國立臺灣大學生物資源暨農學院生物科技研究所 博士論文
Institute of Biotechnology
College of Bioresources and Agriculture
National Taiwan University Doctoral Dissertation
可促進植物生長之光合菌 Rhodopseudomonas palustris PS3 的基因體分析與培養條件優化
Genome analysis and fermentation optimization of plant growth promoting strain Rhodopseudomonas palustris PS3
羅凱軍 Kai-Jiun Lo
指導教授:劉啓德 博士 Advisor: Chi-Te Liu, Ph.D.
中華民國 109 年 7 月
July 2020
謝辭
當這本博士論文完成時,也代表著我的博士求學生涯告一段落了。這本論文 得以完成要感謝的人實在太多了。首先,要感謝我的指導教授劉啓德博士,啓德 老提供了一個很好的實驗環境讓我進行論文研究,並在我遇到困難時協助我解決 難題。在學術研究外上,也給予我生活上很大的支持。時常關心我的生活狀況。
從我決定攻讀博士學位到撰寫計畫書、進行實驗設計至發表研究論文的一路上,
老師都是正向的鼓勵我一路往前,讓我嘗試任何想做的事情。老師給予的實驗自 由度更讓我可以自由自在的進行研究,又在我迷失方向時適時拉回我。老師也提 供了許多合作的機會,讓我在博士生涯裡累積了各種經驗。和老師一起經歷的事 情都會是我博士班求學過程中難以忘懷並且感恩的事情。另外,也謝謝詩舜老師,
雖然我們有些目標還未完成,但您是帶領我進入生物資訊領域的一個重要的人。
也感謝郭志鴻老師再基因體的解序與研究上給予學生很好的指導與協助。仁治老 師在分子實驗上的建議替我解決的實驗上的問題。在每週與昆達老師實驗室的會 議裡,昆達老師的寶貴的建議也讓我的研究更完整。當然還要謝謝口試委員老師 們仔細的閱讀我的論文、給予建議與修正,使得我的博士論文更完整。
在這段研究的路上還要感謝實驗室的成員,在實驗上提供協助、包容以及建 議,也感謝這些人陪伴我渡過這些日子,這些將會是很好的經驗與回億。特別是 我的戰友夥伴筱涵、郁盛、YOYO、爾傑、球球、怡儒、Aniket,雖然大家往後 各奔東西,但我們的友誼將會常存。謝謝孟薇在這段時間裡,替我打理了許多事 情,陪我渡過博士求學生活的日子。謝謝睦惠陪我渡過最後的衝刺階段。
最後要感謝我的弟弟凱胤,未來的日子我們一定會更好。而我最愛的母親黃 瓊雀女士,是您的支持讓我得以繼續攻讀博士學位。從小到大,您一直以來都是 我最大的後盾。雖然您來不及看到我穿上博士袍、無法參與我的畢業,但我完成 了。這本論文也將獻給您做為禮物,您會永遠活在我的心底。
凱軍謹誌於 中華民國一百零九年七月
中文摘要
沼澤紅假單胞菌 (Rhodopseudomonas palustris) PS3 菌株是一株由台灣水稻 田土壤所篩選出的紫色非含硫光合菌。在先前研究中已證明該菌株具有促進各種 作物生長的能力,並且可以提升植物氮肥使用效率。本論文的第一部份是從微生 物全基因體的角度來探討光合菌與促進植物生長的關係。藉由與另一株不具促進 植物生長功能之 YSC3 光合菌 (Rhodopseudomonas palustris)做比較分析,依基因 構造、微生物生理反應以及基因表現的結果找出 PS3 菌株可能參與促進植物生 長的相關基因。本研究結合了短片段與長片段定序技術,並完成序列組裝與註解 後,分別得到 PS3 與 YSC3 菌株的基因體全長為 527 萬與 537 萬鹼基對,含有
4,799 以及 4,907 編碼序列。PS3 與 YSC3 菌株有極高的序列相似度 (約 95.11%),
而且大部分的基因群的組成與排列方式類似,都有固氮、溶磷、吲哚乙酸合成、
氨基環丙烷羧酸脫胺酶等代表性的植物生長促進相關基因群。雖然 PS3 與 YSC3 菌株的生長速率沒有差異,在微生物生理試驗結果發現,添加植物的根分泌液至 兩株菌的培養液中會促使 PS3 菌株的生物膜生成量以及化學趨向性的相關基因 表現量都較 YSC3 菌株高。這意味著 PS3 菌株對於植物的反應可能是其促進植 物生長的重要關鍵。
PS3 光合菌是具有產業化潛力的菌株,本論文的第二部分藉由數學統計模式 探討最適化發酵條件,找出高產量與低成本的生產方式。在本研究中評估了各種 較低成本的基質,包括了工業發酵常用的碳氮源以及農產加工副產物等作為培養 基配方的可行性,並藉由回應曲面法優化培養條件。實驗結果顯示,當以 39.41 mL/L 的玉米浸漬液加上 32.35 g/L 的蔗糖糖蜜作為氮源和碳源,利用 5 公升桌上 型發酵槽於 38°C,pH 7 以及溶氧濃度 30%的條件下進行 24 小時培養,得到 PS3 菌株的最大生物量約為 2.18 ± 0.01 g/L。該新配方所生產的 PS3 發酵液產量約為 使用傳統光合菌培養條件的 8 倍,成本卻只需原先的 30%,而且在植物盆栽試驗 上證實可以促進作物生長。
在本研究中所建立的基因體資訊,可作為探討光合菌與植物間的交互作用以 及促進生長機制的研究平台。此外,新開發的培養基配方是以農產加工副產物作 為光合菌的主要營養基質,不僅可有效降低生產成本,也促進了農業資源的加值 與循環再利用。
關鍵字:沼澤紅假單胞菌、全基因體比較分析、回應曲面法、農產加工副產物、
玉米浸漬液、糖蜜、促進作物生長
ABSTRACT
Rhodopseudomonas palustris PS3 is one of purple non-sulfur phototrophic bacteria that was isolated from Taiwanese paddy soils. It has been demonstrated that PS3 can promote plant growth and increase the agronomic nitrogen use efficiency of the host plant. The first part of this dissertation focuses on the elucidation of the relationship between the phototrophic bacteria and plant growth-promotion from the view of whole genomes of microorganisms. I conducted comparative analyses of genomic structures, physiological responses of microbes, and gene expression profiles with those of an ineffective R. palustris YSC3 strain. Based on the differential data, many putative genes that were associated with known plant growth promotional traits were identified. In this study, Illumina short-reads and PacBio long-reads technologies were integrated and assembled to obtain the whole genome sequences. PS3 and YSC3 individually contains a one circular chromosome with 5.27 and 5.37 Mb bp in size, with 4,799 and 4,907 protein-coding genes, respectively. The PS3 and YSC3 strains are closely related to each other with high identity (95.11%), and have similar genomic structures and compositions. Both strains contain the genes associated with plant growth-promotion, such as nitrogen fixation, phosphate solubilization, indole acetic acid synthesis, 1-aminocyclopropane-1-carboxylate deaminase, etc. Although there was no difference in the growth rate of PS3 and YSC3 strains, both the production of biofilm and the gene expressions of chemotaxis of PS3 were higher than those of YSC3 by the addition of root exudate in culture broth.
Since PS3 is an elite strain with commercialization potential, the second part of this dissertation focuses on the optimal fermentation conditions for PS3 through mathematical and statistical models to find out a high-yield and low-cost production strategy. In this study, I evaluated various substrates, including the carbon and nitrogen
sources commonly used in industry as well as the agricultural processing by-products.
The culture condition was optimized by response surface methodology. The optimum culture condition was found to be at corn steep liquor, 39.41 mL/L; molasses, 32.35 g/L; temperature, 38°C; pH, 7.0; and DO 30%. Under this condition, the maximum yield of PS3 strain was up to 2.18 ± 0.01 g/L, which was approximately 8-fold higher than that with original medium, and the medium cost was approximate 70% reduced.
Moreover, the beneficial effect of the new PS3 broth on plant growth was verified by pot experiments.
The genomic information established in this study can be used as a research platform to investigate the interaction between phototrophic bacteria and plants as well as the molecular mechanisms of plant growth promotion. In addition, the newly developed medium uses agricultural processing by-products as the main nutrient substrates for phototrophic bacterium growth, which not only effectively reduces production costs, but also promotes the value-added and recycling of agricultural resources.
Keywords: Rhodopseudomonas palustris, whole genomic comparative analysis, response surface methodology, agricultural processing by-products, corn steep liquor, molasses, plant growth promotion
CONTENTS
口試委員會審定書 ... i
謝辭 ... ii
中文摘要 ... iii
ABSTRACT... v
CONTENTS... vii
LIST OF FIGURES ... xi
LIST OF TABLE ... xiii
CHAPTER I ... 1
INTRODUCTION... 1
Plant growth promoting rhizobacteria ... 1
PGPR with nitrogen fixing ability ... 2
PGPR with phosphate solubilizing ability ... 3
PGPR with phytohormone producing ability ... 4
Phototrophic bacteria ... 5
Purple non-sulfur phototrophic bacteria ... 6
Application of purple non-sulfur photosynthetic bacteria ... 7
Rhodopseudomonas palustris ... 9
R. palustris PS3 ... 10
Specific aims ... 11
CHAPTER II... 22
Whole-Genome Sequencing and Comparative Analysis of Two Plant-Associated Strains of Rhodopseudomonas palustris (PS3 and YSC3) ... 22
Summary ... 23
Introduction ... 23
Materials and methods ... 27
Preparation of phototrophic bacterial inoculant ... 27
Genomic DNA preparation ... 27
Whole-genome sequencing ... 28
De novo genome assembly ... 29
Genome annotation ... 29
Phylogenetic analysis ... 30
Biofilm formation assay ... 31
Phosphate-solubilizing activity assay ... 32
Indole acetic acid production of R. palustris ... 32
Comparative genomic analyses ... 33
Collection of Chinese cabbage root exudate solution ... 34
Gene expression analysis of R. palustris in response to root exudates ... 35
Statistical analysis ... 35
Results ... 36
General characteristics of the genomes ... 36
Carbon source utilization ... 38
Nitrogen fixation and nitrogen utilization ... 40
Root colonization ... 41
Deduced plant growth promotion-related genes ... 42
Effect of root exudates of Chinese cabbage on biofilm formation and relative gene expression levels ... 44
Transcriptomic profiling of R. palustris PS3 response to root exudate ... 44
Discussion ... 46
CHAPTER III ... 76 Development of A Low-Cost Culture Medium For Rapid Production of Plant Growth-
Promoting Rhodopseudomonas palustris PS3 Strain ... 76
Summary ... 77
Introduction ... 77
Materials and Methods ... 81
Microorganism and bacterial preparation ... 81
Measurement of cell growth ... 81
Screening of nitrogen and carbon sources for medium optimization ... 82
Evaluate the effects of fermentation factors on the growth of R. palustris by fractional factorial design (FFD) ... 82
Maximum region improvement of factors by steepest ascent method ... 84
Construction of the response surface model by central composite design (CCD) ... 84
Batch-culture experiments in a benchtop bioreactor ... 85
Quantification of total organic carbon ... 86
Quantification of total nitrogen ... 86
In planta experiments to verify the plant-growth promotion effect of the newly developed fermentation broth ... 87
Statistical analysis ... 89
Results ... 89
Selection of appropriate components for optimization of R. palustris strain PS3 medium ... 89
Screening suitable fermentation conditions by FFD... 90
Optimization of culture conditions by RSM ... 92
Effects of various factors on R. palustris PS3 biomass production ... 93
Verification of optimization... 94 Validation of the plant growth-promoting effect of the newly developed
fermentation broth... 94
Discussion ... 95
Chapter IV ... 118
Concluding remarks and discussion ... 118
References...125
APPENDIX ...155
LIST OF FIGURES
Figure 1-1. Deduced modes of action of PGPR. ... 13
Figure 1-2. Overview of the biosynthetic pathway of IAA in bacteria. ... 14
Figure 1-3. The four types of metabolism of R. palustris that support its growth ... 15
Figure 2-1. Assembly scheme and annotation pipeline in this study ... 54
Figure 2-2. Genome map of R. palustris PS3 (a) and YSC3 (b)... 55
Figure 2-3. Phylogenetic tree of R. palustris strains based on housekeeping, functional genes and conserved amino acid sequences. ... 56
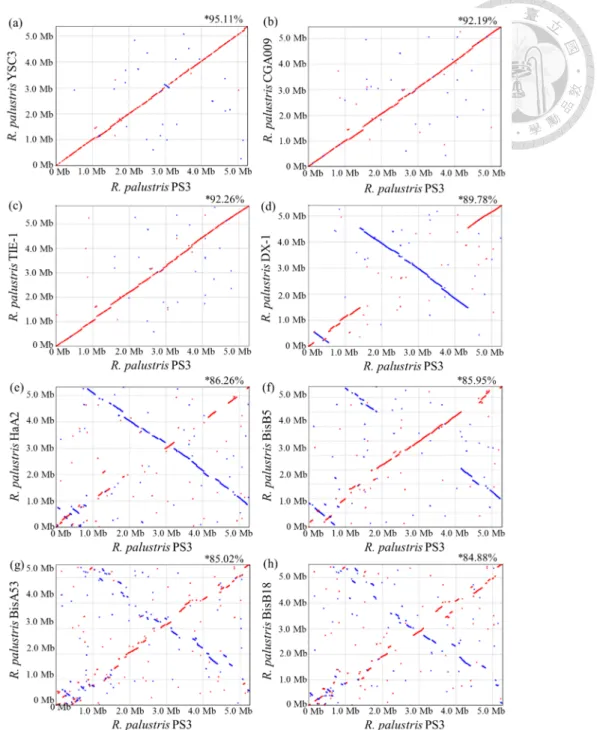
Figure 2-4 Pairwise genome alignments between R. palustris PS3 and other related strains. ... 57
Figure 2-5. Distribution patterns of homologous gene clusters. ... 58
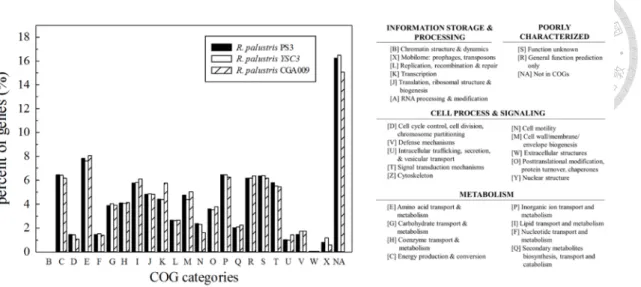
Figure 2-6. COG categories in the three R. palustris strains. ... 59
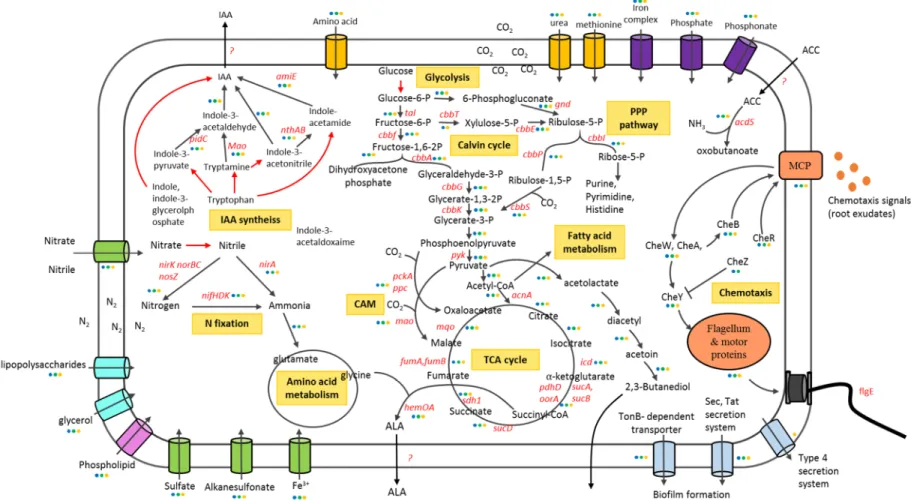
Figure 2-7. Schematic depiction of genes involved in metabolism (PPP pathway, TCA cycle, glycolysis, nitrogen assimilation), rhizosphere adaptation and plant growth promotion in R. palustris. ... 60
Figure 2-8. Putative genes related to plant growth-promotion in the genomes and biofilm production of PS3, YSC3 and CGA009 strains. ... 61
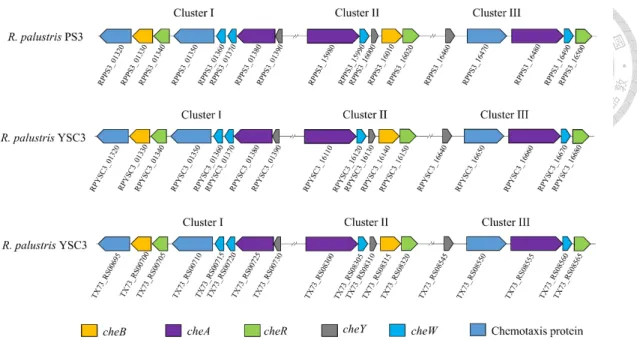
Figure 2-9. Comparison of the chemotaxis (che) gene clusters in R. palustris PS3, YSC3 and CGA009 strains. ... 62
Figure 2-10. Qualification and quantification of biofilm formation assay in R. palustris PS3, YSC3 and CGA009. ... 63
Figure 2-11. The phosphate solubilizing activity assay of R. palustris strains on the dissolve phosphorus agar (DPA) medium. ... 64
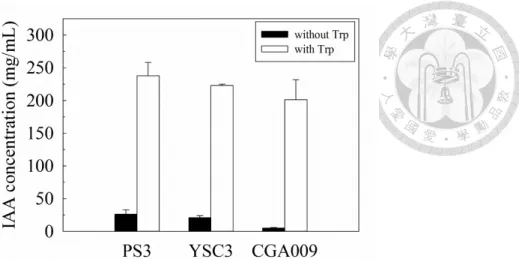
Figure 2-12 Indole-3-acetic acid production of R. palustris PS3, YSC3 and CGA009 strains. ... 65
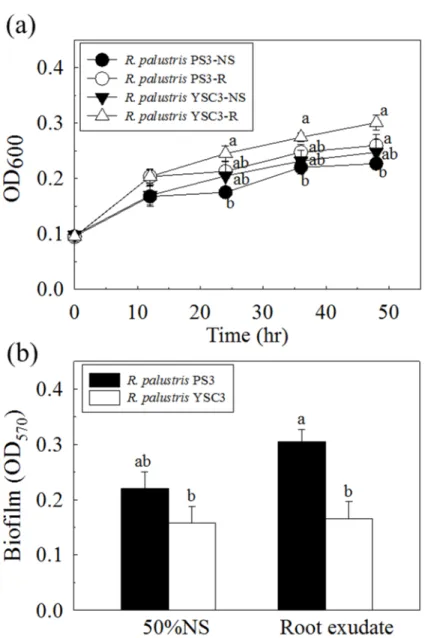
Figure 2-13. Effects of Chinese cabbage root exudates on growth and biofilm
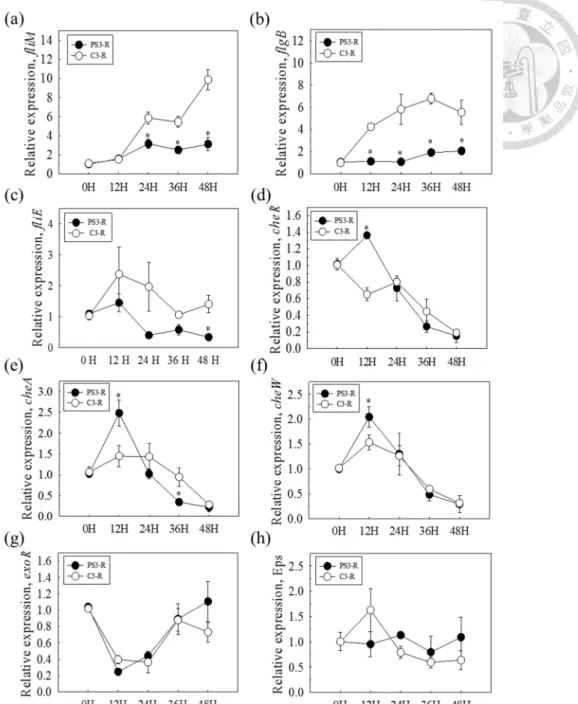
formation of the R. palustris PS3 and YSC3 strains. ... 66 Figure 2-14. Genes expression patterns of R. palustris strains in response to Chinese cabbage root exudate solution. ... 67 Figure 2-15. Genome-wide analysis of gene expression patterns in R. palustris PS3 (a) and YSC3 (b) strains in the presence of root exudates at 24 h. ... 68 Figure 2-16. Gene expression profiles of R. palustris PS3 and YSC3 strains in
response to root exudate for overall view and several representative categories. ... 69 Figure 3-1. Scheme of RSM process. ...103 Figure 3-2. Selection of appropriate components for an optimized R. palustris
medium. ...104 Figure 3-3. Three-dimensional response surfaces and contour plots of the effects of three factors on R. palustris PS3 biomass production. ...105 Figure 3-4. Plant growth-promoting effects of R. palustris PS3 incubated with the newly developed culture conditions on leafy vegetable in hydroponic system. ...106 Figure 3-5. Plant growth-promoting effects of R. palustris PS3 incubated with the newly developed culture conditions on leafy vegetable in soil system. ...107
LIST OF TABLE
Table 1-1 PGPR as potential inoculants for agricultural uses. ... 17
Table 2-1 Primer sequences used and product sizes in this study. ... 70
Table 2-2 Whole genome information of PGPR strains. ... 71
Table 2-3 Functional genes encoding PGPR traits ... 73
Table 2-4 General features of sequenced strains of R. palustris. ... 75
Table 3-1. Level and code of variables in the FFD experiments. ...108
Table 3-2. Level codes of the variables, experimental design and results from the FFD. ...109
Table 3-3. The experimental design and results from the path of steepest ascent method. ... 110
Table 3-4. Level and code of variables in the CCD experiments. ... 111
Table 3-5. The coded levels and real values for the experimental design and results of CCD. ... 112
Table 3-6. The variance analysis of the first-order model regression to biomass production. ... 113
Table 3-7. The variance analysis of the second-order regression model for biomass production. ... 114
Table 3-8. Comparison of PNSB medium and optimal medium parameters for R. palustris PS3 biomass production for 24 hours. ... 115
Table 3-9. The cost of different medium component for R. palustris PS3 fermentation. ... 116
Table 3-10. Quantification of total organic carbon and total nitrogen. ... 117
CHAPTER I INTRODUCTION
Recently, food supply has been a major concern in the word because global warming and rise in human population greatly increase crop demand. Therefore, how to increase crop productivity is an urgent issue. Traditionally, excessive use of chemical fertilizers is a general and simple approach for farmers to increase the harvest yields of crops (Tilman, 1998). However, only a small part of the fertilizers (10% to 40%) is taken up by plants, it means more than 60% of fertilizer is lost during farming (Adesemoye and Kloepper, 2009). The excessive application of chemical fertilizers in agriculture occurs universally and results in soil acidification and reduction of arable lands (Barak et al., 1997). In order to raise crop yields and reduce environmental impact, development of sustainable agriculture is required.
Plant growth promoting rhizobacteria
In 1978, Kloepper and Schroth suggested a concept of plant growth-promoting rhizobacteria, abbreviated as PGPR (Kloepper and Schroth, 1978). PGPR are diverse subgroup of rhizosphere-colonizing bacteria that exert beneficial effects on plant growth by various mechanisms (Adesemoye and Kloepper, 2009; Ahemad and Kibret, 2014; Beneduzi et al., 2012; Lugtenberg and Kamilova, 2009). These bacteria are able to colonize on the roots of plants (rhizosphere), to enhance plant growth directly or indirectly. PGPR not only provides enhancement on plant growth, some PGPR can also systemically activate plant defense mechanisms (Akhtar and Siddiqui, 2011). PGPR offers an alternative way to replace chemical fertilizer, pesticide, and supplements, and can be classified as three groups: (1) biofertilizers, (2) biocontrol agents, or biocontrollers or biopesticides, (3) bioremediators (Lugtenberg and Kamilova, 2009;
Vessey, 2003). The deduced modes of actions of PGPR include (1) facilitating the uptake of certain plant nutrients from soil, such as fixing nitrogen, solubilizing phosphate; (2) producing phytohormones, such as auxin and cytokinins; (3) preventing abiotic or biotic effects, by bioremediation and biocontrol traits (Goswami et al., 2016).
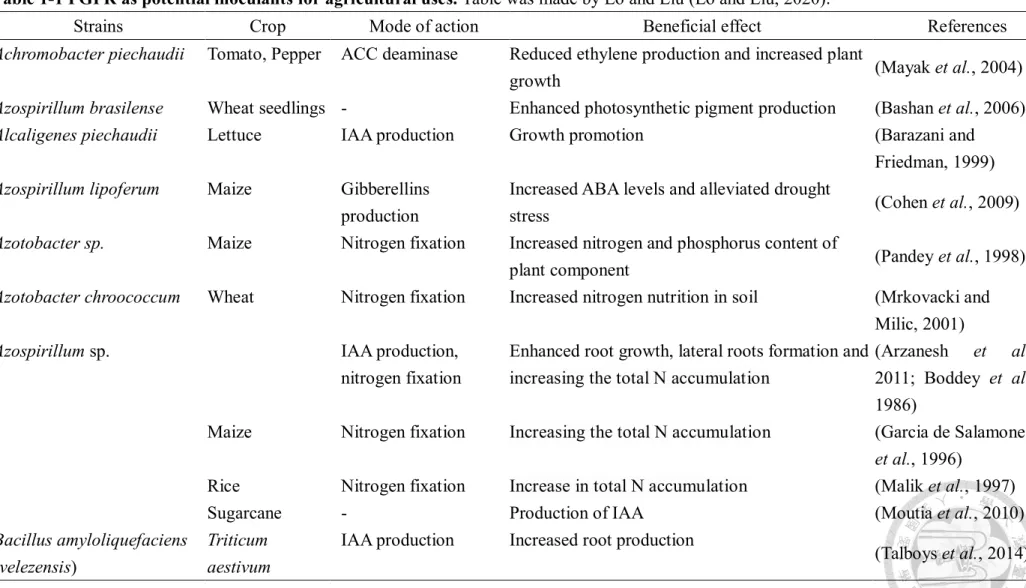
Figure 1-1 shows the general growth promoting modes of action from PGPR. To date, there are many bacterial species considered to be PGPR, such as Azotobacter, Bacillus, Burkholderia, Gluconacetobacter, Klebsiella, Pseudomonas, Rhizobium, etc (Kaymak, 2011). Further, several microbial species have been commercialized such as nitrogen fixation bacteria (Azospirillum spp., Azotobacter spp., Rhizobium spp., etc.) and phosphate solubilization bacteria (Bacillus spp., Pseudomonas spp., Aspergillus spp., Penicillium spp., etc.). The major products are based on Bacillus spp., including B.
thuringiensis, B. subtilis, B. amyloliquefaciens, etc. Other species such as Streptomyces spp., Pseudomonas spp. and Trichoderma spp. are also used in the common products of biopesticides. Table 1-1 shows the microorganisms as potential inoculants for agricultural uses.
PGPR with nitrogen fixing ability
Biological nitrogen fixation is a process that nitrogen is converted to ammonia by nitrogenase complex of microorganisms (Kim and Rees, 1994). PGPR fix nitrogen to facilitate plant growth have been widely investigated (Garcia de Salamone et al., 1996; Oberson et al., 2013; Stueken et al., 2015; Vessey, 2003). In general, nitrogen fixation occurs in root-nodules of leguminous plants that harbor rhizobia. It also can be carried out by the non-symbiotic bacteria, such as cyanobacteria, Azospirillum etc.
(Kim and Rees, 1994; Oberson et al., 2013). According to the metal cofactor, nitrogenase can be classified as Fe-nitrogenase, Mo-nitrogenase and V-nitrogenase (Bishop and Joerger, 1990). The gene clusters encoding nitrogenase are identified in
either symbiotic or non-symbiotic bacteria (Kim and Rees, 1994). Nitrogenase complex consist of two nitrogenase, Fe nitrogenase and Mo-Fe nitrogenase (homologous to V- Fe protein). The Fe protein is encoded by nifH and vnfH (for V-nitrogenase system), respectively. While, Mo-Fe and V-Fe are encoded by nifDK and vnfDK genes, respectively. Fe-protein offers electrons with reducing power while Mo-Fe protein uses these electrons to conserve nitrogen as ammonia (Kim and Rees, 1994). For rhizobia, because these bacteria invade plant tissue to form nodule, they can directly supply the nitrogen to plants. In contrast, the free-living nitrogen fixers are able to provide nitrogen source in the rhizosphere for nourishing plants (Bhattacharyya and Jha, 2012).Nitrogen-fixation are considered an important mechanism of PGPR, and the nitrogen fixing bacteria are also used as biofertilizer in agriculture.
PGPR with phosphate solubilizing ability
Phosphorus is an important limiting nutrient for plants and abound in soil.
Despite of a large quantity of phosphorus in earth’s crust, low available forms can be used in soils by plants. The critical factor for this low availability is due to insoluble forms of phosphorus (Bhattacharyya and Jha, 2012). Some PGPR are reported to increase the availability of phosphorus by solubilizing phosphate from either organic or inorganic phosphates, thereby promoting plant growth (Bhattacharyya and Jha, 2012;
Vassilev et al., 2006). Phosphate solubiliztion is carried out by various phosphatases and organic acids which are secreted by PGPR (Rodriguez et al., 2006). Comparison of both methods, bacteria secrete organic acid may be the primary mechanism, because PGPR colonize in rhizosphere can utilize sugars from root exudates to produce organic acids (Goswami et al., 2014). Among the phosphate solubilizing PGPR, Pseudomonas and Bacillus have been described as effective phosphate solubilizers (Goswami et al., 2015).
PGPR with phytohormone producing ability
PGPR has been demonstrated that can produce phytohormones, such as auxins, cytokinins, gibberellins and ethylene, etc (Ahemad and Kibret, 2014). Particularly, auxin has been regarded as critical hormone for PGPR to promote plant growth. In plants, auxin implicates in several stages of plant growth and development, including cell elongation, cell differentiation, root development, and so on (Davies, 2010). Indole- 3-acetic acid (IAA) is a most common auxin produced by PGPR. It is demonstrated that the IAA producing PGPR can increase plant growth (Ahemad and Kibret, 2014).
Applying such PGPR in rhizosphere increases the endogenous IAA concentration of plants, therefore, it shows beneficial effect on plant growth (Liu et al., 2016b). Bacterial IAA mainly affects the development of root system, such as increasing root size and lateral root number. All these changes result in an enhancement in its ability to uptake the nutrient from soil, therefor, improving growth capacity of plants. (Lee et al., 2011;
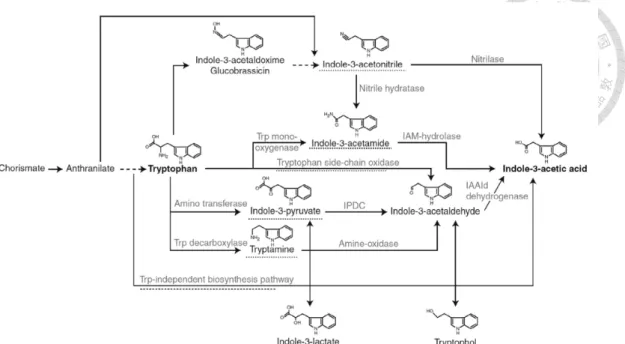
Liu et al., 2016b; Ramos Solano et al., 2008). IAA biosynthesis in bacteria via tryptophan dependent and independent pathways (Figure 1-2). Starting with tryptophan which is a major precursor, at least three tryptophan dependent pathways are identified (Spaepen et al., 2007). First, IAA synthesized via indole-3-acetic aldehyde. The conversion of tryptophan into indole-3-acetic aldehyde via indole-3-pyruvic acid or an alternative pathway in which tryptamine, finally conversed to IAA. This pathway is found in bacteria like Pseudomonas (Patten and Glick, 2002). Second, IAA biosynthesis via indole-3-acetamide which are found in various bacteria such as, Agrobacterium tumefaciens, Rhizobium and Bradyrhizobium (Morris, 1995; Sekine et al., 1989; Theunis et al., 2004). Third, IAA biosynthesis via indole-3-acetonitrile is shown in A. tumefaciens and Rhizobium spp (Kobayashi et al., 1995).. Although some intermediates may be different, these pathways share high similarity to those found in
plants (Spaepen et al., 2007). In tryptophan independent pathways, IAA is synthesized from indole-3-glycerolphosphate or indole. However, no critical enzyme related to this pathway has been identified yet. Azospirillum brasilense was reported to synthesize IAA through this pathway (Prinsen et al., 1993).
Ethylene is another important plant hormone involved in stress response, such as salinity, drought and pathogenicity (Abeles et al., 1992). It is well known that high concentration of ethylene has detrimental effect on plants, such as senescence, wilting and so on (Abeles et al., 1992). PGPR have been reported that can deteriorate the biosynthesis of ethylene due to the production of 1-aminocyclopropane-1-carboxylate (ACC) deaminase (Adesemoye and Kloepper, 2009; Ahemad and Kibret, 2014). PGPR take up ACC, i.e the precursor of ethylene, and convert it into 2-oxobutanoate and NH3
(Arshad et al., 2007). Accordingly, ACC deaminase producing PGPR may protect plants from the detrimental effects of ethylene induced by various stresses (Arshad et al., 2007).
Phototrophic bacteria
Phototrophic bacteria, including cyanobacteria, green bacteria and purple bacteria, convert light energy to chemical energy by photosynthesis (Pfennig, 1977).
Due to the ability of photosynthesis, these bacteria have a capability to survive suing photoautotrophic and photoheterotrophic, whereas most of bacteria can only support their life by chemoheterotrophic (Van Niel, 1954). For cyanobacteria, it is similar to plants that aerobic photosynthesis is generally used. In contrast, most of phototroph bacteria such as green bacteria and purple bacteria are different from plants. They don’t use water, but H2S and H2 as the electron donors in photosynthesis, and therefore no oxygen is produced in the reaction. Thus, this kind of photosynthesis also called as anaerobic photosynthesis and its efficiency is lower than that of aerobic photosynthesis
(Allen, 2005; Van Niel, 1954).
Purple bacteria show diversity of cell colors (red, orange and yellow) due to the various photosynthetic pigment such as bacteriochlorophyll a, bacteriochlorophyll b or carotenoid and etc. The purple bacteria that use H2S as reductant called purple sulfur bacteria, and the bacteria that perform photosynthesis without using H2S as electron donor, that are categorized as purple non-sulfur bacteria (Madigan and Jung, 2009). In purple bacteria, the light reaction is performed by two membrane protein complex, light harvesting complex 1 and 2 (LH1 and LH2). LH2 is responsibility for collecting incoming light then the energy is transferred to LH1 where the mainly photosynthetic reaction center locates (Scheuring et al., 2006).
Purple non-sulfur phototrophic bacteria
Purple non-sulfur phototrophic bacteria (PNSB) is one of the major groups of phototrophic microorganisms, which has a metabolic diversity allows them to growing in a broad range of environments (Madigan and Jung, 2009). According to their morphology, PSNB can be divided as Rhodospirillum, Rhodobacter, Rhodopila, Rhodomicrobium, Rhodopseudomonas and Rhocyclus (Madigan et al., 2006). Compare to purple sulfur phototropic bacteria, mainly PNSB can’t use H2S as electron donor but these strains still can survive under the lower concentration of H2S. PNSB can grow under the dark by using common organic compounds or hydrogen as reductant to perform the anaerobic respiration (Brock et al., 2003). Most of PNSB stains can use acetic acid as energy source, however, the metabolic pathway was quite different. The major metabolism of acetic acid was acetyl-CoA production (Laguna et al., 2011).
Mostly, the assimilation of acetic acid in PNSB was performed by TCA cycle and the key enzymes were isocitrate lyase and malate synthase (Albers and Gottschalk, 1976).
Nevertheless, some PNSB such as Rhodospirillumrubrum, which lack isocitratelyase,
so they conserve acetic acid as pyruvate by carboxylation, further conserved to oxaloacetate (Albers and Gottschalk, 1976). Moreover, the assimilation of acetic acid in R. gelatinosus is carried out via serine-hydroxypyruvate pathway (Albers and Gottschalk, 1976). It also found that PNSB can use NO3-, NO2- and N2O as electron donor in electron transport chain of cellular respiration (Griffin et al., 2007). All of PNSB have the ability to fix nitrogen from air. Most of PNSB can use NH4+, N2 and serval organic nitrogen as nitrogen sources. In contrast, few of PNSB utilize NO3- as nitrogen source. However, existence of NH4 and glutamic acid will inhibit the reaction of NO3- assimilation in PNSB (Imhoff and Trüper, 1992). When PNSB use N2 and glutamic acid as nitrogen sources, the PNSB can utilize various carbon to generate CO2
and H2 (Imhoff and Trüper, 1992). Exactly, the hydrogen gas production of PNSB is controlled by hydrogenase and nitrogenase (Kim et al., 1980). Some studies suggest that PNSB fix carbon through C4 pathway. In this pathway, CO2 and pyruvate or phosphoenolpyruvate (PEP) will be conserved to oxaloacetate (OAA) by pyruvate, orthophosphate dikinase (PPDK), then enter to TCA cycle. Moreover, it was found that PNSB can use reduce inorganic or organic substrate, donating electron to generate NADPH by reverse electron transport (Yoch, 1978). Therefore, it was considered that electron donor is a critical factor to determine the metabolism model in PNSB (Yoch, 1978).
Application of purple non-sulfur photosynthetic bacteria
Polyhydroxybutyrate (PHB) is a polyhydroxyalkanoate (PHA) that is a form of energy storage molecule and produced via fermentation (Jendrossek and Pfeiffer, 2014).
Most of PNSB have ability to produce or metabolize PHAs. On the other hand, PNSB can also accumulate PHAs and store them in intracellular (Doudoroff and Stanier, 1959;
Merrick and Doudoroff, 1961). It has been reported that when R. sphaeroides used
acetate as single carbon source or combined with malate, pyruvate, glucose or propionate in fermentation, the PHAs accumulation can approximate to 50% cell dry weight (Brandl et al., 1991). Although the PHA production by PNSB is not high, using PNSB to produce PHA is still a potential strategy. Because PHA can be produced from waste materials, such as waste water of sugar refineries and palm oil waste (Ali Hassan et al., 1996; Yigit et al., 1999).
Many studies have shown that PNSB can be applied for waste water treatment.
For example, combining the PNSB fermentation tank, aeration tank and algae culture tank in wastewater treatment program, the BOD concentration could be reduced from 1000 mg/L to 50 mg/L (Kobayash.M and Nakanish.H, 1971). In antibiotic production wastewater, lipid concentration was decreased from 1500 mg/L to 180 mg/L (about 90% removal), by R. capsulata and algae mixture treatment in aerobic condition (Sawada and Rogers, 1977). Harwood and Gibson found that R. palustris strains can metabolize diverse aromatic compounds under anaerobic and aerobic conditions (Harwood and Gibson, 1988). on the other hand, phototrophic bacterium R. capsulate which produces an antiviral substance that removed 97.4% of coliphage during the purification process of pigpen wastewater (Hirotani et al., 1990). Recently, it was reported that PNSB can used in the application of microbial fuel cells (MFC) for electron generation (Xing et al., 2008).
It has also been reported that suppling PBSB into animal feed can decrease the levels of serum cholesterol. R. palustris showed the potential to reduce the serum cholesterol in rats fed with high cholesterol diet (Lee et al., 1990). Similar results also observed in the case that feeding diet supplemented with 2% R. capsulatus or 2% R.
palustris significantly reduce the serum cholesterol levels in rats (Tsujii et al., 2007).
Feeding 0.04% R. capsulatus dramatically removed the levels of cholesterol and triglyceride from serum, liver and muscle in Broiler Meat, while the high-density
Lipoprotein cholesterol cholesterol (HDL-C) in serum and unsaturated fatty acid such as oleic acid, linoeic acid, linolenic aicd in muscle were significantly increased (Salma et al., 2007).
Rhodopseudomonas palustris
Rhodopseudomonas palustris, an α-poteobacteria species is one of purple nonsulfur photosynthetic bacteria, which belong to α-Proteobacteria and is widely distributed in environment (Imhoff, 2006). Because R. palustris has extraordinary metabolic versatility, so it can inhabit in a variety of conditions (Larimer et al., 2004).
These exceptional abilities of metabolism including four major modes (Figure 1-3):
photoautotrophic (using light as energy sources and carbon dioxide as carbon sources), photoheterotrophic (using light as energy sources and organic compounds as carbon sources), chemoheterotrophic (using organic compounds as carbon and energy sources) and chemoautotrophic (using inorganic compounds as energy source and carbon dioxide as carbon source). Moreover, R. palustris is well known to inhabit in microaerobic environment with light and also grows aerobically in dark. According to these characteristics, R. palustris has been broadly used in industry for bioremediation, sewage treatment, and removal of phytotoxic compounds (e.g., hydrogen sulfide)(Idi et al., 2015) . In addition, this bacterium can convert complex organic compounds into biomass and bioenergies, no matter the substrates are plant-derived, pollutants or aromatic compounds (Larimer et al., 2004; Liu et al., 2015; Oda et al., 2003; Shi et al., 2014; Zhang et al., 2015a). On the other hand, R. palustris was also applied as biofertilizer to improve crop yield (Kornochalert et al., 2014; Wong et al., 2014).
Several studies reported that R. palustris can utilize citric acid, aromatic organic compounds, such benzoate, 3-hydroxybenzoate and 1,3,5-trihydozybenzene as carbon sources (Dutton and Evans, 1969). Moreover, R. palustris also can use acrylamide as
carbon source. In contrast, it was found Rhodospirillum rubrum strain UR 1 and Rhodobacter capsulatus strain B10 can catabolize acrylamide. This result indicated that acrylamide metabolism is not a ubiquitous characteristics of PNSB (Wampler and Ensign, 2005). So far, it has suggested that R. palustris can catabolize various carbon sources, such as acetate, arabinose, ribose, xylose, glucose, fructose, mannose, sorbose, turanose, lyxose, arabitol, tagatose, benzoate, butyrate, caproate, caprylate, ethanol, formate, fumatate, glycerol, glycoate, lactate, malate, malonate, propionate, pyruvate, succmate, valerate, casammo acids and acrylamide, taurine, potassium 5-ketogluconate (Imhoff and Trüper, 1992; Novak et al., 2004; Wampler and Ensign, 2005; Wong et al., 2014).
R. palustris PS3
R. palustris PS3 was isolated from Taiwanese paddy soil (Wong et al., 2014). It forms rod-shaped cells with approximately 1.0 µm in length and presents round, convex and glossy colony on the nutrient agar plate (Figure 1-4). The colonies are pearl-white under aerobic condition and turn to blood red when grow under anaerobic with illumination (Figure 1-4 b). It has been demonstrated that PS3 can utilize serval carbon sources such as glycerol, D-glucose and D-fructose, and fix atmospheric nitrogen into ammonia (Wong et al., 2014). PS3 possesses the PGP traits like production of IAA and synthesis of phosphatase, etc (Wong et al., 2014). PS3 not only showed beneficial effects on plant growth, but also increased the agronomic nitrogen use efficiency of plants (Wong et al., 2014). Moreover, PS3 can reduce the nitrate contents of host plant, and improve both nitrogen and carbon metabolic efficiencies of plant in hydroponic system (Hsu et al., 2015; Shen, 2016). It has already been proved that neither medium nor dead PS3 cells was able to promote plant growth (Wong et al., 2014), and both viability (i.e., culturability) and vitality (i.e., metabolic activity) of this bacterium are
crucial for the plant beneficial traits (Lee et al., 2016).
Specific aims
According to the traits mentioned above suggest that PS3 can serve as a potential PGPR for agricultural applications. However, some issues remain to be elucidated: (1) the underlying mechanisms of PS3 to promote plant growth still unknown, and (2) the scale-up fermentation is required for commercialization of R. palustris PS3. Therefore, I would like to elucidate these two themes in this study. The outlines of the contents are as follows:
Chapter II: Whole-Genome Sequencing and Comparative Analysis of Two Plant- Associated Strains of Rhodopseudomonas palustris (PS3 and YSC3)
According to the 16S rDNA analysis, the phylogenetic tree indicated that PS3 has a highly close relationship with other R. palustris isolates. However, only PS3 showed significantly plant growth promoting effects (Wong et al., 2014). To elucidate the underlying mechanisms of PS3 for promoting plant growth, I have performed the whole genome sequencing of PS3 to understand the genetic background and identify the potential genes associated with plant growth promoting by high-throughput DNA sequencing in the first part of my study. Hybrid de novo assembly was carried out by combination of shotgun (Illumina) and single molecule real-time (PacBio) reads. In addition, I compared the genome of PS3 with inefficient strain YSC3 strain to identify unique gene from PS3 strain involving in plant growth promoting functions. Moreover, I focused on genes involved in carbon and nitrogen metabolism as well as plant growth promoting genes. Through this study, I addressed the unique features of the PS3, which were attributed to its beneficial traits.
Chapter III: Development of A Low-Cost Culture Medium For Rapid Production of Plant Growth-Promoting Rhodopseudomonas palustris PS3 Strain
To scale-up the fermentation of R. palustris PS3 for its commercialization, I optimized the culture conditions of R. palustris PS3 by response surface methodology.
Firstly, “one-factor-at-a-time” technique was applied to screen the nitrogen and carbon source. Subsequently, the effects of selective nitrogen and carbon source as well as pH values, temperatures and dissolved oxygen were respectively evaluated for PS3 growth by fractional factorial design, and the suitable range of individual factor was estimated by steepest ascent path. Finally, according to the above data, I constructed a response surface model by central composite design. The optimization of fermentation conditions was analyzed by RSM. Besides, I also verified the effect of newly developed PS3 fermentation broth on the plat growth promotion.
Figure 1-1. Deduced modes of action of PGPR. PGPR can promote plant growth through various mechanisms, such as production of pytohormones and secondary metabolites. These factors enhance the development of roots, especial in lateral roots and root hairs. PGPR also affect nutrition availability through nitrogen fixation or phosphorus solubilization. Besides they are also able to mediate the physiology of plants by regulating gene expression in plant cells (Adapted from (Vacheron et al., 2013)).
Figure 1-2. Overview of the biosynthetic pathway of IAA in bacteria. The intermediate referring to the name of the pathway or the pathway itself is underlined with a dashed line. IAAld, indole-3-acetaldehyde; IAM, indole-3-acetamide; IPDC, indole-3-pyruvate decarboxylase; Trp, tryptophan (Adapted from Spaepen et al.
(2007)).
Figure 1-3. The four types of metabolism of R. palustris that support its growth.
The multicolored circle in each cell represents the enzymatic reactions of central metabolism (Adapted from Larimer et al. (2004)).
Figure 1-4. Morphology characteristics of R. palustris strain PS3. (a) colonies grown under aerobic condition for 4 days; (b) colonies developed under anaerobic condition for 7 days; (c) vegetative cells incubated aerobically. Scales bars equal 0.5 cm in (a) and (b), 10μm in (c). This figure was adapted from (Wong et al., 2014).
doi:10.6342/NTU20200342217
Table 1-1 PGPR as potential inoculants for agricultural uses. Table was made by Lo and Liu (Lo and Liu, 2020).
Strains Crop Mode of action Beneficial effect References
Achromobacter piechaudii Tomato, Pepper ACC deaminase Reduced ethylene production and increased plant
growth (Mayak et al., 2004)
Azospirillum brasilense Wheat seedlings - Enhanced photosynthetic pigment production (Bashan et al., 2006)
Alcaligenes piechaudii Lettuce IAA production Growth promotion (Barazani and
Friedman, 1999) Azospirillum lipoferum Maize Gibberellins
production
Increased ABA levels and alleviated drought
stress (Cohen et al., 2009)
Azotobacter sp. Maize Nitrogen fixation Increased nitrogen and phosphorus content of
plant component (Pandey et al., 1998)
Azotobacter chroococcum Wheat Nitrogen fixation Increased nitrogen nutrition in soil (Mrkovacki and Milic, 2001)
Azospirillum sp. IAA production,
nitrogen fixation
Enhanced root growth, lateral roots formation and increasing the total N accumulation
(Arzanesh et al., 2011; Boddey et al., 1986)
Maize Nitrogen fixation Increasing the total N accumulation (Garcia de Salamone et al., 1996)
Rice Nitrogen fixation Increase in total N accumulation (Malik et al., 1997)
Sugarcane - Production of IAA (Moutia et al., 2010)
Bacillus amyloliquefaciens (velezensis)
Triticum aestivum
IAA production Increased root production
(Talboys et al., 2014)
doi:10.6342/NTU20200342218
Cucumber IAA production Plant growth promotion (Shao et al., 2014)
Tomato - Inhibited infection of Tomato mottle virus (Murphy et al., 2000)
Wheat Improvement in homeostatic mechanisms (Kasim et al., 2013)
Canola Production of lipopeptide antibiotics
Produced the iturin A, bacillomycin D and surfactin to suppress growth of Leptosphaeria maculans causing Blackleg disease
(Ramarathnam et al., 2011)
Capsicum Bacteriocins production
Bacterial antagonism to Ralstonia solanacearum (Hu et al., 2010)s Bacillus thuringiensis Wheat ACC deaminase Reduced volatile emissions and increased
photosynthesis
(Timmusk et al., 2014)
Bacillus subtilis Platycladus orientalis
Cytokinin production Increased ABA levels in shoots and enhanced the stomatal conductance
(Liu et al., 2013b) Soybean IAA production Increased production of root hairs (Araújo et al., 2005) Pepper Production of
antibiotics
Suppression of growth of Myzus persicae (Kokalis–Burelle et al., 2002)
Tomato Induce systemic resistance
Enhanced activities of chitinases and β-1,3- glucanase to inhibited growth of Fusarium oxysporum f. sp. lycopersici causing Tomato wilt disease
(Shanmugam and Kanoujia, 2011)
Surfactin production Surfactin production induced the activation of lipxygenase to against Botrytis cinerea growth
(Ongena et al., 2007) Bacillus pumilus Ocimum
sanctum
IAA production Plant growth promotion (Murugappan et al.,
2013)
doi:10.6342/NTU20200342219
Bradyrhizobium sp. Radish IAA production Increased the dry matter yield of radish (Antoun et al., 1998) Burkholderia sp. Rice Nitrogen fixation Increase in N content in plant (Divan Baldani et al.,
2000) Enterobacter cloacae Rapeseed
(Brassica.
napus)
ACC deaminase Increases in root and shoot lengths (Saleh and Glick, 2001)
Methylobacterium fujisawaense
Canola (Brassica campestris)
ACC deaminase Promoted root elongation (Madhaiyan et al.,
2006)
Pseudomonas fluorescens Pisum sativum ACC deaminase Induced longer roots and uptake of water (Zahir et al., 2008)
Soybean Cytokinin Plant growth regulation (García de Salamone
et al., 2001) Wheat ACC deaminase Increased NPK uptake and inhibited ethylene
production
(Nadeem et al., 2010;
Shaharoona et al., 2008)
Tobacco Induce systemic resistance
Induced salicylic acid- dependent
activation of PR-1 gene against Pseudomonas syringae pv. tabaci
(Park and Kloepper, 2000)
Green gram (Vigna radiata)
- Regulated the catalase and peroxidase (Saravanakumar et
al., 2011) Rice Induction of systemic
resistance
Against Xanthomonas oryzae pv.Oryzae in rice leaves
(Vidhyasekaran et al., 2001)
Pseudomonas putida Vigna radiata L ACC deaminase Inhibited ethylene production (Mayak et al., 1999)
doi:10.6342/NTU20200342220
Rice IAA production and phosphate
solubilization
Increased plant height and root length of rice (Ashrafuzzaman et al., 2009)
Soybean Gibberellins production
Plant growth promotion (Kang et al., 2014)
Chickpea Phosphate solubilization, siderophore
production, and IAA production
Improved the growth and the saline tolerance of plant
(Patel et al., 2012)
Cotton - Regulated the ion uptake and improved
production of endogenous indole acetic acid (IAA) content and reduced abscisic acid (ABA) content
(Yao et al., 2010)
Rhizobium leguminosarum Rape and lettuce Cytokinin production Plant growth promotion with possible involvement of the plant growth regulators indole-3-acetic acid and cytokinin
(Noel et al., 1996)
Rhizobium tropici Bean (Phaseolus vulgaris L.)
- Increased nodulation as well as nitrogen fixation (Figueiredo et al., 2008)
Rhodobacter sphaeroides Tomato - Enhanced quality of tomato fruit and increased ascorbic acid content
(Kondo et al., 2010) Rhodopseudomonas Chinese - Plant growth promotion, increased the nitrogen use (Hsu et al., 2015;
doi:10.6342/NTU20200342221
palustris cabbage
(Brassica rapa chinensis)
efficiency and reduced the nitrate content in plant Wong et al., 2014)
Tobacco Indole‐3‐acetic acid and 5‐aminolevulinic acid production
Promote growth and germination (Su et al., 2017)
Induces systemic resistance
Induces systemic resistance of plant to against tobacco mosaic virus
(Su et al., 2017) Pakchoi
(Brassica rapassp.
Chinensis)
- Enhanced photosynthesis and crop yield (Xu et al., 2016)
CHAPTER II
Whole-Genome Sequencing and Comparative Analysis of Two Plant-Associated Strains of Rhodopseudomonas
palustris (PS3 and YSC3)
The content in Chapter II has been published in Scientific Reports as shown below. This first author publication and its quality full-filled the Ph.D. thesis examination application requirements of Institution of Biotechnology, National Taiwan University.
This desertion or any part of it has not been submitted for any degree, diploma, or other qualification at any other university. It is the result of my own work except where mentioned in the text.
Lo, K.J., S.S. Lin, C.W. Lu, C.H. Kuo and C.T. Liu. 2018. Whole-genome sequencing and comparative analysis of two plant-associated strains of Rhodopseudomonas palustris (PS3 and YSC3). Sci. Rep. 8(1): 12769. doi: 10.1038/s41598-018-31128-8
Author Contributions:
K.J. Lo carried out the experiment, experimental data analysis, bioinformatic data analysis and manuscript writing. C.W. Lu performed the bioinformatics analysis for the genome assembly. S.S. Lin provided the bioinformatic platform for data analysis. C.H.
Kuo performed the bioinformatics for gene prediction and annotation as well as manuscript writing. C.T. Liu is the corresponding authors in charge of the project design and manuscript writing.
Summary
Rhodopseudomonas palustris strains PS3 and YSC3 belong to purple non-sulfur phototrophic bacteria that were isolated from Taiwanese paddy soils. Strain PS3 showed beneficial effects on plant growth and enhances the agronomic nitrogen using efficiency of host plant. However, strain YSC3 has no significant effect on plant growth.
According to whole genomic analyses, PS3 and YSC3 strain showed similar genomic structures, individually contains a one circular chromosome with 5,269,926 or 5,371,816 bp in size, with 4,799 or 4,907 protein-coding genes, respectively. In this study, a large class of genes associated with plant-growth promotion, such as nitrogen fixation-, IAA synthesis-, phosphate solubilization and ACC deamination-related genes, were annotated. The growth rate, biofilm formation, and the relative expression levels of several chemotaxis-associated genes were significantly higher for PS3 than for YSC3 upon treatment with root exudates. These results suggested that PS3 has a better response to the host plants, which may contribute to the successful interactions between PS3 and plant hosts. In addition, these findings indicate that the existence of gene clusters associated with plant growth promotion is required but not sufficient for bacteria to exhibit the ability of plant-growth promotion.
Introduction
In 1978, a concept of plant growth-promoting rhizobacteria (PGPR) was proposed by Kloepper and Schroth (Kloepper and Schroth, 1978). It refers as diverse soil bacteria colonize in rhizosphere, and provide beneficial effects on plant growth via various mechanisms (Kloepper and Schroth, 1978). The promotional activity of PGPRs including increasing nutrient availability (e.g., nitrogen fixation), nutrient solubilization (e.g., phosphate solubilization) as well as production of phytohormones (e.g., indole acetic acid (IAA), 2,3-butanediol, and cytokinins) (Ahemad and Kibret,
2014; Goswami et al., 2016; Lugtenberg and Kamilova, 2009). Moreover, PGPR can strengthen plant tolerance against environmental stress by metabolizing 1- aminocyclopropane-1-carboxylic acid (ACC), a precursor of stress hormone - ethylene (Ahemad and Kibret, 2014; Goswami et al., 2016; Lugtenberg and Kamilova, 2009).
In addition, PGPR can also protect plants form pathogen infection by secreting antibiotics or activating induced systemic resistances (Beneduzi et al., 2012). Due to these properties of PGPR, so far, these bacteria are widely used as biofertilizers or biocontrol agents in agriculture (Lugtenberg and Kamilova, 2009). So far, several micrograms were identified as PGPR, such as Azospirillum humicireducens (Yu et al., 2018), Bacillus amyloliquefaciens (Zhang et al., 2015b), Bacillus velezensis (Chen et al., 2007), Bradyrhizobium japonicum (Kaneko et al., 2002), Pseudomonas fluorescens (Loper et al., 2007), Pseudomonas putida (Ponraj et al., 2012), Rhizobium leguminosarum (Young et al., 2006), etc. These microbes were regarded as PGPR not only verified by planta experiments, but also speculated by their functional genes in genome.
Rhodopseudomonas palustris is a phototrophic purple non-sulfur bacterium (PNSB), which has the innate and extraordinary metabolic versatility. It can sustain itself by one of the four modes of metabolism, including photoautotrophic, photoheterotrophic, chemoautotrophic and chemoheterotrophic states (Larimer et al., 2004). Due to this diverse metabolic property, R. palustris is widely distributed in nature, including river, pond water, sediments, wetlands, and paddy fields (Hiraishi and Kitamura, 1984; Oda et al., 2002).
This bacterium has been widely used in industrial applications for bioremediation and sewage treatment and for the removal of phytotoxic compounds (Austin et al., 2015; Idi et al., 2015). In addition, this bacterium can convert complex organic compounds into biomass and bioenergy using substrates that are plant-derived
compounds, pollutants, or aromatic compounds (Larimer et al., 2004; Liu et al., 2015;
Oda et al., 2003; Shi et al., 2014; Zhang et al., 2015a). Some studies have indicated that R. palustris can also be used as a biofertilizer to improve crop yields (Kornochalert et al., 2014; Nunkaew et al., 2014; Wong et al., 2014). In our previous study, we isolated the R. palustris strain PS3 (abbreviated as PS3) from Taiwanese rice paddy soil (Wong et al., 2014). Strain PS3 can have beneficial effects on plant growth and can enhance the utilization efficiency of fertilizers in either soil or hydroponic cultivation system (Hsu et al., 2015; Wong et al., 2014). Although these studies showed that strain PS3 is a promising PGPR, genomic information and the underlying molecular mechanisms for plant growth promotion (PGP) by PS3 are yet to be ascertained.
Systematic analysis of whole-genome sequences is a powerful strategy to identify either causal genes that contribute to plant growth-promoting activities or potential PGPR candidates (Gupta et al., 2014; Liu et al., 2016a; Magno-Perez-Bryan et al., 2015;
Shen et al., 2013; Taghavi et al., 2010). Scientists can obtain more genomic information and deduce the underlying promoting mechanisms of PGPR from whole genome sequence. As shown in Table 2-2, I listed up the whole genomic information of several PGPR strains. Furthermore, the genes associated with plant growth-promoting functions were summarized in Table 2-3.
Next-generation sequencing (NGS) technologies provide a quick and convenient approach to resolve the whole-genome sequence and investigate the transcriptomes of PGPR. The NGS technologies such as Roche/454, Illumina are widely used to study the genes of eukaryotes and prokaryotes (MacLean et al., 2009). These methods used short-read (~150-300 bp) sequencing and containing some advantages, such as cost- effective, accurate, and diversity of analysis tools (Heather and Chain, 2016). However, there were some limitations of short-read sequencing, especially in highly repetitive and complex genome, including high guanine-cytosine (GC) contents, or multiple
homologous elements in sequences (Nagarajan and Pop, 2013; Treangen and Salzberg, 2011). It may result in sequencing error and lose certain genomic regions (Ashley, 2016;
Delaneau et al., 2013; Mavromatis et al., 2012). Although using the long-read sequencing technologies such as Oxford Nanopore Technologies (ONT)(Deamer et al., 2016) or single-molecule sequencing technology (SMRT) Pacific Biosciences (PacBio) sequencing platforms (Levene et al., 2003; Quail et al., 2012) can resolve the above issues, these technologies have high relative error in sequencing with about 11-14%
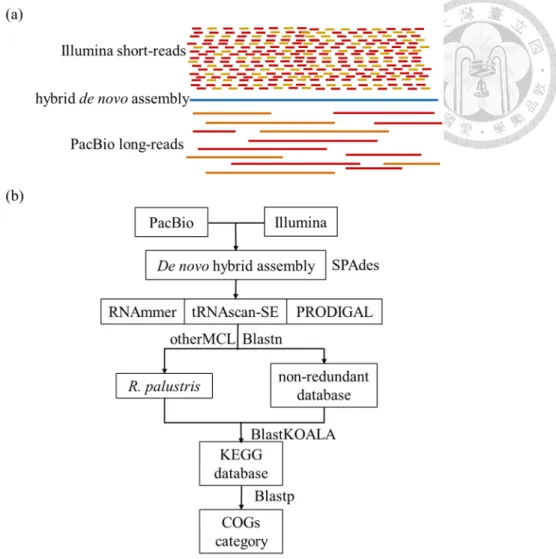
(Pollard et al., 2018). Therefore, it is hard to assemble the complete genomic sequences with high-quality by individual sequencing technology. To address these issues, short- reads and long-reads hybrid assembly techniques were proposed to improve the genome assembly (Utturkar et al., 2014). In this strategy, long reads provide the information for genomic structure and short reads improve the detailed assembly at sequencing, and can be used to correct the errors from long reads. Therefore, combination of short-read and long-read sequencing datasets appear a promising approach to completely resolve the genome assemblies with accuracy (Berbers et al., 2020; De Maio et al., 2019; Risse et al., 2015; Sovic et al., 2016; Wick et al., 2017a; b).
Although few studies conducted the whole-genome sequencing of R. palustris strains, such as CGA009, HaA2, BisB18, and TIE (Larimer et al., 2004; Oda et al., 2008; Oda et al., 2005), none of these strains are plant-associated. Here I focus on the elite strain R. palustris PS3, which was isolated from Taiwanese paddy soil and displayed plant growth-promoting (Hsu et al., 2015; Wong et al., 2014). In order to elucidate the potential modes of action via which R. palustris PS3 has beneficial effects on plants, I compared the genomic characterizations of two plant-associated R. palustris strains- PS3 and YSC3. They are the effective PGPR strain and the ineffective strain, respectively (Wong et al., 2014). For whole-genomic sequencing, I applied two NGS techniques to obtain the sequencing reads, then performed the hybrid de novo assembly.
Identification of genes associated with plant growth-promotion and genomic comparative analyses are very important to understand the underlying mechanisms of PS3. Moreover, I also compared the genomic compositions of these two strains with the genomic representative strain for this species, R. palustris CGA009. Finally, I focused on genes involved in carbohydrate, nitrogen metabolism, phosphate solubilization, phytohormone production, biofilm formation, chemotaxis, and plant colonization. The flow chart of the whole genomic analysis in this chapter was described in Figure 2-1.
Materials and methods
Preparation of phototrophic bacterial inoculant
The R. palustris strains PS3 and YSC3 are PNSB and were both isolated from Taiwanese paddy soils (Wong et al., 2014). PS3 is an effective PGPR, whereas YSC3 is not. For bacterial inoculant preparation, a single colony was picked and inoculated into 3 mL of PNSB broth as described previously (Wong et al., 2014). The culture was then incubated for 24 h at 37°C (200 rpm). Subsequently, 2.5 mL of these cultures were transferred into 250-mL Erlenmeyer flasks containing 50 mL of fresh PNSB broth. The cultures were incubated under the conditions described above, and the log-phase bacterial cells were harvested for genomic DNA extraction.
Genomic DNA preparation
A 1-mL suspension of the log-phase bacterial culture was collected in a 2.0-mL Eppendorf tube and centrifuged at 3,000 × g at 4°C. Subsequently, the supernatant was removed, and the Eppendorf containing the cell pellet was snap-frozen in liquid nitrogen. The cell pellet was homogenized by adding sterile steel beads and rapidly shaking the microcentrifuge tubes back and forth at 9,000 rpm for 1 min in a SH-100 homogenizer (Kurabo, Japan). The homogenization process was repeated three times.
The Gentra® Puregene® Kit (QIAGEN) was used for genomic DNA purification according to the manufacturer’s protocol. The quantity and quality of the total DNA was assessed using UV spectrophotometry (Nanodrop ND-1000, J & H technology Co., Ltd.), and the OD260/280 value of the DNA was higher than 1.80. Agarose gel electrophoresis (0.75%) was used to ensure that the gDNA was intact. Samples containing greater than 25 μg of gDNA were used to perform whole-genome sequencing.
Whole-genome sequencing
We utilized the MiSeq (Illumina) and the PacBio RSII (Pacific Biosciences) platforms to perform whole-genome shotgun sequencing. The sequencing service was provided by Genomics BioSci & Tech Co., Ltd. (New Taipei City, Taiwan). For Illumina MiSeq sequencing, the DNA library was constructed using the Illumina TruSeq Nano DNA HT Sample Prep Kit according to the TruSeq DNA Sample Preparation protocol (Illumina). This library was diluted and sequenced with 600 paired-end cycles on the Illumina MiSeq instrument by following the standard protocol.
For the PS3 strain, the insert size was 500 bp, and 10,471,982 read-pairs and ~3.6 Gb of raw data were obtained; and for the YSC3 strain, the insert size was 500 bp, and 11,242,474 read-pairs and ~3.8 Gb of raw data were obtained. For PacBio SMRT sequencing, the DNA library was constructed according to the PacBio SampleNet – Shared Protocol (Pacific Biosciences). After dilution, the library was loaded onto the instrument with the DNA Sequencing Kit 4.0 v2 (part number PB100-612-400) and a SMRT Cell 8 Pac for sequencing. A primary filtering analysis was performed with the RS instrument, and a secondary analysis was performed using the SMRT analysis pipeline, version 2.1.0. For the PS3 strain, the average length of the reads was 7,112 bp, and 164,831 reads and ~1.1 Gb of raw data were obtained; and for the YSC3 strain, the
average length of the reads was 6,342 bp, and 192,795 reads and ~1.2 Gb of raw data were obtained.
De novo genome assembly
The de novo genome assembly was based on the paired-end Illumina reads and the PacBio reads. The raw Illumina reads were trimmed at the first position from both the 5’- and 3’-ends that had quality scores lower than 20. After discarding these reads, all Illumina reads were shorter than 210 bp, and high-quality sets of 10,470,949 (PS3 strain, ~2.6 Gb of raw data) and 11,241,446 paired reads (YSC3 strain, ~2.8 Gb of raw data) were obtained. These trimmed reads were individually matched with the corresponding PacBio reads and used as the input for SPAdes Genome Assembler, version 3.5(Bankevich et al., 2012) with the default parameters. Finally, the whole- genome sequences were obtained, and the genomic sizes of PS3 and YSC3 were 5,269,926 bp and 5,371,816 bp, respectively.
Genome annotation
Annotations of the PS3 and YSC3 genomes were based on the procedures described by Cho et al. (2015). The programs RNAmmer (Lagesen et al., 2007), tRNAscan-SE (Lowe and Eddy, 1997), and PRODIGAL (Hyatt et al., 2010) were used for gene prediction. The genomic sequence of R. palustris CGA009 (Larimer et al., 2004) was used as the reference, and the initial annotation of each protein-coding gene was conducted by OrthoMC (Li et al., 2003) with a BLAST (Camacho et al., 2009) e- value cutoff of 1e-15 and an inflation value of 1.5. Then, BLASTP(Camacho et al., 2009) searches against the NCBI non-redundant (nr) protein database, BlastKOALA (Kanehisa et al., 2016), and the PATRIC platform (Wattam et al., 2017) were used for manual curation to improve the annotation. For functional categorization, all protein-
coding genes were used to run BLASTP (Camacho et al., 2009) searches against the Clusters of Orthologous Groups (COGs) functional category database as described by Galperin et al (Galperin et al., 2015) with an e-value cutoff of 1e-10. The program CIRCOS (Krzywinski et al., 2009) was used to plot the gene locations, GC-skew and GC content.
Phylogenetic analysis
To infer the relatedness among the R. palustris strains, phylogenetic trees were constructed base on multilocus sequence typing (MLST) analysis and puf genes. For MLST analysis, three housekeeping genes, recA, rpoB and dnaK, were selected. The sequences of these three genes were retrieved from GenBank. Then, individual gene sequences were validated by alignment using ClustalW multiple alignment program (Thompson et al., 1994) with the default settings. Subsequently, these genes were combined to form a recA-rpoB-dnaK concatenated sequence by BioEdit (Hall, 1999).
MEGA7.0.14 (Kumar et al., 2016) was used to construct the topological tree using the maximum likelihood program (Martens et al., 2008). The general time-reversible model and gamma distributed with invariant model (GTR+G+I)(Tavaré, 1986) were evaluated from the alignment in the maximum likelihood framework. To estimate the level of support for each branch, the 1,000 bootstrap (Felsenstein, 1985) samples of the alignment were generated by using the maximum likelihood program (Martens et al., 2008) in MEGA7.0.14 (Kumar et al., 2016). The puf genes consisted of pufL and pufM, which encode the core proteins of the photosynthetic reaction center. The sequences of the puf genes from other R. palustris strains were downloaded from GenBank. The individual gene sequences were aligned using BioEdit (Hall, 1999) with the ClustalW multiple alignment program (Thompson et al., 1994) with the default settings. After gene concatenated, the resulting multiple sequence alignment was used to construct the
phylogenetic trees by using the maximum likelihood program (Martens et al., 2008) with general time-reversible model and gamma distributed with invariant model (GTR+G+I)(Tavaré, 1986). The 1,000 bootstrap (Felsenstein, 1985) replicates were used to estimate the level of support for each internal branch. For the molecular phylogeny based on all single-copy protein-coding genes conserved within the genus Rhodopseudomonas, the procedure was based on that described by Cho, et al (Cho et al., 2015). Briefly, OrthoMCL (Li et al., 2003) was used for homologous gene cluster identification; gene clusters that contained exactly one entry from each of the Rhodopseudomonas genomes were selected. Multiple protein sequence alignment was processed for each individual gene cluster using MUSCLE version 3.8.31 (Edgar, 2004) and then concatenated. The maximum likelihood phylogenetic inference was performed using PHYML version 20120412 (Guindon and Gascuel, 2003). The proportion of invariable sites and the gamma distribution parameter were estimated from the dataset, and the number of substitution rate categories was set to four. Bootstrap supports were estimated based on 1,000 replicates.
Biofilm formation assay
Biofilm formation assay was performed according to a protocol proposed by Tram, et al (Tram et al., 2013) with some modifications. A single bacterial colony was selected, inoculated into a 10-mL sterile plastic tube containing 3 mL of PNSB broth, and then incubated at 37°C and 200 rpm for 24 h. Subsequently, 0.3 mL of the above broth was inoculated into a 10-mL sterile plastic tube containing 3 mL of fresh PNSB broth. This tube was incubated in a static state at 25°C for 5 days. After that, the broth was slowly emptied, and the residual suspension was carefully removed by pipette. The tube was rinsed with sterile distilled, deionized water (DDW) to remove the incomplete biofilm. The tube was air dried for 5 min, and then the biofilm was stained with 4 mL