F & S 17946-R1 decline and resubmit
Hepatitis B Virus Infection and the Risk of Male Infertility: A
Population-Based Analysis
Fu-Hsiung Su, BMBS, PhD a,b, Shih-Ni Chang, MSPH c,d,e, Fung-Chang Sung, PhD,
MPH e,f, Chien-Tien Su, MD, PhD a,g, Ying-Hua Shieh, MD b,h, Cheng-Chieh Lin,
MD, PhD i, Chih-Ching Yeh, PhD, MSPH f,g,*
a Department of Family Medicine, Taipei Medical University Hospital, Taipei,
Taiwan.
b Department of Family Medicine, School of Medicine, Taipei Medical University,
Taipei, Taiwan
c The PhD Program for Cancer Biology and Drug Discovery, China Medical
University, Taichung, Taiwan
d Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan
e Management Office for Health Data, China Medical University Hospital, Taichung,
Taiwan
f Department of Public Health, China Medical University, Taichung, Taiwan g School of Public Health, College of Public Health and Nutrition, Taipei Medical
University, Taipei, Taiwan
h Department of Family Medicine, Taipei Medical University,Wan Fang Hospital,
Taipei, Taiwan
i Division of Family Medicine, China Medical University Hospital, Taichung, Taiwan
Address correspondence to:
Chih-Ching Yeh, PhD, MSPH Professor
School of Public Health, College of Public Health and Nutrition, Taipei Medical University
FAX: 886-2-2738-4831 EMAIL: ccyeh@tmu.edu.tw
Financial disclosures
The author reports no financial or commercial conflicts of interest.
Acknowledgments
The authors would like to thank all participants and research staff for their support of this study. This work was partially funded by Taipei Medical University Hospital, Taipei, Taiwan (Grant no. 101TMU-TMUH-13).
Capsule:
Our population-based study demonstrated a significantly increased risk of developing male infertility among HBV carriers (HR = 1.52).
ABSTRACT
Objective: To evaluate the risk of male infertility among patients with hepatitis B
virus (HBV) infection.
Design: A nationwide population-based cohort study.
Setting: Insurance claims data from the Taiwan National Health Insurance Research
Database from 2000 to 2005.
Patient(s): HBV-infected men (n = 5,138) and men without HBV infection (n =
25,690).
Intervention(s): None.
Main Outcome Measure(s): Male infertility, as defined by the International
Classification of Diseases, Ninth Revision, Clinical Modification.
Result(s): The incidence of infertility was 1.59-times higher in patients with HBV
infection than in those without HBV infection (2.21 vs 1.39 per 1000 person-years). The risk of developing infertility remained significant among patients with HBV infection (HR = 1.52, 95% confidence interval (CI) = 1.20–1.92) after adjusting for covariates in a multivariate Cox proportional hazards model.
Conclusion(s): Our data show an increased incidence and risk of infertility among
men with HBV infection compared with men without HBV.
Hepatitis B virus (HBV) infection is a considerable global health problem, particularly in the Asia-Pacific region . Two billion people have been estimated to be infected with the virus worldwide, with approximately 350 million people being chronic HBV carriers. One million people die annually from HBV-related diseases, including acute hepatitis, chronic hepatitis, cirrhosis of the liver, and hepatocellular carcinoma (HCC) .
In Taiwan, HBV infection is a major cause of chronic hepatitis, cirrhosis of the liver, and HCC . Prior to the introduction of the national HBV vaccination program in 1984, the hepatitis B surface antigen (HBsAg) carrier rate among the Taiwanese population ranged from 15% to 20% . However, HBV-related diseases remain among the leading causes of death in Taiwan . Thus, chronic HBV infection remains a major health problem in Taiwan.
It is well-known that bacterial and viral infections are deleterious to human infertility . As previous studies have identified HBsAg and HBV DNA in the body fluids of both men and women with HBV, including the semen of male patients, it is possible that HBV infection may influence male infertility . In fact, Huang et al. have reported that HBV infection increases chromosomal instability in sperm , with other prior studies having found impaired sperm quality in HBV-infected men . Although these results indicate an association between HBV infection and male reproductive
performance, the effects of HBV on seminal quality are yet to be fully elucidated. As a hyperendemic region of chronic HBV infection, Taiwan provides a unique setting in which to investigate the association between HBV infection and male infertility. In this study, we used a nationwide population-based insurance dataset to evaluate this putative association, comparing HBV-infected men with men without HBV.
Materials and Methods
Data sources
In 1995, Taiwan initiated its state-run National Health Insurance (NHI) program . Since its establishment, this compulsory-enrollment and single-payer insurance program has covered approximately 99% of Taiwan’s population of more than 23 million. This study’s data were obtained from the Taiwan National Health Insurance Research Database (NHIRD), which contains original claims data for 1,000,000 beneficiaries randomly selected from all the NHI insurants during the period between 1996 and 2000. This database is managed by the Taiwan National Health Research Institute (NHRI). The NHRI has reported that this sample represents the original medical claims for all islanders under the NHI program. The Taiwan NHIRD consists of a registry of contracted medical facilities, board-certified physicians, and catastrophically ill patients, in addition to monthly summaries of
inpatient and ambulatory care claims and orders. With the approval of the NHRI, this study used data on all ambulatory care claims, inpatient claims, and updated registries for beneficiaries from 1996 to 2010.
In the dataset, diagnoses were coded according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). The data used can be interlinked by the scrambled and unique personal identification number (PIN). The NHRI safeguards the privacy and confidentiality of all beneficiaries, and transfers the health insurance data to health investigators only after receiving ethical approval. This study was exempted from full review by the International Review Board (IRB) of the China Medical University and Hospital Research Ethics Committee (IRB permit number: CMU-REC-101-012).
Study sample
The Taiwan NHIRD data were obtained from the NHRI, Department of Health. The data consisted of registries and claims reported from contracted healthcare facilities between 1996 and 2010. To limit the study sample to men of reproductive age, male patients 20 to 50 years of age were selected for analysis. Patients newly identified with sole chronic HBV (ICD-9-CM: 070.2, 070.3, V02.61) infection between 2000 and 2005 were evaluated as the exposure group. We excluded those with only one diagnosis of acute or unspecified HBV (ICD-9-CM: 070.20, 070.21,
0.70.30, 070.31) concurrent with a diagnosis of liver function impairment (ICD-9-CM: 794.8). Patients who received a second diagnosis of HBV infection six months after being diagnosed with acute or unspecified HBV were classified as chronic cases. The index date for patients with chronic HBV infection was the first instance of having chronic HBV identified.
Of the 513,872 men included in the 1,000,000 patients in the database, 8,130 were newly identified with HBV between 2000 and 2005. Patients with a diagnosis of male infertility (ICD-9-CM: 606.X, excluding ICD-9-CM: 606.0 Azoospermia and ICD-9-CM: 606.8, infertility due to extratesticular causes) prior to the index date, and patients missing information on either age or sex were excluded. Patients with a diagnosis of human immunodeficiency virus (HIV) infection (ICD-9-CM: 042, 043, 044, V08, and 795.8), chronic hepatitis (ICD-9-CM: 571.4, 571.8, 571.9, and 573.3) were also excluded. After the exclusion of six HIV patients, 15 patients with acute HBV infection, 183 chronic hepatitis patients, 72 patients who had been diagnosed as infertile prior to the index date, 636 patients younger than 20 years, and 2,080 patients older than 50 years, a total of 5,138 male patients with newly identified HBV infection were enrolled.
For the comparison group, a systematic random sampling method was used to select five insured people without viral hepatitis (acute or chronic) infection for every
insured person with an HBV infection during the same period. The comparison group was frequency matched on age (in five-year intervals) and index date. To ensure that the comparison patients were free from viral hepatitis infection, patients with ICD-9-CM diagnostic codes of viral hepatitis ICD-9-CM: 070.X), chronic hepatitis (ICD-9-CM: 571.4, 571.8, 571.9, and 573.3), and carriers or suspected carriers of viral hepatitis (ICD-9-CM: V02.6) were all excluded. Originally, 437,081 male insurants without any identification of HBV, HCV, HAV, or any ICD-9-CM diagnostic codes for chronic hepatitis, and without missing information on either age or sex were retrieved from the NHIRD. After applying the same exclusion criteria used for the HBV exposure group, a non-HBV exposure group of 25,690 patients was enrolled.
Sociodemographic factors, comorbidities, and medication usage
The sociodemographic factors selected for analysis in this study included age, occupation, monthly income, and urbanization level of the community in which the patient resided. Occupation was divided into blue collar, white collar, and retired and others. Patient monthly income was divided into three levels: ≤NT$15,840, NT$15,841–$25,000, and ≥NT$25,001. An income of NT$15,840 was the government-stipulated minimum wage for full-time employees in Taiwan during the inclusion of the HBV cohort. Urbanization levels were stratified into three levels, namely urban (levels 1-2), suburban (levels 3-4) and rural (levels 5-7) areas, based on
population density. Baseline comorbidities were also identified for each subject, including, malignancy (ICD9-CM: 140-239), chronic obstructive pulmonary disease (ICD9-CM: 490-496), hypertension (ICD9-CM: 401-405), hyperlipidemia (ICD-9-CM: 272), diabetic mellitus (ICD9-(ICD-9-CM: 250), coronary artery disease (ICD-9-(ICD-9-CM: 410-414), congestive heart failure (ICD-9-CM: 428, 398.91, 402.x), chronic renal disease (ICD-9-CM: 580-589), cirrhosis of liver (ICD-9-CM: 571.2, 571.5, 571.6), obesity CM: 278.00, 278.01), stroke CM: 430-438), epilepsy (ICD-9-CM: 345.0-345.9), sexually transmitted diseases (genital herpes (ICD-9-(ICD-9-CM: 054.1, 054.10, 054.13, 054.19), genital warts (ICD-9-CM: 078.11), chlamydia (ICD-9-CM: 078.88, 079.88, 079.98, 099.41, 099.53-099.55), gonorrhea (ICD-9-CM: 098.0, 098.1, 08.10- 098.19, 098.2, 098.30-098.34), syphilis (ICD-9-CM: 091.0, 095.4, 095.8), epididymitis/orchitis (ICD-9-CM: 604, 604.0, 604.9, 098.13, 098.33)), psychiatric disorders (depressive disorder (ICD-9-CM: 296.2, 296.3, 300.4, and 311), anxiety disorder (ICD-9-CM: 300.0, 300.2, 300.3, 308.3, 309.81)), alcohol abuse/dependence syndrome (ICD-9-CM: 305.0, 303.0-303.9), tobacco use disorder (ICD-9-CM: 305.1), drug abuse/dependence (ICD-9-CM: 305.2-305.93, 304.00-304.93), and varicocele (ICD-9-CM: 465.4). Medications included chemotherapeutics (cytarabine, vinblastine sulfate, cyclophosphamide, cisplatin, chlorambucil, busulfan), anti-epileptic medications (carbamazepine, oxcarbazepine, valproate sodium), cimetidine,
sulfasalazine, nitrofurantoin, and testosterone replacement therapy .
Statistical analysis
The distributions of sociodemographic factors, comorbidities, as well as medication usage were compared between the HBV cohort and non-HBV cohort using a chi-square test or Fisher’s exact test. The incidence rates of male infertility were calculated during the follow-up period until the end of 2010. Person-years of follow-up time were calculated for each patient until a diagnosis of male infertility or censoring for death, migration, or discontinued enrollment from the NHI program. Crude and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for factors associated with the risk of male infertility were estimated using univariate and multivariate Cox proportional hazards regression models. The sociodemographic factors, chronic hepatitis B infection status, comorbidities, and medications which demonstrated a significant association with male infertility in the univariate analysis, were selected as covariates to estimate adjusted hazard ratios (aHR) in a multivariate Cox proportional hazards model. All models were examined for adherence to the proportional hazards assumption by assessing the log-minus-log survival plots and by performing the Schoenfeld test. The results of the log-minus-log survival plots and the Schoenfeld test showed no violations of the proportionality assumption. The Kaplan-Meier method was used to estimate the cumulative risk of male infertility during the
10-year follow-up. The log-rank test was used to evaluate the differences between the two cohorts. All analyses were performed using SAS statistical software for Windows (Version 9.3; SAS Institute, Inc, Cary, NC, USA), with P < .05 considered significant.
RESULTS
Participant characteristics
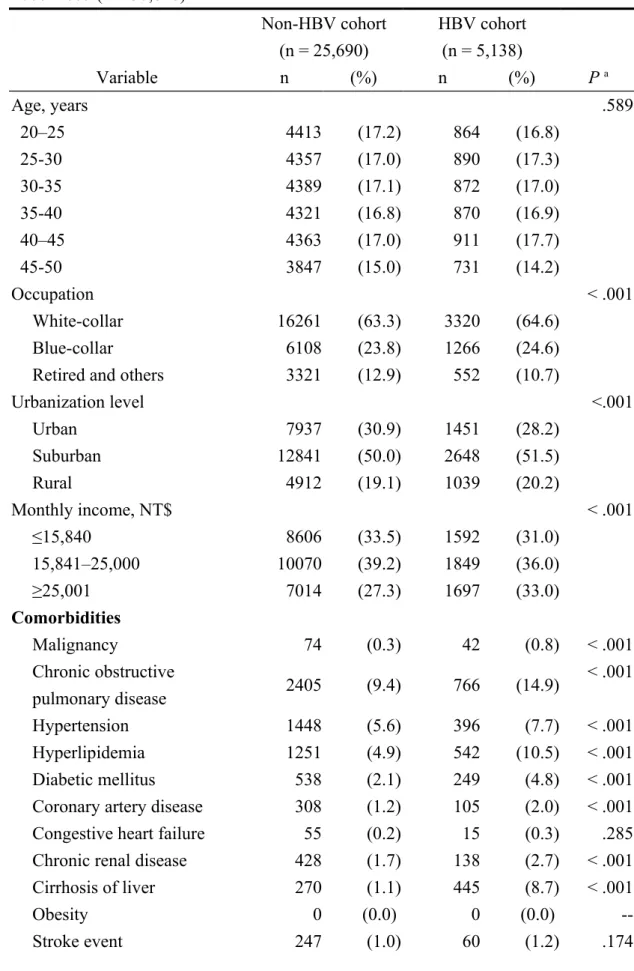
Table 1 shows the sociodemographic characteristics, comorbidities, and medication usage of the newly identified 5,138 HBV-infected patients and the 25,690 non-HBV comparison patients. The two groups showed significant differences in urbanization level, occupation, and monthly income status. The HBV-infected patients also were more likely to have been diagnosed with malignancy, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, diabetic mellitus, coronary artery disease, chronic renal disease, cirrhosis of the liver, psychiatric disorders, alcohol abuse/dependence syndrome, and varicocele at baseline than the comparison patients. In addition, the HBV-infected patients had a higher use of chemotherapeutics and cimetidine than their comparison counterparts.
Risk of male infertility in patients with HBV infection
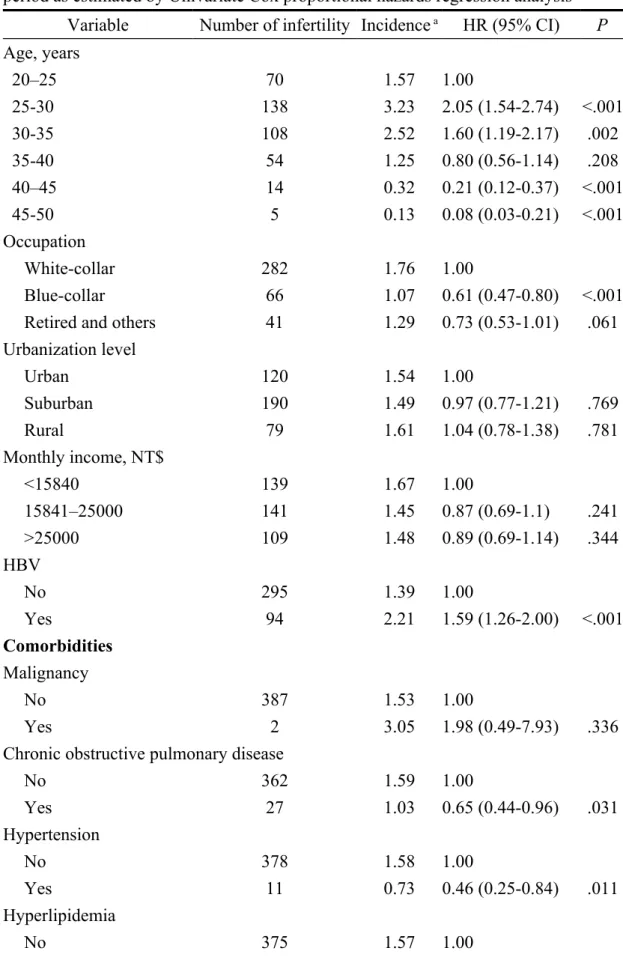
Table 2 lists the incidence densities and HRs of male infertility in the HBV-infected and non-HBV cohorts. Overall, the incidence of male infertility was 1.59-times higher in the HBV cohort than in the non-hepatitis cohort (2.21 vs 1.39 per
1000 person-y; HR = 1.59, 95% CI = 1.39–2.21). The HBV group also had a significantly higher cumulative risk of male infertility than that of the non-HBV group (Fig. 1; log-rank test, P < .001). The 5-year and overall cumulative risk of male infertility were 1.09 % (56/5,138) and 1.83% (94/5,138) among the HBV cases. Within the non-HBV group, the 5-year and overall cumulative risk of male infertility were 0.68% (175/25,690) and 1.15% (295/25,690), respectively.
Risk of male infertility among HBV patients during a 10-year follow-up period as estimated by Univariate Cox proportional hazards regression analysis
Table 2 also shows the incidence densities and HRs of the risk factors for male infertility calculated by univariate Cox proportional hazards regression analyses. After stratifying by age, the HRs for male infertility among patients with HBV remained statistically significant across age groups, with the highest risk being detected among patients aged 25–30 years (HR = 2.05, 95% CI = 1.54–2.74) when compared with patients aged 20–25 years. Having an occupation classified as blue-collar showed a protective effect against infertility when compared with white-collar workers (HR = 0.61, 95% CI = 0.47–0.80). In terms of comorbidities, males with chronic obstructive pulmonary disease, hypertension, and diabetic mellitus tended to have a lower risk of infertility than those without the diseases. The corresponding HRs were 0.65 (95% CI = 0.44–0.96), 0.46 (95% CI = 0.25–0.84), and 0.30 (95% CI = 0.10–0.95),
respectively. However, patients with varicocele were more prone to being diagnosed with male infertility (HR = 11.6, 95% CI = 8.29–16.2).
Adjusted risk of male infertility among HBV patients during a 10-year follow-up period as estimated by Multivariate Cox proportional hazards regression analysis
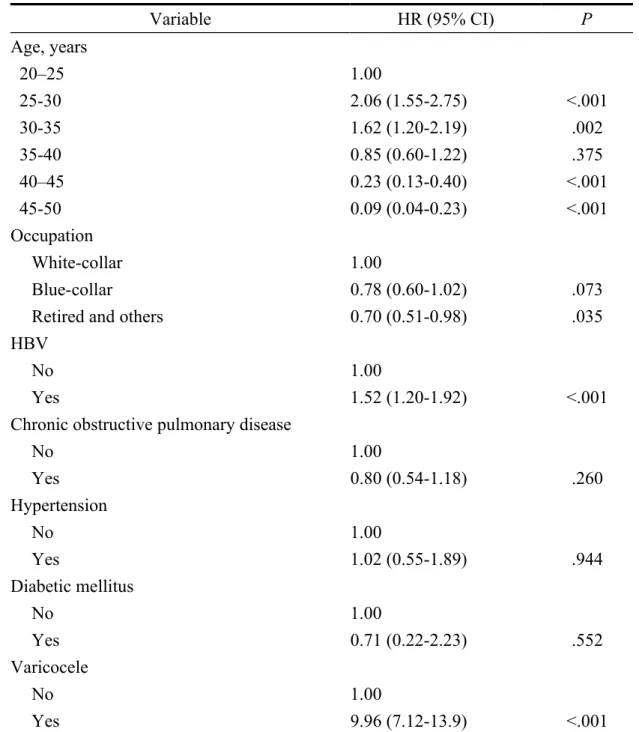
Table 3 shows the selected risk factors in a multivariate Cox proportional hazards model. After adjusting for covariates, the increased risk of developing male infertility in the HBV-infected patients remained significant (aHR = 1.52, 95% CI = 1.20–1.92). Subjects aged 25–30 years (HR = 2.06, 95% CI = 1.55-2.75) and those diagnosed with varicocele (HR = 9.96, 95% CI = 7.12-13.9) still had significantly increased risks of infertility.
DISCUSSION
The results from our large-scale population-based study conducted in a region with a high prevalence of HBV infection demonstrate a significant association between HBV infection and male infertility. In Taiwan, the majority of HBV infections are acquired through vertical transmission during the neonatal period and possibly horizontal transmission during early childhood (5, 6). Therefore, the subjects investigated in this study had longer exposure periods to the virus when compared to most individuals from western populations which generally become infected with HBV at later ages
and through other mechanisms. This difference provides this study with a great observational advantage allowing us to investigate the long-term cumulative risks of extrahepatic manifestations associated with the viral infection, such as male infertility. Several previous studies have evaluated the possible association between male fertility and HBV infection by analyzing sperm quality . However, our population-based study is the first to evaluate the association between male infertility and HBV infection. Bacterial and viral infections have been shown to be deleterious to human fertility . Results from previous studies have shown that HBV infection affects several conventional semen parameters .
Lee et al. evaluated couples undergoing in vitro fertilization (IVF) treatment, and observed that HBsAg-positive male partners had a significantly lower percentage of normal sperm morphology compared to HBsAg-negative male partners . In a study performed by Moretti et al., men with HBV infection were shown to have normal sperm concentrations, but to exhibit some spermatogenetic alterations, such as reduced sperm motility and higher sperm pathologies (apoptosis and necrosis). The authors also observed a lower fertility index (expressed as the number of healthy spermatozoa) among HBV-infected men than among men without HBV . An investigation conducted by Lorusso et al. demonstrated HBV-seropositive men to have significantly reduced sperm concentrations, motility, and viability when
compared with healthy men. HBV-seropositive men were also noted to have significantly reduced normal sperm morphology . In a case-control study, sperm motility before selection was significantly reduced among HBV-infected men seeking IVF assistance compared with the non-HBV group (36.3% ± 11.6% vs 45.3% ± 14.4%, P = .003). Furthermore, a low fertilization rate was more frequently observed in the HBV group (34.4% versus 15.6%, P = .036) . Similarly, Zhou et al. compared HBV-infected and matched HBV-negative men seeking fertility assistance, and determined that the HBV-infected men had reduced semen volume, lower total sperm count, and poorer progressive sperm motility and morphology compared with their matched HBV-negative counterparts . Chen et al. further showed that sperm concentration and forward motility was significantly lower in men with HBV infection than in men without HBV infection (P < .01) . All of these studies have reported a negative correlation between HBV infection and semen quality.
In addition to the effects of HBV infection on conventional semen parameters, previous studies have reported HBV infection to be associated with substantial damage to sperm DNA . In 1985, Hadchouel et al. identified HBV DNA in sperm cells and suggested that HBV may cause male infertility by damaging the spermatozoa . Since then, other research groups have also detected HBV DNA in semen and spermatozoa . Deng et al. recently showed that the integrity of sperm DNA
in HBV-infected men differed significantly from that of normal fertile men. The authors observed a higher sperm DNA fragmentation index (DFI) (28.17% ± 13.06% vs 15.67% ± 4.73%) and higher DNA stainability (10.83% ± 5.60% vs 8.04% ± 2.25%) in HBV-infected men than normal fertile men (30). Chen et al. showed significantly higher DFI scores in men with HBV infection than normal men without HBV infection (23.4% ± 10.2% vs 11.6% ± 5.9%) .
HBV infection can also have mutagenic effects on sperm chromosomes. HBV is believed to pass the blood-testis barrier and enter male germ cells, and then integrate viral DNA into the sperm chromosomes (nonspecific and multisited) resulting in a reduction of genomic stability . HBV infection can thus induce chromosomal aberrations, leading to hereditary defects in male germinal cells and spermatogenic irregularities . Several investigators have suggested that HBV can be transmitted vertically to the next generation (father-to-infant) through the germ line . Moretti et al. also showed that HBV infection increases the percentage of phenotypic sperm pathologies, such as necrosis, apoptosis, and immaturity . In an in vitro study on hepatitis B virus S protein and sperm function, sperm treated with surface protein exhibited a lower fertilization rate and index because of reduced sperm motility resulting from mitochondrial injury (33). The results from Kang et al. also suggested that hepatitis B virus S protein exposure causes a series of deleterious events in sperm
cells, such as the generation of reactive oxygen species, lipid peroxidation, reduction of total antioxidant capacity, phosphatidylserine externalization, activation of caspases, and DNA fragmentation, resulting in increased sperm cell apoptosis, the loss of sperm membrane integrity, and sperm dysfunction (34).
In our analysis, males with varicocele were more prone to infertility than men without varicocele. We also found that HBV-infected patients were significantly more likely to have varicocele than their healthy counterparts. Previous studies had found that varicocele might further impair semen output in HBV patients . However, the incidence of varicocele in our study seemed low (0.9% and 1.3%) in both the HBV and non-HBV groups, respectively. The association might simply reflect an under-diagnosis of varicocele in the general population, and a higher diagnostic rate among men presenting with male infertility. Further investigations are required to confirm this association. After stratifying by age, our results showed that HBV-infected men aged 25-30 years were most likely to present with infertility. This is comparable with the average age of infertile men in Taiwan who might start seeking reproductive assistance.
Strengths and Limitations
This study’s chief strength was the use of a nationwide, population-based dataset. This allowed us to observe the risk of infertility in a large, representative,
sample of male patients with chronic HBV, thus allowing us to calculate stable estimates based on a statistically robust analysis. It also permitted for the adjustment of sociodemographic factors, medication usage, and comorbidities which may have otherwise confounded our results. However, when interpreting our study findings the following limitations must be considered. First, information on some risk factors for male infertility that are associated with HBV infection, such as smoking, alcohol, and social drug use, were unavailable in the insurance claims database. We did adjust for diagnoses of tobacco use disorder, alcohol abuse/dependence syndrome, and drug abuse/dependence to minimize the potential confounding effects associated with these factors. But, subjects with these diagnoses may represent a sub-population of substance abusers as they sought out medical assistance .
Second, some of our populations have overlapped (e.g. hepatitis A (HAV) and hepatitis C (HCV) infections) and the findings should be limited to the population studied. In this investigation, a total of 148 cases of acute HAV infection were identified during the study period. None of them were identified with a diagnosis of male infertility during the following-up period. Many previous reports have suggested that HCV infection may cause sperm alterations, hence, possibly affecting male fertility (21, 22, 39, 40). However, no significant association between male infertility and HCV was observed in the 1,235 cases with HCV infection in this study (HR =
1.64, 95% CI = 0.95–2.84; P = .077), among whom 18 were coded with a diagnosis of male infertility during the following-up period. We speculated that the lack of significance may be due to the small HCV case numbers in our study. Further studies should be conducted to further explore this association. As a result, HAV and HCV populations were purposely not studied in this investigation.
Third, some HBV-infected patients without obvious clinical symptoms do not submit claims for medical services. Therefore, some HBV-infected patients may have been included in the comparison group. However, to ensure that our comparison group contained a minimal number of HBV-infected patients, we excluded all patients with an ICD-9-CM diagnosis of acute or chronic viral hepatitis. If HBV infection is associated causally with male infertility, patient misclassification would bias the estimated HRs toward the null. Therefore, we are confident of the presence of an association between HBV and male infertility.
Fourth, the diagnoses of male infertility, HBV infection, and other comorbid medical conditions were dependent on ICD-9 coding, and might be less accurate than those obtained through standardized examinations and laboratory procedures. However, the NHI Bureau of Taiwan randomly samples a fixed percentage of claims from every hospital, randomly interviews patients, and reviews charts annually to verify the validity of diagnoses and the quality of care. These national quality control
procedures aim to ensure the accuracy of diagnoses.
The use of ICD-9 coding in epidemiological studies has also been suggested to introduce a severity bias in which patients with a given diagnosis are likely to represent a subset of patients with a more severe clinical manifestation. As the effect of such a severity bias would likely work to push the estimated HRs away from the null it would represent a significant limitation. However, since HCC is one of the leading causes of cancer deaths in Taiwan, public free-of-charge community screening programs, self-paid pre-employment health checks, and private health checks have served to extensively screen for chronic viral hepatitis. Most HBV cases are thus asymptomatically identified through such screening programs rather than diagnosed in hospitals. This should help to ensure that the diagnoses present in the dataset better represent all the cases of HBV in Taiwan.
Finally, the vast majority of the residents in Taiwan are of Chinese ethnicity. Therefore, our results may not be generalizable to other ethnic groups because the transmission routes of HBV infection might differ across various populations.
Conclusion
This nationwide population-based cohort study conducted in a region with high HBV prevalence demonstrated that the incidence of male infertility is significantly higher in HBV-infected patients than in patients without HBV. From the results of previous
clinical observations and our population-based study, chronic HBV-infection should be considered as one possible risk factor contributing to couples experiencing infertility, especially in HBV endemic regions.
Acknowledgments
The authors would like to thank all participants and research staff for their support of this study. This work was partially funded by Taipei Medical University Hospital, Taipei, Taiwan (Grant no. 101TMU-TMUH-13).
Reference:
1. Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol 2000;15:1356-61.
2. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009;373:582-92.
3. Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;2:1129-33.
4. Beasley RP. Hepatitis B virus as the etiologic agent in hepatocellular carcinoma: epidemiological considerations. Hepatology 1982;2:21S–6S.
5. Chen DS, ed. Hepatitis B virus infection, its sequelae, and prevention in Taiwan. Tokyo,Japan: Springer-Verlag Inc, 1987.
6. Chen DS, Sung JL. Hepatitis B virus infection and chronic liver disease in Taiwan. Acta Hepatogastroenterol (Stuttg) 1978;25:423-30.
7. Wu JS, Chen CH, Chiang YH, Lee YC, Lee MH, Ko YC et al. Hepatitis B virus infection in Taiwan with reference to anti-HBc versus HBsAg and anti-HBs. Taiwan Yi Xue Hui Za Zhi 1980;79:760-7.
8. Sung JL. Hepatitis B virus infection and its sequelae in Taiwan. Gastroenterol Jpn 1984;19:363-6.
9. Ministry of Health and Welfare. 2012. Statistics of Causes of Death 2012. Taipei: Ministry of Health and Welfare, Executive Yuan, Taiwan, ROC. Available: http://www.mohw.gov.tw/cht/DOS/Statistic.aspx?f_list_no=312&fod_list_no=2747. Accessed: 2012 August 1.
10. Collodel G, Baccetti B, Capitani S, Moretti E. Necrosis in human spermatozoa. I. Ultrastructural features and FISH study in semen from patients with uro-genital infections. J Submicrosc Cytol Pathol 2005;37:67-73.
11. Masarani M, Wazait H, Dinneen M. Mumps orchitis. J R Soc Med 2006;99:573-5.
12. Ye F, Yue Y, Li S, Chen T, Bai G, Liu M et al. Presence of HBsAg, HBcAg, and HBVDNA in ovary and ovum of the patients with chronic hepatitis B virus infection. Am J Obstet Gynecol 2006;194:387-92.
13. Chen LZ, Fan XG, Gao JM. Detection of HBsAg, HBcAg, and HBV DNA in ovarian tissues from patients with HBV infection. World J Gastroenterol 2005;11:5565-7.
14. Hadchouel M, Scotto J, Huret JL, Molinie C, Villa E, Degos F et al. Presence of HBV DNA in spermatozoa: a possible vertical transmission of HBV via the germ line.
15. Heathcote J, Cameron CH, Dane DS. Hepatitis-B antigen in saliva and semen. Lancet 1974;1:71-3.
16. Jenison SA, Lemon SM, Baker LN, Newbold JE. Quantitative analysis of hepatitis B virus DNA in saliva and semen of chronically infected homosexual men. J Infect Dis 1987;156:299-307.
17. Qian WP, Tan YQ, Chen Y, Peng Y, Li Z, Lu GX et al. Rapid quantification of semen hepatitis B virus DNA by real-time polymerase chain reaction. World J Gastroenterol 2005;11:5385-9.
18. Davison F, Alexander GJ, Trowbridge R, Fagan EA, Williams R. Detection of hepatitis B virus DNA in spermatozoa, urine, saliva and leucocytes, of chronic HBsAg carriers. A lack of relationship with serum markers of replication. J Hepatol 1987;4:37-44.
19. Huang JM, Huang TH, Qiu HY, Fang XW, Zhuang TG, Liu HX et al. Effects of hepatitis B virus infection on human sperm chromosomes. World J Gastroenterol 2003;9:736-40.
20. Vicari E, Arcoria D, Di Mauro C, Noto R, Noto Z, La Vignera S. Sperm output in patients with primary infertility and hepatitis B or C virus; negative influence of HBV infection during concomitant varicocele. Minerva Med 2006;97:65-77.
21. Lorusso F, Palmisano M, Chironna M, Vacca M, Masciandaro P, Bassi E et al. Impact of chronic viral diseases on semen parameters. Andrologia 2010;42:121-6. 22. Moretti E, Federico MG, Giannerini V, Collodel G. Sperm ultrastructure and meiotic segregation in a group of patients with chronic hepatitis B and C. Andrologia 2008;40:173-8.
23. Chiang TL. Taiwan's 1995 health care reform. Health policy 1997;39:225-39. 24. Wald M. Male infertility: Causes and cures. Sex Reprod Menopause 2005;3:83-7.
25. Lee VC, Ng EH, Yeung WS, Ho PC. Impact of positive hepatitis B surface antigen on the outcome of IVF treatment. Reprod Biomed Online 2010;21:712-7. 26. Oger P, Yazbeck C, Gervais A, Dorphin B, Gout C, Jacquesson L et al. Adverse effects of hepatitis B virus on sperm motility and fertilization ability during IVF. Reprod Biomed Online 2011;23:207-12.
27. Zhou XP, Hu XL, Zhu YM, Qu F, Sun SJ, Qian YL. Comparison of semen quality and outcome of assisted reproductive techniques in Chinese men with and without hepatitis B. Asian J Androl 2011;13:465-9.
28. Chen JW, Cui Y, Zhang XX. [Investigate the impact of hepatitis B virus infection on sperm DNA integrity]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011;25:345-7.
integration of hepatitis B virus DNA sequence in human sperm chromosomes. Asian J Androl 2002;4:209-12.
30. Deng TQ, Huang YH, Zhen JY, Lu JT, Li YC, Tan XY et al. [Sperm DNA integrity of infertile males with hepatitis B virus infection]. Zhonghua Nan Ke Xue 2013;19:72-6.
31. Wang S, Jiang P, Peng G. [Detection of S-gene mutation strain in vertical transmission of HBV and its significance]. Zhonghua liu xing bing xue za zhi 1999;20:204-7.
32. Wang S, Peng G, Li M, Xiao H, Jiang P, Zeng N et al. Identification of hepatitis B virus vertical transmission from father to fetus by direct sequencing. Southeast Asian J Trop Med Public Health 2003;34:106-13.
33. Zhou XL, Sun PN, Huang TH, Xie QD, Kang XJ, Liu LM. Effects of hepatitis B virus S protein on human sperm function. Hum Reprod 2009;24:1575-83.
34. Kang X, Xie Q, Zhou X, Li F, Huang J, Liu D et al. Effects of hepatitis B virus S protein exposure on sperm membrane integrity and functions. PloS one 2012;7:e33471.
35. Hsueh KC, Chen CY, Yang YH, Huang CL. Smoking cessation program in outpatient clinics of Family Medicine Department in Taiwan: a longitudinal evaluation. Eval Health Prof 2010;33:12-25.
36. Huang CL, Lee CW. Factors associated with mortality among heroin users after seeking treatment with methadone: a population-based cohort study in Taiwan. J Subst Abuse Treat 2013;44:295-300.
37. Hofny ER, Ali ME, Taha EA, Nafeh HM, Sayed DS, Abdel-Azeem HG et al. Semen and hormonal parameters in men with chronic hepatitis C infection. Fertil Steril 2011;95:2557-9.
38. Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol 2013;100:20-9.
Tables and Figure Legends
Table 1. Baseline characteristics of HBV and non-HBV patients identified during
2000–2005 (n = 30,828)
Table 2. Risk of male infertility among HBV patients during a 10-year follow-up
period as estimated by Univariate Cox proportional hazards regression analysis
Table 3. Adjusted risk of male infertility among HBV patients during a 10-year
follow-up period as estimated by Multivariate Cox proportional hazards regression analysis
Figure 1. Cumulative risk of male infertility in HBV and non-HBV groups during a
Table 1. Baseline characteristics of HBV and non-HBV patients identified during 2000–2005 (n = 30,828) Non-HBV cohort (n = 25,690) HBV cohort (n = 5,138) Variable n (%) n (%) P a Age, years .589 20–25 4413 (17.2) 864 (16.8) 25-30 4357 (17.0) 890 (17.3) 30-35 4389 (17.1) 872 (17.0) 35-40 4321 (16.8) 870 (16.9) 40–45 4363 (17.0) 911 (17.7) 45-50 3847 (15.0) 731 (14.2) Occupation < .001 White-collar 16261 (63.3) 3320 (64.6) Blue-collar 6108 (23.8) 1266 (24.6)
Retired and others 3321 (12.9) 552 (10.7)
Urbanization level <.001 Urban 7937 (30.9) 1451 (28.2) Suburban 12841 (50.0) 2648 (51.5) Rural 4912 (19.1) 1039 (20.2) Monthly income, NT$ < .001 ≤15,840 8606 (33.5) 1592 (31.0) 15,841–25,000 10070 (39.2) 1849 (36.0) ≥25,001 7014 (27.3) 1697 (33.0) Comorbidities Malignancy 74 (0.3) 42 (0.8) < .001 Chronic obstructive pulmonary disease 2405 (9.4) 766 (14.9) < .001 Hypertension 1448 (5.6) 396 (7.7) < .001 Hyperlipidemia 1251 (4.9) 542 (10.5) < .001 Diabetic mellitus 538 (2.1) 249 (4.8) < .001
Coronary artery disease 308 (1.2) 105 (2.0) < .001
Congestive heart failure 55 (0.2) 15 (0.3) .285
Chronic renal disease 428 (1.7) 138 (2.7) < .001
Cirrhosis of liver 270 (1.1) 445 (8.7) < .001
Obesity 0 (0.0) 0 (0.0)
Epilepsy 99 (0.4) 25 (0.5) .295
Sexual transmitted disease 85 (0.3) 21 (0.4) .384
Psychiatric disorders 672 (2.6) 262 (5.1) < .001
Alcohol abuse/dependence
syndrome 106 (0.4) 51 (1.0)
< .001
Tobacco use disorder 14 (0.1) 4 (0.1) .527
Drug abuse/dependence 29 (0.1) 4 (0.1) .483 Varicocele 230 (0.9) 68 (1.3) .004 Medication Chemotherapeutics 55 (0.2) 23 (0.4) .002 Cimetidine 1098 (4.3) 328 (6.4) < .001 Sulfasalazine 0 (0.0) 0 (0.0) --Anti-epileptic medication 99 (0.4) 23 (0.4) .516 Nitrofurantoin 13 (0.1) 5 (0.1) .206 Testosterone replacement therapy 4 (0.0) 1 (0.0) .842
Table 2. Risk of male infertility among HBV patients during a 10-year follow-up period as estimated by Univariate Cox proportional hazards regression analysis
Variable Number of infertility Incidence a HR (95% CI) P
Age, years 20–25 70 1.57 1.00 25-30 138 3.23 2.05 (1.54-2.74) <.001 30-35 108 2.52 1.60 (1.19-2.17) .002 35-40 54 1.25 0.80 (0.56-1.14) .208 40–45 14 0.32 0.21 (0.12-0.37) <.001 45-50 5 0.13 0.08 (0.03-0.21) <.001 Occupation White-collar 282 1.76 1.00 Blue-collar 66 1.07 0.61 (0.47-0.80) <.001
Retired and others 41 1.29 0.73 (0.53-1.01) .061
Urbanization level Urban 120 1.54 1.00 Suburban 190 1.49 0.97 (0.77-1.21) .769 Rural 79 1.61 1.04 (0.78-1.38) .781 Monthly income, NT$ <15840 139 1.67 1.00 15841–25000 141 1.45 0.87 (0.69-1.1) .241 >25000 109 1.48 0.89 (0.69-1.14) .344 HBV No 295 1.39 1.00 Yes 94 2.21 1.59 (1.26-2.00) <.001 Comorbidities Malignancy No 387 1.53 1.00 Yes 2 3.05 1.98 (0.49-7.93) .336
Chronic obstructive pulmonary disease
No 362 1.59 1.00 Yes 27 1.03 0.65 (0.44-0.96) .031 Hypertension No 378 1.58 1.00 Yes 11 0.73 0.46 (0.25-0.84) .011 Hyperlipidemia No 375 1.57 1.00
Yes 14 0.95 0.61 (0.36-1.04) .068 Diabetic mellitus
No 386 1.56 1.00
Yes 3 0.47 0.30(0.10-0.95) .040
Coronary artery disease
No 383 1.53 1.00
Yes 6 1.83 1.19 (0.53-2.68) .665
Chronic renal disease
No 385 1.54 1.00
Yes 4 0.88 0.57 (0.21-1.53) .268
Cirrhosis of liver
No 385 1.55 1.00
Yes 4 0.79 0.51 (0.19-1.37) .180
Alcohol abuse/dependence syndrome
No 389 1.54 --Yes 0 0.00 Varicocele No 351 1.39 1.00 Yes 38 16.17 11.59 (8.29-16.2) <.001 Medication Chemotherapeutics No 388 1.50 --Yes 1 1.93 Cimetidine No 375 1.55 1.00 Yes 14 1.19 0.77 (0.45-1.31) .335 a Per 1000 person-years.
Table 3. Adjusted risk of male infertility among HBV patients during a 10-year follow-up period as estimated by Multivariate Cox proportional hazards regression analysis Variable HR (95% CI) P Age, years 20–25 1.00 25-30 2.06 (1.55-2.75) <.001 30-35 1.62 (1.20-2.19) .002 35-40 0.85 (0.60-1.22) .375 40–45 0.23 (0.13-0.40) <.001 45-50 0.09 (0.04-0.23) <.001 Occupation White-collar 1.00 Blue-collar 0.78 (0.60-1.02) .073
Retired and others 0.70 (0.51-0.98) .035
HBV
No 1.00
Yes 1.52 (1.20-1.92) <.001
Chronic obstructive pulmonary disease
No 1.00 Yes 0.80 (0.54-1.18) .260 Hypertension No 1.00 Yes 1.02 (0.55-1.89) .944 Diabetic mellitus No 1.00 Yes 0.71 (0.22-2.23) .552 Varicocele No 1.00 Yes 9.96 (7.12-13.9) <.001