C for 5 h (the heating rate of the entire calcination process is 2 °C /min), to remove the F127 block copolymer template. The mesostructure of WO3 obtained then was investigated by thermo- gravimetric analysis (TGA; TA 5100, Thermal Analysis System), Fourier transform infrared spectrophotometer (FTIR; Perkin-Elmer, Los Angeles, CA, Spectrum GX), x-ray powder diffractometry (XRD; Rigaku, Osaka, Ja- pan, D/max-IV, using Cu K␣ radiation), transmission electron microscopy (TEM; Hitachi Model, Tokyo, Ja- pan, HF-2000, 200 keV), and N2 adsorption/desorption isotherm measurements (Micromeritics, Norcross, GA, ASAP 2010). The grain size was calculated from XRD results according to the Scherrer’s equation14
D= 共0.9 兲Ⲑ共 cos 兲 , (1) where D is the average crystallite size in nanometers, is the radiation wavelength (0.154 nm),  is the corrected half-width at half-maximum (FWHM), and is the diffraction peak angle. The FWHM was obtained by de- convolution with a peak-fit procedure (Profile Fitting Software) to separate peaks and to calculate the crystal- line size. Gaussian–Lorentzian functions were used to fit the crystalline peaks, and the peak parameters were iter- ated to achieve a good fit with the experimental data.
III. RESULTS AND DISCUSSION
The result of the TGA curve for the mesoporous WO3, which was carried out from room temperature to 800 °C in the air, is presented in Fig. 1. The gravimetric loss below 100 °C is attributed to water, and to the combus- tion of organic compounds for the removal of the block
copolymer template between 150 and 250 °C. This indi- cates that as the samples were heated at the temperature higher than 150 °C for a long time, it is able to gain an insight into the structural stability, crystallization behav- ior and the grain growth of the mesoporous structure.
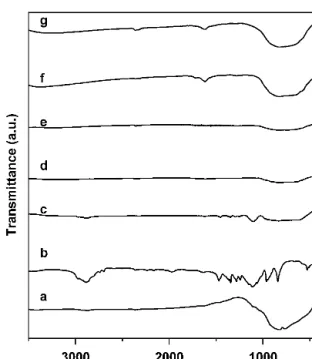
Figure 2 presents the FTIR spectra of WO3 (Aldrich, Milwaukee, WI) block copolymer, as-synthesized pow- ders calcined at 200, 250, 300, and 350 °C. In the spectra of the as-synthesized powder, some bands are observed in the region of 2800–3000 cm−1and attributed to CH2
vibrations. The bands in the region of 1450–1500 cm−1 are attributed to the deformation of –CH2– and –CH3.15 The bands centered at 1621 cm−1found on all samples
FIG. 1. TGA curve of mesoporous WO3.
FIG. 2. FTIR spectra of samples: (a) WO3from Aldrich, (b) block copolymer, (c) as-synthesized, (d) after calcination at 200 °C, (e) 250 °C, (f) 300 °C, and (g) 350 °C for 5 h.
are assigned to O–H stretching and deformation vibra- tions of weak-bound water.16The broad bands between 500 and 1000 cm−1are attributed to the framework vi- brations of WO3.17,18The peaks related to the copolymer disappeared after 5 h calcination at 200 °C, indicating that the template was completely removed and the sample was carbon-free. Moreover, Cl− ions are sug- gested to be removed by calcination. As shown by the FTIR spectra, we could not see any peak related to the hydrogen chloride of as-synthesized sample in Fig. 2(c) (the stretching vibration of HCl is at 2719.5 cm−1).
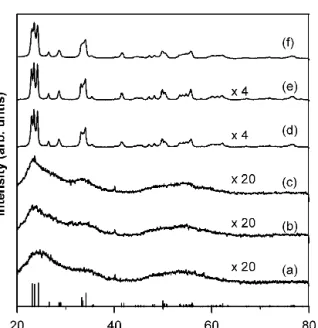
Figure 3 shows the XRD patterns for the mesoporous WO3 calcined at different temperatures. The samples heated below 300 °C were characterized with broad peaks, indicating that the framework of mesopores may consist of nanocrystallite WO3. Above 350 °C, the for- mation of the marked monoclinic diffraction peaks sug- gests that the mesoporous structure may collapse and the wells with crystallized grains present. In comparison with the result of TGA analysis, one can see that the mesoporous structure was stable at the temperatures up to 350 °C after repelling the copolymer template from 150 to 250 °C, which provides a temperature range to investigate the microstructure stability of the mesoporous structure. Three peaks can be identified at 2 values of 23.06°, 23.34°, and 24.33° by a deconvolution analysis of the XRD pattern. These peaks correspond to mono- clinic crystalline structure with 002, 020, and 200 diffractions, respectively. The average grain sizes determined from Scherrer’s equation were about 1.4, 3.2, 7.6, 15.5, 19.0, and 20.9 nm as the sample calcined at 200, 250, 300, 350, 400, and 450 °C, respectively. These results are consistent with TEM observations, as shown
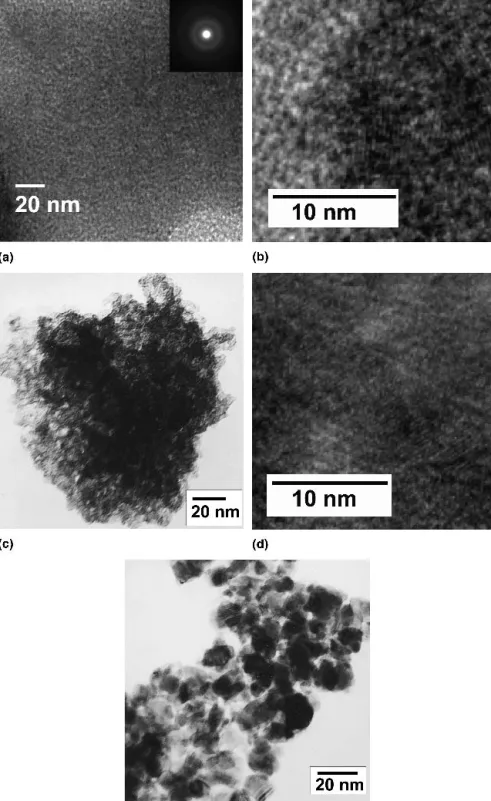
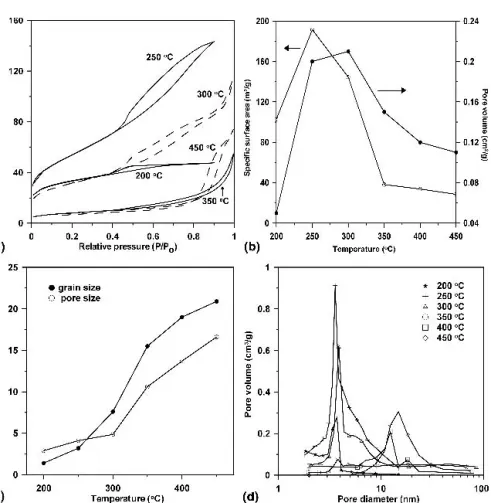
in Fig. 4. The samples before mesopore structure col- lapsed are clearly characterized with a feature of a dis- ordered mesoporous structure or the wormhole-like structure [Figs. 4(a) and 4(c)]. There is no diffraction peak in the low angle region due to the lack of long-range order. For mesoporous transition metal oxides, similar results are found in the references.2,3,19Additional high- resolution TEM (HRTEM) images were recorded to re- solve the lattice fringes in crystallites within the walls of the mesopores. Although the diffraction pattern [inset in Fig. 4(a)] indicates the poor crystallinity, the micro- graphs presented in Figs. 4(b) and 4(d) reveal several randomly oriented nanocrystallites with clearly resolved lattice fringes, which demonstrates that the walls are par- tially crystallized. Since the TEM images show only the local regions, the overall mesoporosity of the samples is also measured by N2 adsorption/desorption isotherms, shown in Fig. 5(a). The calcination temperature was set from 200 to 450 °C. The shape of the curves exhibiting a type IV curve which is the characteristic of mesoporous WO3.20 The hysteresis loop of the isotherm before the mesopores collapsed is a typical H2-type isotherm, and after the mesopores collapsed exhibited H1-type iso- therms. Figure 5(b) illustrates the specific area and total pore volume as a function of the calcined temperatures.
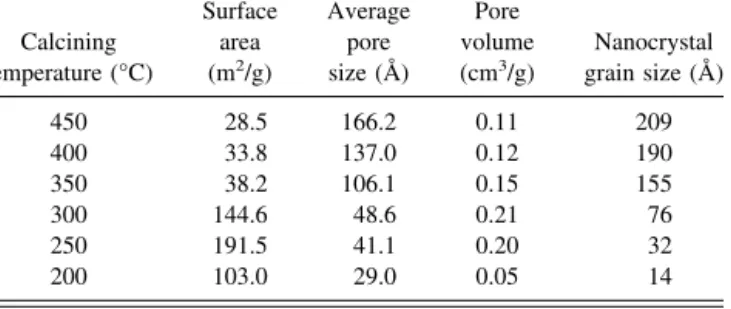
The evaluation of the specific surface areas of the 250 and 300 °C calcined materials allows us to compare them with the previously reported mesoporous WO3materials, by Yang et al. (125 m2/g)12,13 and by Cheng et al.
(135 m2/g).3 The detailed values are also presented in Table I. The Brunauer–Emmett–Teller (BET) specific surface area increases between 200 and 250 °C, reaching a maximum value of 191.5 m2/g, and the pore volume increases between 200 and 300 °C. The phenomenon may be due to the removal of copolymer template. The dependence of grain size and pore size on calcined tem- perature is depicted in Fig. 5(c). From 200 to 450 °C, the grain size increased from 1.4 to 20.9 nm, and the average pore size increased from 2.9 to 16.62 nm. It can be seen that by increasing the calcined temperature a larger crystallite size and larger average pore size of WO3are favored, indicating that as the calcination tem- perature increases, the total number of pores decrease as a result of sintering while the amount of larger pore size increases. Moreover, as revealed by N2 adsorption/
desorption isotherms, the pore size increase is not too large, from about 2.9 to 4.86 nm in the range of 200 and 300 °C before the mesoporous structure collapsed. This may be attributed to the incomplete removal of the block copolymer at 200 °C. It also can be concluded that as the calcination temperature increases to 350 °C, the meso- pore structure collapses due to the crystallization of the WO3inorganic wall, which leads to a decrease in specific surface area, and an increase in average pore size and nanocrystal size. Furthermore, the pore-size distribution
FIG. 3. XRD patterns of mesoporous WO
3 calcined at (a) 200 °C, (b) 250 °C, (c) 300 °C, (d) 350 °C, (e) 400 °C, and (f) 450 °C for 5 h.
of the samples calcined at different temperatures is shown in Fig. 5(d). As calcination temperature increases, the pore size shifts to a large value, and the pore-size distribution broadens. These results also demonstrate that the larger pores arose from the collapse of mesopores.
Figure 5(c) also shows that a pronounced increase in
the grain size is apparent between 300 °C (573 K) and 350 °C (623 K), and a further increase is observed after this transition, which corresponds to the temperature the mesoporous structure completely collapsed. This might be an indication for different mechanisms being respon- sible for grain growth before and after the mesostructure
FIG. 4. Bright-field TEM images of mesoporous WO3after calcined at (a) 200 °C, (c) 300 °C, and (e) 350 °C for 5 h; (b) and (d) show HRTEM images of (a) and (c), respectively.
collapsed. To calculate activation energy of grain growth, the Arrhenius-type equation can be utilized21,22: D= D0exp(−QⲐRT) , (2) where D is the calculated grain size, D0the initial grain size, T the absolute temperature, Q the activation energy, and R the ideal gas constant.
A plot of log D versus the reciprocal of absolute tem- perature (1/T) is presented in Fig. 6. The slope of the resulting Arrhenius plot is −Q/(2.303R) and the activa- tion energy of grain growth can be obtained. The grain growth investigated seems to be governed by two differ- ent sets of mechanisms. The region before mesoporous structure collapse exhibits activation energy of 38.1 kJ/
mol, while the other one, after mesoporous structure col- lapse, reveals activation energy of 11.3 kJ/mol. It was found that higher activation energy is needed to activate the process of grain growth and pore growth. When the pore disappeared gradually and the following sintering occurred, smaller activation energy is needed to continue grain growth. Continuous grain growth and secondary recrystallization are similar to the main processes occur- ring in ceramic materials during the thermal treatment.23 In general the addition of impurities may weaken grain
growth and inhibit sintering effects.23,9For nanocrystal- line ceramic grains the activation energy has been ob- served to be much smaller than the bulk ceramics.24 Abundant oxygen vacancies are suggested to account for the reduced activation energy.22However, contrary to the previous work, this study showed that the activation en- ergy increased as grain size reduced. Our measurement shows that the activation energy for grain growth is at least 3-fold in magnitude before the mesopores col- lapsed. We attributed this to the presence of mesopores around the nanocrystalline. In the case of mesoporous zirconia-based oxide, other researchers also demon- strated that the grain growth might be hindered by the wall-pore boundaries.25 The results described above shows another way besides impurity effect to inhibit the grain growth, which is the key feature for gas sensor as previously demonstrated.1
IV. CONCLUSIONS
WO3with mesoporous structure and crystalline frame- work consisting of monoclinic phase was synthesized.
The grain sizes of WO3were about 1.4–20.9 nm, and the activation energy for grain growth were about 38.1 kJ/mol before mesoporous structure collapse and 11.3 kJ/mol after collapse. These two values are thought to be possibly
FIG. 5. (a) N2adsorption–desorption isotherms for calcined mesoporous WO3, (b) specific surface area and pore volume, (c) pore size and grain size as a function of the calcined temperature, and (d) BJH pore-size distributions of WO3sample calcined at 200–450 °C.
due to two different mechanisms governing the process of grain growth. The larger activation before pore col- lapse is attributed to the presence of mesopores. The pore can be related to the impurity inhibited grain growth. The mesoporous WO3 is shown to have a high specific sur- face area of 191.5 m2/g, and the mesoporous structure was thermally stable up to 300–350 °C. As the calcina- tion temperature was larger than 350 °C, the mesoporous structure collapsed and the activation energy needed for grain growth was reduced, as evidenced by XRD, TEM, and BET analyses.
ACKNOWLEDGMENT
This work was financially supported by the National Science Council of Taiwan, ROC, Grant No. NSC 90- 2216-E-006-064, which is gratefully acknowledged.
REFERENCES
1. L.G. Teoh, I.M. Hung, J. Shieh, W.H. Lai, and M.H. Hon: High sensitivity semiconductor NO2gas sensor based on mesoporous WO3thin film. Electrochem. Solid-State Lett. 6, 108 (2003).
2. E. Ozkan, S.H. Lee, P. Liu, C.E. Tracy, F.Z. Tepehan, J.R. Pitts, and S.K. Deb: Electrochromic and optical properties of meso- porous tungsten oxide films. Solid State Ionics 149, 139 (2002).
3. W. Cheng, E. Baudrin, B. Dunn, and J.I. Zink: Synthesis and electrochromic properties of mesoporous tungsten oxide. J. Mater.
Chem. 11, 92 (2001).
4. C. Santato, M. Odziemkowski, M. Ulmann, and J. Augustynski:
Crystallographically oriented mesoporous WO3films: Synthesis, characterization, and applications. J. Am. Chem. Soc. 123, 10639 (2001).
5. C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, and J.S.
Beck: Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359, 1710 (1992).
6. S. Forster and M. Antonietti: Amphiphilic block copolymers in structure-controlled nanomaterial hybrids. Adv. Mater. 10, 195 (1998).
7. D. Zhao, Q. Huo, J. Feng, B.F. Chmelka, and G.D. Stucky:
Nonionic triblock and star diblock copolymer and oligomeric sur- factant syntheses of highly ordered, hydrothermally stable, meso- porous silica structures. J. Am. Chem. Soc. 120, 6024 (1998).
8. C.J. Brinker, Y. Lu, A. Sellinger, and H. Fan: Evaporation- induced self-assembly: Nanostructures made easy. Adv. Mater.
11,579 (1999).
9. J. Shieh, H.M. Feng, M.H. Hon, and H.Y. Juang: WO3 and W-Ti-O thin film gas sensors prepared by sol-gel dip-coating.
Sens. Actuators B 86, 75 (2002).
10. N.L. Wu, S.Y. Wang, and I.A. Rusakova: Inhibition of crystallite growth in the sol-gel synthesis of nanocrystalline metal oxides.
Science 285, 1375 (1999).
11. E.R. Leite, A.P. Maciel, I.T. Weber, P.N. Lisboa-Filho, E. Longo, C.O. Paiva-Santos, A.V.C. Andrade, C.A. Pakoscimas, Y. Mani- ette, and W.H. Schreiner: Development of metal oxide nanopar- ticles with high stability against particle growth using a metastable solid solution. Adv. Mater. 14, 905 (2002).
12. P. Yang, D. Zhao, D.I. Margolese, B.F. Chmelka, and G.D.
Stucky: Generalized syntheses of large-pore mesoporous metal oxides with nanocrystalline walls. Nature 396, 152 (1998).
13. P. Yang, D. Zhao, D.I. Margolese, B.F. Chmelka, and G.D.
Stucky: Block copolymer templating syntheses of mesoporous metal oxides with large ordering lengths and semicrystalline framework. Chem. Mater. 11, 2813 (1999).
14. B.D. Cullity: Elements of X-ray Diffraction (Addison-Wesley, Reading, MA, 1978).
15. L.J. Bellamy: The Infrared Spectra of Complex Molecules, Vol. 1, 3rd ed. (Chapman Hall, New York, 1975).
16. G.J. Li and S. Kawi: Synthesis, characterization and sensing ap- plication of novel semiconductor oxides. Talanta 45, 759 (1998).
17. M.F. Daniel, B. Desbat, J.C. Lassegues, B. Gerand, and M. Fi- glarz: Infrared and Raman study of WO3tungsten trioxides and WO3, xH2O tungsten trioxide hydrates. J. Solid State Chem. 67, 235 (1987).
18. L.H.M. Krings and W. Talen: Wet chemical preparation and char- acterization of electrochromic WO3. Sol. Energy Mater. Sol. Cells 54,27 (1998).
19. B. Lee, T. Yamashita, D. Lu, J.N. Knodo, and K. Domen: Single- crystal particles of mesoporous niobium-tantalum mixed oxide.
Chem. Mater. 14, 867 (2002).
20. S.J. Gregg and K.S.W. Sing: Adsorption, Surface Area and Po- rosity (Academic, London, U.K., 1982).
21. M. Jarcho, C.H. Bolen, M.B. Thomas, J. Bobick, J.F. Kay, and FIG. 6. A plot of activation energy for mesoporous and nanoparticle
WO3.
TABLE I. Surface area, pore volume, pore size, and nanocrystal size of the mesoporous WO3calcined at different temperature.
Calcining temperature (°C)
Surface area (m2/g)
Average pore size (Å)
Pore volume (cm3/g)
Nanocrystal grain size (Å)
450 28.5 166.2 0.11 209
400 33.8 137.0 0.12 190
350 38.2 106.1 0.15 155
300 144.6 48.6 0.21 76
250 191.5 41.1 0.20 32
200 103.0 29.0 0.05 14
R.H. Doremus: Hydroxyapatite synthesis and characterization in dense polycrystalline. J. Mater. Sci. 11, 2027 (1976).
22. S. Shukla, S. Seal, R. Vij, and S. Bandyopadhyay: Reduced aci- tivation energy for grain growth in nanocrystalline yttria-stablized zirconia. Nano Lett. 3, 397 (2003).
23. M. Ferroni, V. Guidi, G. Martinelli, E. Comini, G. Sberveglieri, D. Boscarino, and G. Della Mea: Electron microscopy and Ruth- erford backscattering study of nucleation and growth in nanosized W-Ti-O thin films. J. Appl. Phys. 88, 1097 (2000).
24. A.P. Hynes, R.H. Doremus, and R.W. Siegel: Sintering and char- acterization of nanophase zinc oxide. J. Am. Ceram. Soc. 85, 1979 (2002).
25. E.L. Crepaldi, G.J. de, A.A. Soler-Illia, A. Bouchara, D. Grosso, D. Durand, and C. Sanchez: Controlled formation of cubic and hexagonal mesoporous yttria-zirconia and ceria-zirconia thin films exhibiting unprecedented thermal stability. Angew. Chem.
Int. Ed. Engl. 42, 347 (2003).