FULL PAPER
Asymmetric organocatalytic synthesis of highly substituted cyclohexenols via
domino double Michael reactions of 1-hydroxy-1,4-dien-3-ones and 2-alkylidene
malononitriles
Yeong-Jiunn Jang,
[a,b,c]Yu-Shan Chen,
[a,c]Chia-Jui Lee,
[a]Chi-Han Chen,
[a]Ganapuram
Madhusudhan Reddy,
[a]Chi-Ting Ko
[a]and Wenwei Lin*
[a]Keywords: Domino reactions / Michael addition / Cinchona alkaloids / Enones / Carbocycles
A facile asymmetric synthetic protocol to afford 4-hydroxy-3-keto-2,6-disubstituted-cyclohex-3-ene-1,1-dicarbonitriles has been developed through domino double Michael addition of 1-hydroxy-1,5-disubstituted-1,4-dien-3-ones to 2-alkylidene malononitriles using quinine as the catalyst. This simple organocatalytic domino
process provides access to various highly functionalized chiral 4-hydroxy-3-keto-cyclohex-3-enes, which are the rarely reported chiral diketo cyclohexane analogues, in moderate to good yields and enantioselectivities with excellent diastereoselectivities (> 25:1 dr).
____________
[a] Department of Chemistry, National Taiwan Normal University, No. 88, Sec. 4, Tingchow Road, Taipei, Taiwan 11677, R.O.C.
E-mail: wenweilin@ntnu.edu.tw
[b] School of Pharmacy, College of Pharmacy, China Medical University, No. 91 Hsueh-Shih Road, Taichung, Taiwan 40402, R.O.C.; Chinese Medicine Research and Development Center, China Medical University Hospital, No. 2 Yuh-Der Road, Taichung, Taiwan 40447, R.O.C.
[c] Y.-J. Jang and Y.-S. Chen made equal contributions to this work. Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/ejoc.xxxxxxxxx.
Introduction
The 1,3-dicarbonyl moieties and their derivatives have
been paid much attention for a long while as they are known
to act as the HIV integrase (IN) inhibitors and are
considered the key structures which interact with the metal
ion (Mg
2+) in the IN active site.
1Thus, some of the
analogous compounds, such as curcumin
2and S-1360
3, have
already been thoroughly investigated in pharmacological
field.
2Although few modifications of 1,3-dicarbonyls have
been attempted,
4there is still a major scope for further
development in this area.
On the other hand, the 6-membered nonbenzenoid
carbocycles, related cyclohexanones and isomeric
cyclohexenols are the common core structures found in
biologically active natural products and drugs.
5Different
synthetic strategies and asymmetric protocols such as
cyclization
via
palladium-catalyzed intramolecular
hydroalkylation,
6carbo [3+3] cycloaddition reaction via
domino Michael/Knoevenagel condensation,
7phosphine-catalyzed [4+2] annulation,
8vinylogous
Mukaiyama-Michael reaction,
9conjugate addition of β-ketoesters to
α,β-unsaturated ketones,
10proline catalyzed Robinson
annulation,
11addition of ɛ-nitro-α,β-unsaturated esters to
conjugated cyanosulfones,
12addition of malanonitrile to
1,5-disubstituted pentadien-3-ones
13etc. have been reported for
the generation of highly functionalized cyclohexanone
derivatives. The extensive use of nazarov-type reagents has
also been well documented to gain access to such
carbocycles.
14In continuation of our earlier work on the
synthesis of highly diastereoselective polysubstituted
4-hydroxy-3-keto-cyclohex-3-enes,
15we herein report its
organocatalytic enantioselective version for the synthesis of
such cyclohexanone derivatives.
Figure 1. Structure of beta-diketo acid derivatives.
In the present work, we tried to exploit one of the most
common organocatalysts, quinine, to initiate our asymmetric
domino double Michael reaction of
1-hydroxy-1,4-dien-3-ones and 2-alkylidene malononitriles. Using this protocol,
different
4-hydroxy-3-keto-2,6-disubstituted-cyclohex-3-ene-1,1-dicarbonitriles were obtained in good yields with
high diastereoselectivities and good enantiomeric excesses.
Results and Discussion
To begin with, our first concern was to identify the type
of organocatalyst suitable to initiate the pro-nucleophile,
1-hydroxy-1,4-dien-3-one. Based on our earlier work,
15we
could envisage that a tertiary amine was most suitable to
activate the pro-nucleophile. Hence, we selected the
cinchona category of alkaloids (I-XII) for our preliminary
study (Table 1) of the asymmetric domino double Michael
reaction of 2-alkylidene malanonitrile 1a and 1,3-diketone
2aa. While the cinchona catalysts with unsubstituted aryl
group resulted in low enantioselectivities of 21% and 2%
(entries 2 & 7), the methoxy substituted cinchona derivative
displayed better enantioselectivities, 63% and 51% (entries
1 & 6). Few other modifications of the cinchona catalyst
such as reduction of alkene, transformation of the hydroxyl
group to an amine, combination of thiourea or protection
with aroyl group (entries 3-5 & 8-10) failed to give better
results. The significantly different results with catalysts I
and V indicated that the free hydroxyl center was a key
function for the reaction to proceed smoothly (entries 1 &
3). It not only controlled the activity of reactants but also
demonstrated
its significance for
the
better
enantioselectivity: the NMR yield was merely 9% with a
racemic result when the hydroxyl group was masked (entry
3). In addition, the well-known diphenylprolinol silyl ether
catalyst (XIII) and the Takemoto-type catalyst (XIV & XV)
also failed to deliver better results (entries 11-13).
Table 1. Catalyst screening of asymmetric domino double Michael reaction of malanonitrile 1aanddiketone2aa.[a]
Entry Cat. Time (d) Product/SM[c] ee (%)[d]
1 I 29 h 93/0 63 2 II 29 h 96/0 21 3 V 29 h 9/81 -4 VI 5 28/47 34 5 VII 5 40/32 23 6 VIII 29 h 92/0 51[e] 7 IX 29 h 91/0 2[e] 8 X 3 89/0 53[e] 9 XI 5 58/20 45[e] 10 XII 5 58/28 17 11 XIII 5 38/28 14 12 XIV 2 0/100 -13 XV 2 14/77 20
[a] Unless otherwise specified, all the reactions were carried out using 1a (0.12 mmol, 1.2 equiv) and 2aa (0.1 mmol) with 20 mol % of catalyst in CH2Cl2 (0.8 mL) and stirred at room temperature (30 °C). [b]
Diastereomeric ratio was determined by the 1H NMR analysis of crude
reaction mixture. [c] NMR yield. [d] Determined by HPLC analysis on a chiral stationary phase. [e] Enantiometric excess of ent-3aaa.
The importance of hydroxyl group on quinine
demonstrates that the reaction was facilitated by the
hydrogen bonding interactions between the reagent and
cinchona catalyst. These interactions would be further
affected by the solvent selected. So we screened a series of
solvents to analyze their effect on the reaction (Table 2) and
it was evident that the reaction in the polar solvents such as
THF and EA (entries 2 & 3) resulted in lower
enantioselectivities, 37% and 46%, than in non-polar
solvents. Although using other solvents such as chloroform
and 1, 2-dichloroethane gave better yields, the enantiomeric
excess values of the products were not impressive (entries 1
& 5). Carrying out the reaction in non-polar solvents had the
better consequence with good yields and ee values with
xylenes being the best of the lot (entries 6-9). Decreasing the
reaction temperature to either 0 or -20
oC did not yield any
better results (entries 10 & 11). Later on, we tried to
evaluate the importance of methoxy substitution by carrying
out the reaction of malanonitrile 1a and
diketone 2aa in
xylenes using the isopropyl substituted quinine catalyst III.
Although increasing the steric hindrance on the quinoline
group slightly enhanced the enantioselectivity, the reactivity
dropped considerably (entry 12). Further, when diketone
2aa was reacted with other 2-alkylidene malononitriles such
as 1d in presence of III, the enantioselectivity decreased
dramatically. Conversely, when the cinchona catalyst (IV)
with hydroxy substituted aryl group was used to carry out
the reaction of 1a
and
2aa in xylenes, both the yield and
enantioselectvity of the reaction were low (entry 13).
Table 2. Solvent screening and optimization of asymmetric domino double Michael reaction of malanonitrile 1aanddiketone
2aa.[a]
Entry Solv. Time (d) Product/SM[c] ee (%)[d]
1 CHCl3 3 91/0 58 2 THF 3 76/8 37 3 EA 1 77/8 46 4 Dioxane 2 1/83 -5 dichloroethane1,2- 2 100/0 62 6 Toluene 3 85/6 75 7 Mesitylene 2 54/31 70 8 Benzene 2 83/2 74 9 Xylenes 2 84/3 77 10[e] Xylenes 2 66/22 77 11[f] Xylenes 2 4/84 79 12[g] Xylenes 2 75/14 80 13[h] Xylenes 2 23/64 26[i]
[a] Unless otherwise specified, all the reactions were carried out using 1a (0.12 mmol, 1.2 equiv) and 2aa (0.1 mmol) with 20 mol % of catalyst I in the solvent (0.8 mL) and stirred at room temperature (30 °C). [b] Diastereomeric ratio was determined by the 1H NMR analysis of crude
reaction mixture. [c] NMR yield. [d] Determined by HPLC analysis on a chiral stationary phase. [e] 0 °C. [f] -20 °C. [g] 20 mol% of catalyst III was used. [h] 20 mol% of catalyst IV was used. [i] Enantiometric excess of
ent-3aaa.
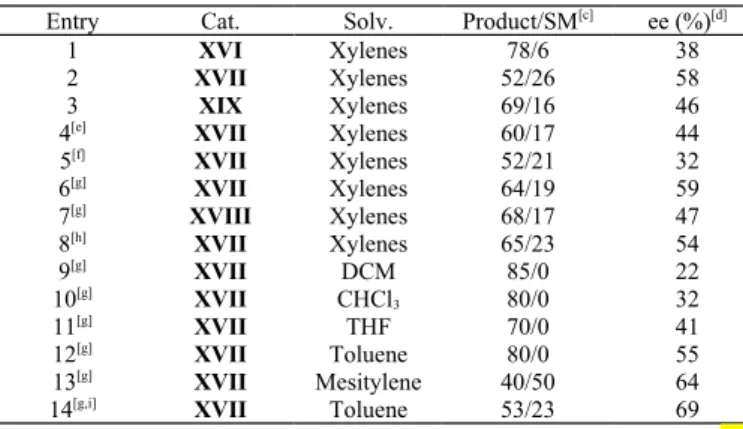
After discovering that quinine and xylenes were the most
suitable catalyst and solvent respectively to carry out this
reaction, we tried to test whether the phase transfer catalysts
(PTCs) derived from quinine would afford even better
results. So, we screened certain PTCs (XVI-XIX) derived
from quinine for the asymmetric domino double Michael
reaction of malanonitrile 1a
and
diketone 2aa in presence
of a base in different solvents and the results are presented
in table 3. The use of PTCs (XVI-XIX) to carry out the
reaction did not seem to work as good as quinine and the
comparitive yields and enantiomeric excesses of the
products were lower. The best result while using a PTC was
obtained using the catalyst XVII in presence of 1.5 equiv of
K
2CO
3at 0 °C yielding the opposite enantiomer ent-3aaa in
69% ee (entry 14) which was lower than that obtained while
using quinine as catalyst.
Table 3. Screening of asymmetric domino double Michael reaction of malanonitrile 1aanddiketone2aa using PTCs.[a]
Entry Cat. Solv. Product/SM[c] ee (%)[d]
1 XVI Xylenes 78/6 38
2 XVII Xylenes 52/26 58
3 XIX Xylenes 69/16 46
4[e] XVII Xylenes 60/17 44
5[f] XVII Xylenes 52/21 32 6[g] XVII Xylenes 64/19 59 7[g] XVIII Xylenes 68/17 47 8[h] XVII Xylenes 65/23 54 9[g] XVII DCM 85/0 22 10[g] XVII CHCl 3 80/0 32 11[g] XVII THF 70/0 41 12[g] XVII Toluene 80/0 55 13[g] XVII Mesitylene 40/50 64
14[g,i] XVII Toluene 53/23 69
[a] Unless otherwise specified, all the reactions were carried out using 1a (0.12 mmol, 1.2 equiv), 2aa (0.1 mmol) and K2CO3 (0.3 mmol, 3.0 equiv)
with 20 mol % of phase transfer catalyst in the solvent (0.8 mL) and stirred at room temperature (30 °C). [b] Diastereomeric ratio was determined by the 1H NMR analysis of crude reaction mixture. [c] NMR yield. [d]
Determined by HPLC analysis on a chiral stationary phase. [e] Cs2CO3 was
used as base. [f] K3PO4 was used as base. [g] K2CO3 (0.15 mmol, 1.5 equiv)
was used. [h] K2CO3 (0.02 mmol, 20 mol%) was used. [i] 0 °C.
In an attempt to enhance the hydrogen bonding
interactions in the transition state, the effect of common
additives has also been investigated in this reaction (please
see the supporting information). But the use of either the
acidic or the basic additives did not seem to enhance the
yields or enantiomeric excesses of the resulting products.
Based on the above investigations, we could conclude that
the optimized conditions to carry out this domino double
Michael reaction are as depicted in entry 9 (Table 2),
utilizing malanonitrile 1a (1.2 equiv) and diketone 2aa (0.1
mmol) with 20 mol % of quinine in xylenes (0.8 mL) at
room temperature (30 °C) in the absence of any additive.
Under the optimized conditions, the extensive scope of
the asymmetric domino double Michael reaction was
explored using different 2-alkylidene malononitriles 1
(Table 4). Although the phenyl, 1-naphthyl or 2-naphthyl
groups showed no obvious steric hindrance and did not
affect the enantioselectivity (entries 1-3), there was a
notable decrease in the yield of the product in case of
2-naphthyl substitution.
When compared to the unsubstituted phenyl group in
malanonitrile 1 (entry 1), the halide substituted ones (entries
4-7) displayed slightly better stereocontrol with ee ranging
between 76-86% without any extensive drop in their
respective yields. Surprisingly, the reaction of 2aa with the
malanonitrile substituted with a stronger electron
withdrawing group such as 4-nitro group resulted in reduced
stereocontrol although the reactivity was slightly better
(entry 12). Introducing an electron-donating methoxy group
on to the aromatic ring of malanonitrile 1 not only required
different ratios of diketone 2aa (1.2 equiv) and malanonitrile
1 (1 equiv) but also prolonged the reaction time to 15 days
(entry 8) to furnish the product in 68% yield and 77% ee.
Heteroaryl R
1substituents were also examined for their
reactivity: 2-thienyl, 2-furyl or 3-pyridinyl substituents
resulted in moderate yields of 58-75% with a reaction time
of 10 days (entries 9-11). Although the 3-pyridinyl
substitution in malanonitrile 1 facilitated a smooth reaction,
the enantioselectivity decreased to 62% ee. Surprisingly,
2-thienyl substituted malanonitrile 1 resulted in the product
with higher enantiomeric excess value of 92% (entry 9). The
aliphatic R
1substitution such as ethyl group on
malanonitrile 1 showed the decomposition of the starting
material (and may be also the product) and the expected
product could not be isolated, while 70% of diketone 2aa
could be recovered.
Table 4. Asymmetric domino double Michael reaction of R1
-substituted malanonitrile 1anddiketone2aa.[a]
Entry R1 (1) Time (d) Yield (%)[c] ee (%)[d]
1 Ph (1a) 3 3aaa, 76 77 2 1-Naphthyl (1b) 3.5 3baa, 80 76 3 2-Naphthyl (1c) 3.5 3caa, 62 72 4 4-ClC6H4 (1d) 2.5 3daa, 74 86 5 4-BrC6H4 (1e) 2.5 3eaa, 75 78 6 2-ClC6H4 (1f) 2 3faa, 72 76 7 2-BrC6H4 (1g) 22 h 3gaa, 86 81 8[e] 4-MeOC 6H4 (1h) 15 3haa, 68 77
9 2-Thienyl (1i) 10 3iaa, 58 92 10 2-Furyl (1j) 10 3jaa, 73 82 11 3-Pyridinyl (1k) 10 3kaa, 75 62
12 4-NO2C6H4(1l) 2.5 3laa, 80 50
[a] Unless otherwise specified, all the reactions were carried out using 1 (0.3 mmol, 1.2 equiv) and 2aa (0.25 mmol) in the presence of quinine (20 mol%) in xylenes (2 mL) at 30 oC. [b] Diastereomeric ratio was determined by the 1H NMR analysis of crude reaction mixture. [c] Isolated yield. [d]
Determined by HPLC analysis on a chiral stationary phase. [e] The reactions were carried out using 2aa (1.2 equiv) and 1h (0.25 mmol) in the presence of quinine (20 mol%) in xylenes (2 mL) at 30 oC.
The asymmetric domino double Michael reaction was
then investigated using different R
2substituents in diketone
2 which could be obtained from benzilideneacetone and
corresponding acid chlorides.
16The results are depicted in
table 5. The varying substituents displayed a notable
impact on the yields of the products with a slight variation
in their enantiomeric excesses. The 4-chloro- and
4-bromo-phenyl substitution of R
2showed similar results with an ee
of 76 or 77% respectively (entries 1 & 2), though the
reaction time of 4-bromo phenyl substitution had to be
prolonged to 10 days. We then tried to compare the results
with a more electron withdrawing moeity such as 4-nitro
group, but the starting material was insoluble in xylenes
and could be recovered completely. Heteroaryl substitution
on R
2, involving 2-thienyl or 2-furyl groups, seemed to
display no significant influence on the enantioselectivity
but showed a considerable difference in the yields of the
respective products (entries 3 & 4). Next, the impact of
different degrees of aliphatic substitutions (ethyl, i-propyl
and t-butyl groups) on R
2of diketone 2 were also
examined. It showed a clear impact not only on the yield
but also on the enantioselectivity. (78-86% ee, entries 5-7).
Interestingly, the t-butyl substituted R
2had the same
impact in the achiral protocol, with the keto-form of the
product appearing as the major isomer 3aha-keto (70%)
and the enolic form being 25% (3aha-enol).
Table 5. Asymmetric domino double Michael reaction of malanonitrile 1a and R2-substituted diketone2.[a]
Entry R2 (2) Time (d) Yield (%)[c] ee (%)[d]
1 4-ClC6H4 (2ba) 4 3aba, 76 76
2 4-BrC6H4 (2ca) 10 3aca, 81 77
3 2-Thienyl (2da) 2 3ada, 64 78 4 2-Furyl (2ea) 16 h 3aea, 80 78
5 n-Ethyl (2fa) 16 h 3afa, 94 78
6 i-Propyl (2ga) 22 h 3aga, 92 85
7 t-Butyl (2ha) 4 3aha-enol, 25 86
3aha-keto, 70 82
[a] Unless otherwise stated, all the reactions were carried out using 1a (0.3 mmol, 1.2 equiv) and 2 (0.25 mmol) in the presence of quinine (20 mol%) in xylenes (2 mL) at 30 °C. [b] Diastereomeric ratio was determined by the
1H NMR analysis of crude reaction mixture. [c] Isolated yield. [d]
Determined by HPLC analysis on a chiral stationary phase.
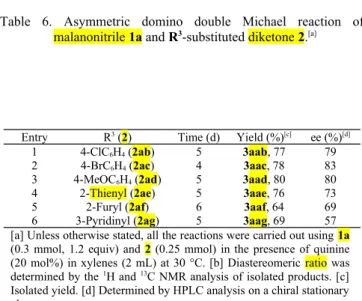
Finally, the effect of varying R
3substituents on diketone
2 were studied (Table 6). The 4-chloro and 4-bromo
substituted diketone 2 resulted in similar yields, 77 and 78%
respectively, with good enantioselectivity (entries 1 & 2).
Similarly, neither the electron-withdrawing bromo group nor
the electron-donating methoxy group seemed to show any
influence on the reactivity and selectivity (entries 1 & 3).
The heteroaryl R
3groups such as 2-thienyl, 2-furyl and
3-pyridinyl, were also tolerated but with a considerable
decrease in the yield and selectivity (entries 4-6). The
product obtained with an aliphatic R
3substitution such as
ethyl group was not stable and decomposed while trying to
purify by column chromatography
.
Table 6. Asymmetric domino double Michael reaction of malanonitrile 1a and R3-substituted diketone2.[a]
Entry R3 (2) Time (d) Yield (%)[c] ee (%)[d]
1 4-ClC6H4 (2ab) 5 3aab, 77 79
2 4-BrC6H4 (2ac) 4 3aac, 78 83
3 4-MeOC6H4 (2ad) 5 3aad, 80 80
4 2-Thienyl (2ae) 5 3aae, 76 73 5 2-Furyl (2af) 6 3aaf, 64 69 6 3-Pyridinyl (2ag) 5 3aag, 69 57 [a] Unless otherwise stated, all the reactions were carried out using 1a (0.3 mmol, 1.2 equiv) and 2 (0.25 mmol) in the presence of quinine (20 mol%) in xylenes (2 mL) at 30 °C. [b] Diastereomeric ratio was determined by the 1H and 13C NMR analysis of isolated products. [c]
Isolated yield. [d] Determined by HPLC analysis on a chiral stationary phase.
The absolute configuration of 3gaa was established by
single crystal X-ray data analysis and that of others was
assigned by analogy.
17Based on the combination of the
experimental data, catagory of catalysts screened and the
X-ray diffraction analysis, the plausible transition state is
presented in Figure 2. The highly acidic diketo group of
diketone 2 is induced by the tertiary amine of quinine while
the hydroxyl group on the 9 position of quinine is a key
function which coordinates with the dicyano substituted
electrophile 1 via hydrogen bonding. The methoxy
substitution on the quinoline also plays an important role in
enhancing the enanioselectivity (Table 1, entries 1 & 2). The
heteroaryl R
3-substitution on diketone 2 has the repulsion
with methoxyl group in quinine which destroys the
transition state. As a result, enantioselectivity is decreased
(Table 6, entries 4-6).
Figure 2. The stereochemical model predicting the asymmetric induction in domino double Michael reaction of malanonitrile 1g and diketone2aa.
Conclusions
In summary, we have successfully demonstrated the
asymmetric domino double Michael reaction of
malanonitrile 1 with diketone 2. The rarely constructed
chiral diketo cyclohexane derivatives,
4-hydroxy-3-keto-2,6-disubstituted-cyclohex-3-ene-1,1-dicarbonitriles, could
be effectively synthesized using this protocol. The simple
and mild reaction conditions provide moderate to good
yields and enantioselectivities of the desired adducts which
are highly potential targets for study in the pharmacological
field.
Experimental Section
General Procedure for the Synthesis of 3. In a glass vial equipped with a
magnetic stir bar, malanonitrile 1 (0.30 mmol, 1.2 equiv), diketone 2 (0.25 mmol) and quinine (20 mol %, 0.05 mmol) were dissolved in 2.0 mL of Xylenes and stirred at 30 °C. After the completion of the reaction, the mixture was directly subjected to flash column chromatography on silica gel to purify the corresponding product 3.
3-benzoyl-4-hydroxy-2,6-diphenylcyclohex-3-ene-1,1-dicarbonitrile (3aaa): White solid, 76.8 mg, yield 76%, Rf 0.21 (DCM/hexanes: 2/3); [α]D30 = -54.89 (c = 0.5 in CHCl3); 77% ee, determined by HPLC analysis
[Daicel chiralpak OD-H, n-hexane/i-PrOH = 80/20, 1.0 mL/min, λ = 298 nm, t (major) = 7.90 min, t (minor) = 45.10 min]; mp 241-242 °C; 1
H-NMR (400 MHz, CDCl3) δ/ppm: 16.41 (s, 1H), 7.44-7.34 (m, 7H),
7.34-7.29 (m, 2H), 7.30 (t, 1H, J = 7.6 Hz), 7.16-7.09 (m, 2H), 7.01 (d, 2H, J = 7.9 Hz), 4.52 (s, 1H), 3.47 (dd, 1H, J = 11.7, 6.2 Hz), 3.32 (dd, 1H, J = 19.7, 11.7 Hz), 3.10 (dd, 1H, J = 19.6, 6.2 Hz). 13C-NMR (100 MHz,
129.3, 129.2, 128.9, 128.32, 128.3, 126.1, 114.4, 113.1, 105.4, 49.1, 44.4, 40.2, 34.7. HRMS (APCI-TOF) for C27H20N2O2Na, [M+Na]+ (427.1422)
found: 427.1415.
3-Benzoyl-4-hydroxy-2-(naphthalen-1-yl)-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3baa): White solid, 90.9 mg, yield 80%, Rf 0.18 (DCM/hexanes: 2/3); [α]D30 = -104.70 (c = 0.5 in CHCl3); 76% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 70/30, 1.0 mL/min, λ = 298 nm, t (major) = 10.34 min, t (minor) = 80.27 min]; mp 207-208 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.21 (s, 1H), 7.87 (d, 1H, J = 8.3 Hz), 7.79 (d, 1H, J = 8.2 Hz), 7.60 (d, 1H, J = 8.6 Hz), 7.50 (t, 1H, J = 7.7 Hz), 7.42-7.37 (m, 2H), 7.35-7.29 (m, 6H), 7.07 (t, 1H, J = 7.4 Hz), 6.92 (t, 2H, J = 7.7 Hz), 6.82 (d, 2H, J = 7.4 Hz), 5.55 (s, 1H), 3.72 (dd, 1H, J = 11.7, 6.4 Hz), 3.38 (dd, 1H, J = 19.8, 11.9 Hz), 3.14 (dd, 1H, J = 19.8, 6.1 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 197.3, 180.5, 136.6, 135.0, 133.7, 132.7, 131.7, 130.3, 130.0, 129.4, 129.1, 128.8, 128.7, 128.4, 128.1, 126.4, 126.3, 125.5, 124.5, 122.8, 114.8, 112.9, 106.8, 43.2, 43.1, 40.4, 34.2. HRMS (ESI-TOF) for C31H22N2O2Na, [M+Na]+
(477.1579) found: 477.1581.
3-Benzoyl-4-hydroxy-2-(naphthalen-2-yl)-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3caa): Yellow solid, 70.5 mg, yield 62%, Rf 0.41 (DCM/hexanes: 1/1); [α]D30 = -76.07 (c = 0.5 in CHCl3); 72% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 70/30, 1.0 mL/min, λ = 298 nm, t (major) = 7.66 min, t (minor) = 88.40 min]; mp 182-183 °C; 1H-NMR (500 MHz, CDCl 3) δ/ppm: 16.54 (s, 1H), 7.87 (d, 1H, J = 8.8 Hz), 7.83 (d, 2H, J = 8.6 Hz), 7.61 (s, 1H), 7.58-7.52 (m, 2H), 7.39-7.30 (m, 4H), 7.28-7.23 (m, 2H), 7.23-7.17 (m, 3H), 7.00 (d, 2H, J = 7.5 Hz), 4.70 (s, 1H), 3.53 (dd, 1H, J = 11.8, 6.4 Hz), 3.36 (dd, 1H, J = 20.0, 11.7 Hz), 3.16 (dd, 1H, J = 20.0, 6.3 Hz). 13C-NMR (125 MHz, CDCl3) δ/ppm: 196.5, 182.6, 136.4, 134.9, 133.5, 133.3, 132.9, 130.7, 129.9, 129.4, 129.1, 128.7, 128.31, 128.29, 128.2, 127.7, 127.19, 127.16, 126.9, 126.1, 114.4, 113.2, 105.5, 49.2, 44.4, 40.3, 34.8. HRMS (ESI-TOF) for C31H21N2O2, [M-H]- (453.1603) found: 453.1594. 3-Benzoyl-2-(4-chlorophenyl)-4-hydroxy-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3daa): White solid, 81.2 mg, yield 74%, Rf 0.43 (DCM/hexanes: 1/1); [1α]D30 = -42.52 (c = 0.5 in CHCl3); 86% ee,
determined by HPLC analysis [Daicel chiralpak AD-H, n-hexane/i-PrOH = 95/5, 1.0 mL/min, λ = 298 nm, t (minor) = 11.54 min, t (major) = 13.59 min]; mp 182-183 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.35 (s, 1H), 7.45-7.37 (m, 4H), 7.37-7.28 (m, 6H), 7.04 (dd, 4H, J = 12.6, 8.4 Hz), 4.51 (s, 1H), 3.42-3.27 (m, 2H), 3.10 (dd, 1H, J = 18.4, 4.9 Hz). 13C-NMR (100 MHz, CDCl3) δ/ppm: 196.9, 182.0, 136.5, 135.6, 134.7, 134.5, 131.4, 130.8, 129.6, 129.3, 129.1, 128.5, 128.3, 126.0, 114.2, 113.1, 105.2, 48.6, 44.3, 40.3, 34.6. HRMS (ESI-TOF) for C27H18N2O2Cl, [M-H]- (437.1057) found: 437.1063. 3-Benzoyl-2-(4-bromophenyl)-4-hydroxy-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3eaa): White solid, 90.6 mg, yield 75%, Rf 0.56 (DCM/hexanes: 1/1); [α]D30 = -54.17 (c = 0.5 in CHCl3); 78% ee,
determined by HPLC analysis [Daicel chiralpak AD-H, n-hexane/i-PrOH = 95/5, 1.0 mL/min, λ = 298 nm, t (minor) = 12.77 min, t (major) = 15.51 min]; mp 244-245 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.35 (s, 1H), 7.50 (d, 2H, J = 8.2 Hz), 7.46-7.36 (m, 4H), 7.36-7.27 (m, 4H), 7.00 (d, 2H, J = 7.6 Hz), 6.99 (d, 2H, J = 8.2 Hz), 4.50 (s, 1H), 3.42-3.26 (m, 2H), 3.09 (dd, 1H, J = 18.3, 4.8 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 196.8, 182.0, 136.4, 135.1, 134.7, 132.1, 131.7, 130.8, 129.6, 129.3, 128.5, 128.3, 126.0, 123.8, 114.2, 113.0, 105.1, 48.6, 44.2, 40.3, 34.6. HRMS (ESI-TOF) for C27H18N2O2Br, [M-H]- (481.0552) found: 481.0545. 3-Benzoyl-2-(2-chlorophenyl)-4-hydroxy-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3faa): White solid, 79.0 mg, yield 72%, Rf 0.57 (DCM/hexanes: 1/1); [α]D30 = -59.39 (c = 0.5 in CHCl3); 76% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 80/20, 1.0 mL/min, λ = 298 nm, t (major) = 13.12 min, t (minor) = 36.64 min]; mp 187-189 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.15 (s, 1H), 7.46-7.32 (m, 7H), 7.32-7.21 (m, 5H) , 6.96 (d, 2H, J = 7.4 Hz), 5.28 (s, 1H), 3.54 (dd, 1H, J = 12.0, 5.8 Hz), 3.36 (dd, 1H, J = 19.5, 12.0 Hz), 3.09 (dd, 1H, J = 19.6, 5.8Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 198.5, 180.5, 136.7, 136.3, 134.7, 134.1, 131.1, 130.44, 130.42, 129.6, 129.3, 128.6, 128.5, 127.0, 125.4, 114.3, 112.3, 105.8, 44.6, 42.8, 40.5, 34.1.
HRMS (ESI-TOF) for C27H18N2O2Cl, [M-H]- (437.1057) found: 437.1055.
3-Benzoyl-2-(2-bromophenyl)-4-hydroxy-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3gaa): White solid, 103.9 mg, yield 86%, Rf 0.57 (DCM/hexanes: 1/1); [α]D30 = -85.10 (c = 0.5 in CHCl3); 81% ee,
determined by HPLC analysis [Daicel chiralpak IB, n-hexane/i-PrOH = 70/30, 1.0 mL/min, λ = 220 nm, t (major) = 9.28 min, t (minor) = 55.93 min]; mp 193-194 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.12 (s, 1H), 7.44 (d, 1H, J = 8.0 Hz), 7.42-7.32 (m, 7H), 7.29 (t, 2H, J = 7.6 Hz), 7.25-7.15 (m, 2H), 6.95 (d, 2H, J = 7.2 Hz), 5.25 (s, 1H), 3.53 (dd, 1H, J = 12.0, 5.8 Hz), 3.35 (dd, 1H, J = 19.6, 12.0 Hz), 3.08 (dd, 1H, J = 19.6, 5.8 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 198.7, 180.3, 136.8, 135.5, 134.7, 133.8, 131.2, 130.5, 130.4, 129.6, 129.2, 128.7, 128.4, 127.6, 127.4, 125.4, 114.2, 112.3, 105.9, 47.0, 42.7, 40.4, 34.0. HRMS (ESI-TOF) for C27H18N2O2Br, [M-H]- (481.0552) found: 481.0555. 3-Benzoyl-4-hydroxy-2-(4-methoxyphenyl)-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3haa): White solid, 73.9 mg, yield 68%, Rf 0.28 (DCM/hexanes: 1/1); [α]D30 = -39.67 (c = 0.5 in CHCl3); 77% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 70/30, 1.0 mL/min, λ = 290 nm, t (major) = 7.62 min, t (minor) = 49.34 min]; mp 144-145 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.42 (s, 1H), 7.42-7.34 (m, 4H), 7.34-7.24 (m, 4H), 7.08-7.00 (m, 4H), 6.88 (d, 2H, J = 8.7 Hz), 4.48 (s, 1H), 3.82 (s, 3H), 3.44 (dd, 1H, J = 11.8, 6.2 Hz), 3.29 (dd, 1H, J = 19.6, 11.8 Hz), 3.07 (dd, 1H, J = 19.7, 6.2 Hz). 13C-NMR (100 MHz, CDCl3) δ/ppm: 196.7, 182.0, 160.1, 136.5, 135.1, 131.3, 130.6, 129.4, 129.2, 128.3, 127.8, 126.1, 114.5, 114.2, 113.3, 105.7, 55.3, 48.5, 44.5, 40.1, 34.6. HRMS (ESI-TOF) for C28H21N2O3, [M-H]- (433.1552) found: 433.1551. 3-Benzoyl-4-hydroxy-6-phenyl-2-(thiophen-2-yl)cyclohex-3-ene-1,1-dicarbonitrile (3iaa): Yellow solid, 59.5 mg, yield 58%, Rf 0.59 (DCM/hexanes: 1/1); [α]D30 = -64.98 (c = 0.5 in CHCl3); 92% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 290 nm, t (major) = 9.19 min, t (minor) = 41.80 min]; mp 106-107 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.41 (s, 1H), 7.48-7.32 (m, 9H), 7.15 (d, 2H, J = 7.8 Hz), 7.05 (dd, 1H, J = 5.2, 3.6 Hz), 6.95 (d, 1H, J = 3.7 Hz), 4.83 (s, 1H), 3.59 (dd, 1H, J = 11.8, 6.4 Hz), 3.29 (dd, 1H, J = 19.7, 11.8 Hz), 3.07 (dd, 1H, J = 19.7, 6.4 Hz).13C-NMR (100 MHz, CDCl3) δ/ppm: 196.4, 182.2, 140.5, 136.3, 135.1, 130.9, 129.6, 129.3, 128.6, 128.3, 128.2, 127.7, 126.2, 114.0, 113.5, 107.1, 44.9, 44.6,
40.8, 34.6. HRMS (ESI-TOF) for C25H17N2O2S, [M-H]- (409.1011) found:
409.1007.
3-Benzoyl-2-(furan-2-yl)-4-hydroxy-6-phenylcyclohex-3-ene-1,1-dicarbonitrile (3jaa): Yellow solid, 72.0 mg, yield 73%, Rf 0.45 (DCM/hexanes: 1/1); [α]D30 = -39.76 (c = 0.5 in CHCl3); 82% ee,
determined by HPLC analysis [Daicel chiralpak IB, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 297 nm, t (major) = 8.21 min, t (minor) = 26.49 min]; mp 226-227 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.44 (s, 1H), 7.48 (t, 2H, J = 7.5 Hz), 7.44-7.35 (m, 7H), 7.16 (d, 2H, J = 7.4 Hz), 6.41 (dd, 1H, J = 3.2, 2.0 Hz), 6.21 (d, 1H, J = 3.3 Hz), 4.66 (s, 1H), 3.53 (dd, 1H, J = 11.8, 6.4 Hz), 3.27 (dd, 1H, J = 19.7, 11.8 Hz), 3.06 (dd, 1H, J = 19.6, 6.4 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 195.3, 183.6, 149.3, 144.2, 136.1, 135.1, 131.0, 129.6, 129.3, 128.6, 128.3, 126.2, 113.7, 113.1, 112.4, 111.2, 103.7, 43.7, 43.5, 41.6, 34.9. HRMS (ESI-TOF) for C25H17N2O3, [M-H]- (393.1239) found: 393.1239. 3-Benzoyl-4-hydroxy-6-phenyl-2-(pyridin-3-yl)cyclohex-3-ene-1,1-dicarbonitrile (3kaa): White solid, 76.0 mg, yield 75%, Rf 0.62 (ethyl acetate /hexanes: 1/1); [α]D30 = -31.26 (c = 0.5 in CHCl3); 62% ee,
determined by HPLC analysis [Daicel chiralpak AD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 210 nm, t (minor) = 13.44 min, t (major) = 24.10 min]; mp 251-252 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.28 (s, 1H), 8.63 (s, 1H), 8.41 (s, 1H), 7.47-7.38 (m, 5H), 7.37-7.27 (m, 5H), 7.02 (d, 2H, J = 7.5 Hz), 4.59 (s, 1H), 3.44-3.30 (m, 2H), 3.22-3.08 (m, 1H). 13 C-NMR (125 MHz, CDCl3) δ/ppm: 196.9, 181.8, 150.4, 149.9, 138.2, 136.4, 134.3, 132.3, 130.9, 129.8, 129.4, 128.6, 128.2, 125.8, 123.6, 113.9, 112.8, 104.3, 47.1, 44.1, 40.4, 34.4. HRMS (ESI-TOF) for C26H18N3O2, [M-H] -(404.1399) found: 404.1400. 3-Benzoyl-2-(4-nitrophenyl)-4-hydroxy-6-phenylcyclohex-3-ene-1,1-dicarbonitrile(3laa): White solid, 94.4 mg, yield 84%, Rf 0.28 (ethyl acetate /hexanes: 1/5); [α]D30 = -50.79 (c = 0.5 in CHCl3); 50% ee,
determined by HPLC analysis [Daicel chiralpak AD-H, n-hexane/i-PrOH = 95/5, 0.8 mL/min, λ = 284 nm, t (minor) = 52.72 min, t (major) = 61.14 min]; mp 147-148.1 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.29 (s, 1H), 8.20 (d, 2H, J = 8.56), 7.44-7.40 (m, 4H), 7.34-7.27 (m, 5H), 7.00 (d, 2H, J = 7.72), 4.66 (s, 1H), 3.42-3.33 (m, 2H), 3.22-3.13 (m, 1H). 13C-NMR (100 MHz, CDCl3) δ/ppm: 197.1, 181.7, 148.2, 143.0, 136.3, 134.2, 131.1, 131.0, 129.9, 129.4, 128.7, 128.2, 125.8, 123.9, 113.8, 112.8, 104.7, 48.7, 44.0, 40.5, 34.3. HRMS (ESI-TOF) for C27H19N3O4, [M+H]+ (450.1448) found: 450.1409. 3-(4-Chlorobenzoyl)-4-hydroxy-2,6-diphenylcyclohex-3-ene-1,1-dicarbonitrile (3aba): White solid, 83.4 mg, yield 76%, Rf 0.59 (DCM/hexanes: 1/1); [α]D30 = -38.90 (c = 0.5 in CHCl3); 76% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 70/30, 1.0 mL/min, λ = 298 nm, t (major) = 6.93 min, t (minor) = 37.30 min]; mp 147-148.1 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.38 (s, 1H), 7.46-7.35 (m, 6H), 7.34-7.28 (m, 2H), 7.28-7.23 (m, 2H), 7.16 (t, 2H, J = 3.6 Hz), 6.96 (d, 2H, J = 3.6 Hz), 4.46 (s, 1H), 3.46 (dd, 1H, J = 11.6, 6.3 Hz), 3.32 (dd, 1H, J = 19.6, 11.6 Hz), 3.11 (dd, 1H, J = 19.7, 6.2 Hz). 13 C-NMR (100 MHz, CDCl3) δ/ppm: 195.4, 182.7, 137.1, 135.8, 134.9, 134.8, 130.2, 129.54, 129.48, 129.3, 129.0, 128.7, 128.3, 127.7, 114.3, 113.0, 105.4, 49.1, 44.4, 40.2, 34.7. HRMS (ESI-TOF) for C27H18N2O2Cl, [M-H] -(437.1057) found: 437.1057. 3-(4-Bromobenzoyl)-4-hydroxy-2,6-diphenylcyclohex-3-ene-1,1-dicarbonitrile (3aca): White solid, 97.9 mg, yield 81%, Rf 0.57 (DCM/hexanes: 1/1); [α]D30 = -23.16 (c = 0.5 in CHCl3); 77% ee,
determined by HPLC analysis [Daicel chiralpak IB, n-hexane/i-PrOH = 75/25, 1.0 mL/min, λ = 295 nm, t (major) = 6.65 min, t (minor) = 24.66 min]; mp 242-243 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.37 (s, 1H), 7.44-7.34 (m, 8H), 7.33-7.27 (m, 2H), 7.18-7.12 (m, 2H), 6.88 (d, 2H, J = 8.4 Hz), 4.46 (s, 1H), 3.45 (dd, 1H, J = 11.6, 6.3 Hz), 3.31 (dd, 1H, J = 19.7, 11.6 Hz), 3.10 (dd, 1H, J = 19.7, 6.3 Hz). 13C-NMR (100 MHz, CDCl3) δ/ppm: 195.3, 182.7, 135.8, 135.2, 134.9, 131.6, 130.2, 129.51, 129.45, 129.2, 129.0, 128.3, 127.8, 125.4, 114.3, 113.0, 105.4, 49.0, 44.3, 40.2, 34.7. HRMS (ESI-TOF) for C27H18N2O2Br, [M-H]- (481.0552) found: 481.0558. 4-Hydroxy-2,6-diphenyl-3-(thiophene-2-carbonyl)cyclohex-3-ene-1,1-dicarbonitrile (3ada): White solid, 65.7 mg, yield 64%, Rf 0.31 (DCM/hexanes: 1/1); [α]D30 = -19.21 (c = 0.5 in CHCl3); 78% ee,
determined by HPLC analysis [Daicel chiralpak AD-H, n-hexane/i-PrOH = 90:10, 1.0 mL/min, λ = 298 nm, t (major) = 11.95 min, t (minor) = 15.04 min]; mp 184-185 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 17.51 (s, 1H), 7.60 (d, 1H, J = 5.0 Hz), 7.54-7.43 (m, 5H), 7.41-7.35 (m, 3H), 7.34-7.27 (m, 3H), 7.00 (t, 1H, J = 4.4 Hz), 5.01 (s, 1H), 3.41 (dd, 1H, J = 11.7, 6.0 Hz), 3.32 (dd, 1H, J = 19.2, 11.7), 3.11 (dd, 1H, J = 19.2, 6.0 Hz). 13 C-NMR (100 MHz, CDCl3) δ/ppm: 184.8, 184.5, 139.6, 135.4, 135.1, 133.5, 132.2, 130.4, 129.7, 129.5, 129.2, 128.3, 128.1, 114.3, 113.4, 103.7, 48.5, 44.8, 40.1, 35.5. HRMS (ESI-TOF) for C25H17N2O2S, [M-H]- (409.1011) found: 409.1012. 3-(Furan-2-carbonyl)-4-hydroxy-2,6-diphenylcyclohex-3-ene-1,1-dicarbonitrile (3aea): Yellow solid, 78.9 mg, yield 80%, Rf 0.34 (DCM/hexanes: 1/1); [α]D30 = -31.28 (c = 0.5 in CHCl3); 78% ee,
determined by HPLC analysis [Daicel chiralpak AD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 298 nm, t (major) = 11.58 min, t (minor) = 13.43 min]; mp 242-243 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 17.37 (s, 1H), 7.51 (s, 1H), 7.47-7.34 (m, 8H), 7.34-7.28 (m, 2H), 7.25 (d, 1H, J = 3.6 Hz), 6.47 (dd, 1H, J = 3.6, 1.6 Hz), 5.47 (s, 1H), 3.47 (dd, 1H, J = 12.2, 6.1 Hz), 3.33 (dd, 1H, J = 19.6, 12.1 Hz), 3.07 (dd, 1H, J = 19.5, 6.1 Hz). 13 C-NMR (100 MHz, CDCl3) δ/ppm: 184.2, 178.7, 150.4, 146.7, 136.5, 135.2, 130.2, 129.4, 129.2, 129.1, 128.6, 128.3, 120.4, 114.6, 113.4, 112.6, 103.4, 47.5, 44.6, 39.5, 35.3. HRMS (ESI-TOF) for C25H17N2O3, [M-H] -(393.1239) found: 393.1238. 4-Hydroxy-2,6-diphenyl-3-propionylcyclohex-3-ene-1,1-dicarbonitrile (3afa): White solid, 83.8 mg, yield 94%, Rf 0.45 (DCM/hexanes: 1/1); [α]D30 = -82.40 (c = 0.5 in CHCl3); 78% ee, determined by HPLC analysis
[Daicel chiralpak AD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 298 nm, t (major) = 8.75 min, t (minor) = 11.15 min]; mp 174-175 °C; 1
H-NMR (400 MHz, CDCl3) δ/ppm: 16.14 (s, 1H), 7.51-7.44 (m, 3H), 7.41-7.34 (m, 5H), 7.33-7.27 (m, 2H), 4.58 (s, 1H), 3.41 (dd, 1H, J = 12.4, 5.9 Hz), 3.24 (dd, 1H, J = 19.5, 12.4 Hz), 2.95 (dd, 1H, J = 19.4, 5.9 Hz), 2.47 (dq, 1H, J = 17.6, 5.9 Hz), 1.95 (dq, 1H, J = 17.7, 5.9 Hz), 0.95 (t, 3H, J =7.2 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 203.8, 176.6, 135.4, 135.0, 130.1, 129.6, 129.4, 129.2, 129.1, 128.2, 114.5, 113.1, 104.9, 48.6, 44.4, 39.7, 33.5, 30.9, 7.6. HRMS (ESI-TOF) for C23H19N2O2, [M-H] -(355.1447) found: 355.1448.
4-Hydroxy-2,6-diphenyl-3-isobutyrylcyclohex-3-ene-1,1-dicarbonitrile (3aga): White solid, 85.2 mg, yield 92%, Rf 0.51 (DCM/hexanes: 1/1); [α]D30 = -80.34 (c = 0.5 in CHCl3); 85% ee, determined by HPLC analysis
[Daicel chiralpak AD-H, n-hexane/i-PrOH = 97/3, 1.0 mL/min, λ = 298 nm, t (minor) = 14.83 min, t (major) = 17.35 min]; mp 196-197 °C; 1H-NMR
(400 MHz, CDCl3) δ/ppm: 16.64 (s, 1H), 7.52-7.43 (m, 3H), 7.42-7.34 (m, 5H), 7.34-7.27 (m, 2H), 4.63 (s, 1H), 3.42 (dd, 1H, J = 12.3, 5.9 Hz), 3.25 (dd, 1H, J = 19.5, 12.3 Hz), 2.97 (dd, 1H, J = 19.5, 5.9 Hz), 2.57 (sept, 1H, J = 6.7 Hz), 1.16 (d, 3H, J = 6.8 Hz), 0.64 (d, 3H, J = 6.5 Hz). 13C-NMR (100 MHz, CDCl3) δ/ppm: 206.9, 179.4, 135.7, 135.1, 130.3, 129.6, 129.4, 129.2, 129.1, 128.2, 114.4, 113.2, 104.1, 48.7, 44.5, 39.7, 34.1, 33.9, 19.7, 17.9. HRMS (ESI-TOF) for C24H21N2O2, [M-H]- (369.1603) found:
369.1599.
4-Hydroxy-2,6-diphenyl-3-pivaloylcyclohex-3-ene-1,1-dicarbonitrile (3aha-enol): White solid, 24.0 mg, yield 25%, Rf 0.49 (DCM/hexanes: 1/1); [α]D30 = +5.06 (c = 0.5 in CHCl3); 86% ee, determined by HPLC
analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 227 nm, t (major) = 6.88 min, t (minor) = 44.90 min]; mp 140-141 °C;
1H-NMR (400 MHz, CDCl 3) δ/ppm: 17.20 (s, 1H), 7.49-7.41 (m, 3H), 7.40-7.32 (m, 5H), 7.31-7.25 (m, 2H), 5.05 (s, 1H), 3.31-3.15 (m, 2H), 3.07-2.93 (m, 1H), 1.15 (s, 9H). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 207.2, 181.0, 135.8, 135.2, 130.3, 129.4, 129.3, 129.2, 129.0, 128.3, 114.3, 113.8, 104.5, 47.7, 44.3, 43.1, 40.0, 34.7, 27.8. HRMS (FAB-magnetic sector) for C25H25N2O2, [M+H]+ (385.1916) found: 385.1917.
4-oxo-2,6-diphenyl-3-pivaloylcyclohexane-1,1-dicarbonitrile(3aha-keto): White solid, 67.3 mg, yield 70%, Rf 0.33 (DCM/hexanes: 1/1); [α]D30
= -49.10 (c = 0.5 in CHCl3); 82% ee, determined by HPLC analysis [Daicel
chiralpak OD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 227 nm, t (minor) = 10.23 min, t (major) = 11.83 min]; mp 166-167 °C; 1H-NMR
(400 MHz, CDCl3) δ/ppm: 7.53-7.43 (m, 3H), 7.42-7.28 (m, 7H), 4.74 (d,
1H, J = 12.5 Hz), 4.14-4.03 (m, 2H), 3.41 (dd, 1H, J = 16.7, 7.2 Hz), 3.02 (dd, 1H, J = 17.0, 1.6 Hz), 0.89 (s, 9H). 13C-NMR (100 MHz, CDCl
3)
δ/ppm: 210.6, 203.5, 134.7, 134.1, 129.8, 129.7, 129.3, 129.1, 115.4, 112.8,
59.2, 48.2, 45.8, 45.0, 44.1, 42.4, 25.7. HRMS (FAB-magnetic sector) for
C25H25N2O2, [M+H]+ (385.1916) found: 385.1915.
3-Benzoyl-6-(4-chlorophenyl)-4-hydroxy-2-phenylcyclohex-3-ene-1,1-dicarbonitrile (3aab): White solid, 84.5 mg, yield 77%, Rf 0.27 (DCM/hexanes: 1/1); [α]D30 = -54.82 (c = 0.5 in CHCl3); 79% ee,
determined by HPLC analysis [Daicel chiralpak IB, n-hexane/i-PrOH = 70/30, 1.0 mL/min, λ = 298 nm, t (major) = 6.16 min, t (minor) = 20.13 min]; mp 166-167 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.43 (s, 1H), 7.45-7.33 (m, 6H), 7.29-7.23 (m, 4H), 7.12 (d, 1H, J = 6.9 Hz), 7.11 (d, 1H, J = 7.9 Hz), 7.04-6.97 (m, 2H), 4.52 (s, 1H), 3.45 (dd, 1H, J = 11.7, 6.4 Hz), 3.26 (dd, 1H, J = 19.6, 11.7 Hz), 3.09 (dd, 1H, J = 19.6, 6.4 Hz). 13 C-NMR (100 MHz, CDCl3) δ/ppm: 196.6, 181.9, 136.4, 135.8, 135.7, 133.5, 130.8, 130.2, 129.7, 129.5, 129.4, 129.0, 128.4, 126.1, 114.2, 113.0, 105.4, 49.0, 44.3, 39.8, 34.6. HRMS (EI-magnetic sector) for C27H19N2O2Cl, [M]+
(438.1135) found: 438.1137.
3-Benzoyl-6-(4-bromophenyl)-4-hydroxy-2-phenylcyclohex-3-ene-1,1-dicarbonitrile (3aac): White solid, 94.3 mg, yield 78%, Rf 0.25 (DCM/hexanes: 1/1); [α]D30 = -58.84 (c = 0.5 in CHCl3); 83% ee,
determined by HPLC analysis [Daicel chiralpak IB, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 298 nm, t (major) = 12.28 min, t (minor) = 92.09
min]; mp 198.6-199.4 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.43 (s, 1H), 7.50 (d, 2H, J = 8.4 Hz), 7.44-7.34 (m, 4H), 7.27 (t, 2H, J = 7.5 Hz), 7.19 (d, 2H, J = 8.4 Hz), 7.14-7.08 (m, 2H), 7.00 (d, 2H, J = 7.8 Hz), 4.52 (s, 1H), 3.43 (dd, 1H, J = 11.7, 6.4 Hz), 3.25 (dd, 1H, J = 19.7, 11.7 Hz), 3.08 (dd, 1H, J = 19.7, 6.4 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 196.6, 181.9, 136.3, 135.8, 134.0, 132.4, 130.7, 130.1, 129.9, 129.4, 128.9, 128.4, 126.1, 123.8, 114.1, 113.0, 105.3, 49.0, 44.2, 39.8, 34.5. HRMS (ESI-TOF) for C27H18N2O2Br, [M-H]- (481.0552) found: 481.0546.
3-Benzoyl-4-hydroxy-6-(4-methoxyphenyl)-2-phenylcyclohex-3-ene-1,1-dicarbonitrile (3aad): White solid, 86.9 mg, yield 80%, Rf 0.47 (DCM/hexanes: 1/1); [α]D30 = -59.63 (c = 0.5 in CHCl3); 80% ee,
determined by HPLC analysis [Daicel chiralpak IB, n-hexane/i-PrOH = 85/15, 1.0 mL/min, λ = 298 nm, t (major) = 12.56 min, t (minor) = 42.75 min]; mp 183-184 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.43 (s, 1H), 7.42-7.33 (m, 4H), 7.20-7.30 (m, 4H), 7.15-7.08 (m, 2H), 7.03-6.97 (m, 2H), 6.88 (d, 2H, J = 8.8 Hz), 4.50 (s, 1H), 3.78 (s, 3H), 3.43 (dd, 1H, J = 11.6, 6.3 Hz), 3.28 (dd, 1H, J = 19.6, 11.6 Hz), 3.07 (dd, 1H, J = 19.6, 6.3 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 196.6, 182.4, 160.3, 136.5, 136.0, 130.6, 130.2, 129.5, 129.2, 128.8, 128.3, 126.9, 126.1, 114.55, 114.47, 113.3, 105.5, 55.3, 49.0, 44.8, 39.5, 34.8. HRMS (ESI-TOF) for C28H21N2O3, [M-H]- (433.1552) found: 433.1556. 3-Benzoyl-4-hydroxy-2-phenyl-6-(thiophen-2-yl)cyclohex-3-ene-1,1-dicarbonitrile (3aae): White solid, 78.0 mg, yield 76%, Rf 0.32 (DCM/hexanes: 1/1); [α]D30 = -46.20 (c = 0.5 in CHCl3); 73% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 298 nm, t (major) = 14.35 min, t (minor) = 30.42 min]; mp 199-200 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.34 (s, 1H), 7.43-7.30 (m, 5H), 7.30-7.24 (m, 2H), 7.14-7.05 (m, 3H), 7.04-6.95 (m, 3H), 4.52 (s, 1H), 3.82 (t, 1H, J = 8.8 Hz), 3.25 (d, 2H, J = 8.8 Hz). 13 C-NMR (100 MHz, CDCl3) δ/ppm: 196.7, 181.2, 137.5, 136.5, 135.8, 130.7, 130.1, 129.3, 128.9, 128.3, 127.8, 127.4, 126.3, 126.0, 114.1, 113.1, 105.4, 48.7, 45.3, 36.5, 36.4. HRMS (ESI-TOF) for C25H17N2O2S, [M-H] -(409.1011) found: 409.1017. 3-Benzoyl-6-(furan-2-yl)-4-hydroxy-2-phenylcyclohex-3-ene-1,1-dicarbonitrile (3aaf): Yellow solid, 63.1 mg, yield 64%, Rf 0.36 (DCM/hexanes: 1/1); [α]D30 = -63.66 (c = 0.5 in CHCl3); 69% ee,
determined by HPLC analysis [Daicel chiralpak OD-H, n-hexane/i-PrOH = 90/10, 1.0 mL/min, λ = 295 nm, t (major) = 10.09 min, t (minor) = 13.18 min]; mp 172-173 °C; 1H-NMR (400 MHz, CDCl 3) δ/ppm: 16.40 (s, 1H), 7.47-7.34 (m, 5H), 7.27 (t, 2H, J = 7.7 Hz), 7.15-7.07 (m, 2H), 7.00 (d, 2H, J = 7.3 Hz), 6.45-6.34 (d, 2H), 4.49 (s, 1H), 3.67 (dd, 1H, J = 11.3, 6.5 Hz), 3.30 (dd, 1H, J = 19.8, 11.4 Hz), 3.12 (dd, 1H, J = 19.8, 6.5 Hz). 13C-NMR (100 MHz, CDCl3) δ/ppm: 196.5, 181.7, 148.5, 143.7, 136.4, 135.8, 130.8, 130.1, 129.4, 129.0, 128.4, 126.2, 113.9, 113.2, 110.9, 109.7, 105.4, 48.6, 43.1, 34.9, 33.2. HRMS (ESI-TOF) for C25H17N2O3, [M-H]- (393.1239) found: 393.1244. 3-Benzoyl-4-hydroxy-2-phenyl-6-(pyridin-3-yl)cyclohex-3-ene-1,1-dicarbonitrile (3aag): Yellow solid, 69.9 mg, yield 69%, Rf 0.24 (DCM/ethyl acetate/hexanes: 1/2/3); [α]D30 = -21.62 (c = 0.5 in CHCl3);
57% ee, determined by HPLC analysis [Daicel chiralpak AS-H, n-hexane/i-PrOH = 80/20, 1.0 mL/min, λ = 295 nm, t (minor) = 22.49 min, t (major) = 36.06 min]; mp 197-198 °C; 1H-NMR (400 MHz, CDCl
3) δ/ppm: 16.45 (s,
7.28 (t, 2H, J = 7.9 Hz), 7.18-7.10 (m, 2H), 7.05-6.97 (m, 2H), 4.55 (s, 1H), 3.53 (dd, 1H, J = 11.6, 6.4 Hz), 3.29 (dd, 1H, J = 19.7, 11.7 Hz), 3.12 (dd, 1H, J = 19.7, 6.4 Hz). 13C-NMR (100 MHz, CDCl 3) δ/ppm: 196.5, 181.5, 150.5, 149.7, 136.2, 135.62, 135.58, 130.8, 130.1, 129.6, 129.1, 128.4, 126.1, 124.1, 114.0, 112.8, 105.3, 48.9, 44.0, 38.1, 34.3. HRMS (EI-magnetic sector) for C26H19N3O2, [M]+ (405.1477) found: 405.1469.
Supporting Information (see footnote on the first page of this article): 1H
and 13C NMR spectra and X-ray crystallographic data for all new
compounds.
Acknowledgments
We thank the Ministry of Science and Technology of the Republic of China (MOST 103-2113-M-003-009-MY3) and National Taiwan Normal University (NTNU 100-D-06) for financial support.
____________
[1] a) R. D. Santo, J. Med. Chem. 2014, 57, 539; b) L. L. Tarrago, M. L. Andreola, M. Fournier, G. A. Nevinsky, V. Parissi, V. R. Soultrait, S. Litvak, Curr. Pharm. Des. 2002, 8, 595; c) R. D. Santo , R. Costi , A. Roux , M. Artico , A. Lavecchia , L. Marinelli , E. Novellino , L. Palmisano , M. Andreotti , R. Amici , C. M. Galluzzo , L. Nencioni , A. T. Palamara , Y. Pommier, C. Marchand, J. Med. Chem. 2006, 49, 1939; d) L. Hu, S. Zhang, X. He, Z. Luo, X. Wang, W. Liu, X Qin, Bioorg. Med. Chem. 2012, 20, 177.
[2] a) O. Vajragupta, P. Boonchoong, G. M. Morris, A. J. Olson,
Bioorg. Med. Chem. Lett. 2005, 15, 3364; b) A. Mazumder, N.
Neamati, S. Sunder, J. Schulz, H. Pertz, E. Eich, Y. Pommier, J.
Med. Chem. 1997, 40, 3057; c) A. Mazumder, N. Neamati, S.
Sunder, J. Schulz, H. Pertz, E. Eich, Y. Pommier J. Med. Chem.
1997, 40, 3057; d) R. D. Santo, R. Costi, M. Artico, E. Tramontano,
P. L. Colla, A. Pani Pure Appl. Chem. 2003, 75, 195.
[3] a) T. Fujishita; T. Yoshinaga, A. Sato, PCT Int. Appl. WO 2000039086A, 2000; b) S. Shimizu, T. Endo, K. Izumi, H. Mikamiyama, Org. Process Res. Dev. 2007, 11, 1055.
[4] a) D. Rogolino, M. Carcelli, C. Compari, D. L. Luca, S. Ferro, E. Fisicaro, G. Rispoli, N. Neamati, Z. Debyser, F. Christ, A. Chimirri,
Eur. J. Med. Chem. 2014, 78, 425; b) M. L. Barreca, S. Ferro, A.
Rao, L. De Luca, M. Zappala, A. M. Monforte, Z. Debyser, M. Witvrouw, A. Chimirri, J. Med. Chem. 2005, 48, 7084; c) L. De Luca, M. L. Barreca, S. Ferro, N. Iraci, M. Michiels, F. Christ, Z. Debyser, M. Witvrouw, A. Chimirri, Bioorg. Med. Chem. Lett. 2008,
18, 2891; d) L. De Luca, S. De Grazia, S. Ferro, R. Gitto, F. Christ,
Z. Debyser, A. Chimirri; Eur. J. Med. Chem. 2011, 46, 756; e) S. Ferro, L. De Luca, M. L. Barreca, N. Iraci, S. De Grazia, F. Christ, M. Witvrouw, Z. Debyser, A. Chimirri, J. Med. Chem. 2009, 52, 569; f) S. Ferro, L. De Luca, M. L. Barreca, S. De Grazia, F. Christ, Z. Debyser, A. Chimirri, Bioorg. Med. Chem. 2010, 18, 5510. [5] a) J. Jiang, J. L. Bunda, G. A. Doss, G. G. Chicchi, M. M. Kurtz, K.
L. C. Tsao, X. Tong, S. Zheng, A. K. Upthagrove, K. Samuel, R. Tschirret-Guth, S. Kumar, A. Wheeldon, E. J. Carlson, R. Hargreaves, D. Burns, T. Ryan, C. Hamill, S. M. Krause, W. Eng, R. J. DeVita, S. G. Mills, J. Med. Chem. 2009, 52, 3039; b) R. Ciochina, R. B. Grossman, Chem. Rev. 2006, 106, 3963.
[6] H. Qian, T. Pei, R. A. Widenhoefer, Organometallics 2005, 24, 287. [7] T. Hayashi, M. Toyoshima, H. Gotoh, H. Ishikawa, Org. Lett. 2009,
11, 45.
[8] R. Zhou, J. Wang, J. Tian, Z. He, Org. Biomol. Chem. 2012, 10, 773. [9] L. Chabaud, T. Jousseaume, P. Retailleau, C. Guillou, Eur. J. Org.
Chem. 2010, 2010, 5471.
[10] F. Wu, H. Li, R. Hong, L. Deng, Angew. Chem. Int. Ed. 2006, 45, 947.
[11] T. Bui, C. F. Barbas, Tetrahedron Lett. 2000, 41, 6951.
[12] S. Rajkumar, K. Shankland, J. M. Goodman, A. J. A. Cobb, Org.
Lett. 2013, 15, 1386.
[13] C. D. Fusco, A. Lattanzi, Eur. J. Org. Chem. 2011, 2011, 3728. [14] a) S. Goudedranche, W. Raimondi, X. Bugaut, T. Constantieux, D.
Bonne, J. Rodriguez, Synthesis 2013, 45, 1909; b) G. Audran, P. Bremond, M, Fauerstein, S. R. A. Marque, M. Santelli Tetrahedron
2013, 69, 8325.
[15] Y. J. Jang, Y. S. Chen, C. J. Lee, C. H. Chen, W. Lin, Synthesis
2014, DOI: 10.1055/s-0034-1379143
[16] F. K. MacDonald, D. J. Burnell, J. Org. Chem. 2009, 74, 6973. [17] The structure of 3gaa was determined by X-ray analysis (CCDC
number: 940048), and more information is provided in the supporting information.
Received: ((will be filled in by the editorial staff)) Published online: ((will be filled in by the editorial staff))
Layout 2:
((Key Topic))
Yeong-Jiunn Jang, Yu-Shan Chen, Chia-Jui Lee, Chi-Han Chen, Ganapuram Madhusudhan Reddy, Chi-Ting Ko and Wenwei Lin* ...1 – 8.
Asymmetric organocatalytic synthesis of highly substituted cyclohexenols via domino double Michael reactions of 1-hydroxy-1,4-dien-3-ones and 2-alkylidene malononitriles
Keywords: Domino reactions / Michael
addition / Cinchona alkaloids / Enones / Carbocycles
An effective asymmetric organocatalytic version for the synthesis of polysubstituted cyclohexenes from 1-hydroxy-1,4-dien-3-one and 2-alkylidene malononitriles via a domino double
Michael reaction sequence using quinine as the catalyst is achieved. The products were obtained in moderate to good yields (up to 96%) and enantioselectivities (up to 92% ee).