A randomized, double-blind,
placebo-controlled comparison study
of sarcosine (N-methylglycine) and
D
-serine
add-on treatment for schizophrenia
Hsien-Yuan Lane1,2, Ching-Hua Lin3, Yu-Jhen Huang1, Chun-Hui Liao1, Yue-Cune Chang4
and Guochuan E. Tsai5
1Departments of Psychiatry, China Medical University and Hospital, Taichung, Taiwan
2Institute of Clinical Medical Science, China Medical University Medical College, Taichung, Taiwan 3Department of Adult Psychiatry, Kai-Suan Psychiatric Hospital, Kaohsiung, Taiwan
4Department of Mathematics, Tamkang University, Taipei, Taiwan
5Department of Psychiatry, Harbor–UCLA Medical Center, and the Los Angeles Biomedical Research Institute, Torrance, CA, USA
Abstract
Recent evidence indicates that enhancing N-methyl-D-aspartate (NMDA) neurotransmission with the
treatment of NMDA/glycine site agonists, such asD-serine, or a glycine transporter-1 (GlyT-1) antagonist,
N-methylglycine (sarcosine), can improve symptoms of schizophrenia. To compare these two novel approaches, 60 patients with chronic schizophrenia were enrolled into a 6-wk double-blind, placebo-controlled trial of add-on treatments at the reported effective dosages (2 g/d). Clinical assessments were conducted every other week. Treatment grouprtreatment duration interaction analysis by multiple linear regression showed that sarcosine was superior to placebo at all four outcome measures of Positive and Negative Syndrome Scale (PANSS) total (p=0.005), Scale for the Assessment of Negative Symptoms (SANS) (p=0.021), Quality of Life (QOL) (p=0.025), and Global Assessment of Functioning (GAF) (p=0.042). However,D-serine did not differ significantly from placebo in any measure. Sarcosine
treat-ment was better thanD-serine in effect sizes for all outcome measures. Sarcosine also surpassed placebo in
most of the measures of five PANSS factors and five SANS subscales. All treatments were well tolerated. These findings suggest that the GlyT-1 inhibitor is more efficacious than the NMDA/glycine site agonist in treatment for schizophrenia, including life quality and global function, at the dosages tested.
Received 24 July 2009 ; Reviewed 2 September 2009 ; Revised 22 September 2009 ; Accepted 6 October 2009 ; First published online 4 November 2009
Key words:D-serine, glycine, N-methylglycine, NMDA, sarcosine, schizophrenia.
Introduction
In addition to monoaminergic theory, glutamatergic dysfunction has been implicated in the pathophysi-ology of schizophrenia on the basis of the psychoto-mimetic action of phencyclidine (PCP) and ketamine, both of which block N-methyl-D-aspartate (NMDA)
subtype glutamate receptor-mediated neurotrans-mission (Javitt, 1987 ; Tsai & Coyle, 2001). Conse-quently, enhancement of NMDA neurotransmission
has been proposed as a potential treatment of schizo-phrenia (Deutsch et al. 1989). Several studies have tar-geted the glycine site of the NMDA receptor (NMDA/ glycine site). The agents included full agonists such as D-serine (Heresco-Levy et al. 2005 ; Tsai et al.
1984), glycine (Heresco-Levy et al. 1996, 1999, 2004),
D-alanine (Tsai et al. 2006), and the partial agonist D-cycloserine (Goff et al. 1999 ; Heresco-Levy et al.
2002). As add-on therapy to antipsychotics, these agonists improve negative and cognitive symptoms, but the efficacy of glycine and D-cycloserine appear
inconsistent (Buchanan et al. 2007 ; Goff et al. 2005). Moreover, both D-serine and D-alanine also reduce
positive symptoms (Heresco-Levy et al. 2005 ; Tsai et al. 1998, 2006).
Address for correspondence : G. E. Tsai, M.D., Ph.D., Department of Psychiatry, Harbor–UCLA Medical Center, HH212,
1000 W. Carson Street, Torrance, CA 90509, USA. Tel. : (1)310-781-1401 Fax : (1) 310-328-5546 Email : etsai@labiomed.org.
Another strategy to improve NMDA neurotrans-mission is increasing the synaptic glycine level by blocking glycine reuptake through the glycine trans-porter-1 (GlyT-1) (Javitt, 2008 ; Johnson et al. 2003). The GlyT-1 is vital in maintaining glycine within syn-apses at a sub-saturating level (Johnson et al. 2003), and its anatomical distribution parallels that of the NMDA receptor (Smith et al. 1992). N-methylglycine (sarcosine) is a potent and prototype endogenous Gly-T1 inhibitor, with IC50at low micromolar range
(Herdon et al. 2001 ; McBain et al. 1989). Sarcosine is present at high concentrations in humans (Glorieux et al. 1971). Sarcosine is also a methyl donor, and there is no other known neurotransmitter system af-fected by sarcosine. Supporting the role GlyT-1 plays in NMDA neurotransmission, N[3-(4k-fluorophenyl)-3-(4k-phenylphenoxy)propyl]sarcosine (NFPS), a sarco-sine analogue and a potent GlyT-1 inhibitor, enhances NMDA neurotransmission (Bergeron et al. 1998 ; Chen et al. 2003). In behavioural studies, the potency of a series of GlyT-1 antagonists for the inhibition of PCP-induced hyperactivity in vivo correlated signifi-cantly with their potency in antagonizing GlyT-1 in vitro (Javitt et al. 1999). In rodents, treatment with NFPS prevents dopaminergic dysregulation follow-ing chronic or subchronic PCP administration (Javitt et al. 2004), and improves MK-801-induced cognitive deficits (Karasawa et al. 2008). Further, Gly-T1 hetero-zygous knockout mice are more resistant to PCP-induced disruption of prepulse inhibition and possess better working memory (Tsai et al. 2004a).
A pilot clinical trial (Tsai et al. 2004b) demonstrated that sarcosine adjuvant therapy improved positive and negative symptoms in patients with chronically stable schizophrenia who were receiving typical or atypical antipsychotics. More recent studies further suggest that sarcosine, superior to D-serine, both at
2 g/d, can benefit the negative symptoms of acutely ill schizophrenia patients on concurrent atypical anti-psychotic therapy (Lane et al. 2005), and that sarcosine can be used as monotherapy in acutely symptomatic patients (Lane et al. 2008). These findings suggest that the GlyT-1 inhibitor may be more efficacious than the NMDA/glycine site agonist for treatment of schizo-phrenia at the dosages tested (Lane et al. 2005). On the other hand, both sarcosine and D-serine improve
comprehensive symptom components in chronically symptomatic patients. To compare the efficacy of these two novel treatments, we conducted a placebo-controlled study of add-on sarcosine and D-serine,
at the reported effective dosages, in chronic patients with schizophrenia, who had been stabilized with atypical antipsychotic therapy for at least 3 months.
Furthermore, we investigated whether these two novel compounds can improve life quality and func-tioning. The design can minimize the confounding of psychotic exacerbation, whose improvement by the add-on study agent may have been obscured by the concomitant antipsychotic treatment in the previous study for patients with acute exacerbation (Lane et al. 2005).
Method Subjects
Patients were recruited from the in-patient units of China Medical University, which is a major medi-cal centre in Taiwan, between 1 January 2005 and 31 December 2006. The research protocol was ap-proved by the Institutional Review Boards (IRB) of the institute. Ethnically Han Chinese patients were screened and evaluated by the research psychiatrists. After complete description of the study to the subjects, written informed consent was obtained in line with the IRB’s guidelines. The Structured Clinical Interview for DSM-IV (APA, 1995) was conducted for the diag-nosis. Patients were enrolled into this study if they : (1) were physically healthy and had all laboratory assessments (including urine/blood routine, bio-chemical tests, and electrocardiograph) within normal limits, (2) aged 18–60 yr, (3) satisfied DSM-IV criteria for schizophrenia (APA, 1994), (4) had no DSM-IV diagnosis of substance (including alcohol) abuse or dependence, (5) remained symptomatic but without clinically significant fluctuation and the antipsychotic doses were unchanged for at least 3 months, and (6) had a minimum baseline total score of 60 on the Positive and Negative Syndrome Scale (PANSS ; Kay et al. 1987).
Study design
The dosing strategy for the concurrent atypical antipsychotics, based upon recent studies (Lane et al. 2000, 2004), is to optimize efficacy while minimizing side-effects, especially extrapyramidal side-effects (EPS). After achieving optimal clinical treatment re-sponse, patients’ antipsychotic doses remained con-stant for at least 3 months prior to enrolment in the study and remained on the same antipsychotic regi-mens for the study period. All patients were treated with atypical antipsychotics, risperidone for the majority (Table 1).
All patients were then randomly assigned under double-blind conditions to receive a 6-wk trial of placebo, or the only reported effective dosages of
D-serine or sarcosine (2 g/d) (Lane et al. 2005, 2008 ;
Tsai et al. 1998, 2004b). Patients were randomized in blocks of six subjects, without stratification, through a computer-generated randomization table to receive placebo or active drug in a 1 : 1 : 1 ratio. Study medi-cation was provided in coded containers with a supply of identically appearing capsules of placebo or either of the active compounds. To ensure concealment of the randomization assignment, the research pharma-cist implemented random allocation and masked treat-ment assigntreat-ment was communicated by telephone to research staff. Patients, caregivers, and investigators (except for the investigational pharmacist) were all masked to the assignment. The doses of both amino acids were equivalent to those used in earlier studies (Lane et al. 2005, 2008 ; Tsai et al. 1998, 2004b). Patient’s compliance and safety were closely monitored by the research psychiatrists and the in-patient nursing staff.
The sample size was similar to that of an earlier trial which has effect sizes between 0.5–0.9 and power of 0.4–0.8 for sarcosine treatment (Lane et al. 2005).
Measures
The outcome measures were psychopathology
changes measured by PANSS (Kay et al. 1987) and Scales for the Assessment of Negative symptoms
(SANS ; Andreasen, 1983) total scores, Quality of Life (QOL) scale (10 items for in-patient use) (Heinrichs et al. 1984 ; Lane et al. 2008), and Global Assessment of Function (GAF) (Axis V in DSM-IV) (APA, 1994). A secondary analysis aimed to explore whether the positive results (if any) from the PANSS or SANS were due to a general effect on all components or to an effect on a specific component(s). Treatment response was defined as a o20% reduction of the PANSS total score.
Originally, the PANSS contained three subscales : positive, negative, and general psychopathology (Kay et al. 1987). However, further factor analyses revealed five components : positive, negative, cognitive, de-pression, and excitement (Lindenmayer et al. 1994). In the present study, we thus applied the five-factor analysis for PANSS. For the assessment of negative symptoms, we a priori chose SANS rather than PANSS negative to avoid multiple comparisons because SANS is more comprehensive, consisted of five subscales : blunted affect, alogia, apathy, anhedonia/asociality, and attention (Andreasen, 1983). Of the original 21 items on the QOL scale (Heinrichs et al. 1984), 10 (social activity, social initiatives, social withdrawal, sense of purpose, motivation, curiosity, anhedonia, aimless inactivity, capacity for empathy, emotional interaction) were selected for the in-patient setting (Lane et al. 2008). The GAF (Axis V in DSM-IV)
Table 1.Demographics, illness and treatment characteristics of the patients assigned to placebo,D-serine, or sarcosine
plus their chronically stable atypical antipsychotic treatments Study groups
p value
Sarcosine (n=20) D-serine (n=20) Placebo (n=20)
Demographics
No. ( %), female 8 (40) 8 (40) 11 (55) 0.55a
Age (yr), mean (S.D.) 30.4 (10.6) 30.7 (9.6) 31.5 (7.9) 0.70b
Body weight (kg), mean (S.D.) 66.6 (11.7) 63.0 (11.4) 67.3 (13.5) 0.53b
Age at onset of psychosis (yr), mean (S.D.) 22.4 (7.3) 20.0 (5.8) 21.6 (5.9) 0.44b
No. of hospitalizations, mean (S.D.) 2.9 (3.2) 2.9 (2.9) 2.8 (2.0) 0.87b
Schizophrenia subtype no. ( %) 0.67c
Paranoid 15 (75) 13 (65) 11 (55)
Disorganized 2 (10) 4 (20) 3 (15)
Undifferentiated 3 (15) 3 (15) 6 (30)
Risperidone dose (mg), mean (S.D.) 4.1 (1.5, n=16) 4.2 (1.5, n=17) 3.9 (1.8, n=17) 0.87b
Olanzapine dose (mg) – 10, 20 20
Quetiapine dose (mg) 400, 400, 500, 600 400 600, 600
ax2test.
bKruskal–Wallis test. cFisher’s exact test.
included clinical symptoms in the anchors (APA, 1994), but the raters ignored the symptom components and focused on the global functioning when using GAF.
Side-effect assessments included the Simpson– Angus Rating Scale for EPS (Simpson & Angus, 1970), Abnormal Involuntary Movement Scale (AIMS) for dyskinesia (Guy, 1976), and Barnes Akathisia Scale (BAS ; Barnes, 1989). Systemic side-effects of treat-ments were evaluated by means of routine physical and neurological examinations, laboratory tests, and reviewed by applying the Udvalg for Kliniske
Undersogelser (UKU) Side-effects Rating Scale
(Lingjaerde et al. 1987).
Clinical ratings were performed by the research psychiatrists who were trained and experienced in the rating scales. Inter-rater reliability was analysed with the ANOVA test. Only raters reaching the intra-class correlation coefficients of o0.90 during pre-study training were allowed to rate the study patients. To maintain high inter-rater reliability and to prevent rater drift, raters met at least once a month for training and reliability re-testing. To minimize inter-rater variability, each individual patient was assessed by the same research psychiatrist throughout the trial. Assessments were completed at baseline and at the end of weeks 2, 4, and 6.
Statistical analysis
The demographic and clinical characteristics of the patients, antipsychotic doses, response rate, and side-effects among groups were compared by Kruskal– Wallis tests (or ANOVA tests if the distribution was normal) for continuous variables and by x2
tests (or Fisher’s exact tests) for categorical variables.
Since the interception random effect in the mixed-effects model was not sufficient to model the corre-lation structure within the subject and might lead to overestimation of treatment effects, we applied an autoregressive structure of random errors in multiple linear regression with the generalized estimating equation (GEE) method (Zeger et al. 1988) for the treatment (sarcosine,D-serine, or placebo) by time (0, 2,
4, 6 wk) interaction analysis, which simultaneously compared the three treatment groups using a single analysis and controlled for baseline psychopathology. Subjects with at least one post-treatment measurement were included. The results of GEE models were ana-lysed by the SAS/STAT (SAS Institute Inc, USA)PROC
GENMODprocedure with AR (autoregressive) (1)
work-ing correlation structure uswork-ing the marginal model. Since there were three comparison groups, the placebo
group was initially selected to be compared with the other two groups in a single analysis. In the next step, for direct comparison of the two active treatment groups, the sarcosine group was selected to be com-pared with the other two groups. Because ANOVA and multiple linear regression can be applied only if the distribution of the response values is normal, we examined the distribution pattern using the Kolmogorov D package in SAS/INSIGHT v. 8.2. Before treatmentrtime interaction analysis, linear change over time was checked for all outcomes. Unlike ANOVA, the GEE model did not obtain a statistical value or a p value among all groups.
We also applied mixed-effects models (Lange & Ryan, 1989) (with intercept as the random effect) for all normally distributed outcomes, with main effects for treatment (sarcosine, D-serine, or placebo), time
(0, 2, 4, 6 weeks), and the treatmentrtime interaction. Significance of treatment effects over time was as-sessed by the significance of the treatmentrtime interaction while controlling for the main effects. The requirement for the mixed-effects model is the same as that for the GEE model, as shown above.
All hypothesis tests were two-sided and conducted at a=0.05 significance level. The p values of the four outcome measures were corrected by Bonferroni cor-rection of multiple comparisons. After the significant findings in PANSS total or SANS total were confirmed by the stringent GEE analysis, secondary analysis on PANSS factors or SANS subscales were conducted. To compare across the treatments, Cohen’s d effect sizes (Rosnow & Rosenthal, 1996) between endpoint and baseline were calculated.
Results
Sixty schizophrenia patients were enrolled and 51 patients completed the double-blind, placebo-con-trolled study. Three patients (one on sarcosine, two on placebo) dropped out after the week-2 assessment, and another six (fourD-serine, two placebo) dropped
out after the week-4 assessment due to non-adherence to protocol (delayed return of day pass) ; not due to symptom change (Fig. 1).
The demographic characteristics, illness course, diagnostic subtype, stable antipsychotic medication, and clinical severity at baseline of the patients were similar in the three treatment groups (Tables 1 and 2). The doses of co-administered risperidone treatment were similar to earlier studies without or with add-on
D-serine or sarcosine treatment (Lane et al. 2000, 2004,
2005 ; Tsai et al. 2004b). The clinical severity of the subjects was also close to that of previous clinical trials
ofD-serine and sarcosine (Lane et al. 2006 ; Tsai et al.
1998, 2004b). Clinical outcomes
Clinical changes in outcomes are presented in Table 2. To determine the efficacy of the sarcosine andD-serine
treatments, we chose placebo group as the reference group to be compared with the other two groups (Table 2). The multiple linear regression with the GEE method (Zeger et al. 1988) was for the treatment (sarcosine,D-serine, or placebo) by time (0, 2, 4, 6 wk)
interaction analysis, which simultaneously compared the three treatment groups using a single analysis and controlled for baseline psychopathology. The results were similar to, but more stringent than the findings from the mixed-effects model. Sarcosine treatment was effective for all outcome measures, including PANSS total (p=0.0052), SANS total (p= 0.021), QOL scale (p=0.025), GAF (p=0.042) (Table 2), butD-serine treatment did not improve any measure
(Table 2).
The sarcosine group was numerically superior to the D-serine group in all outcome domains but the
differences did not reach statistical significance after Bonferroni correction (results not shown). Due to the non-normal distributions of PANSS factors and SANS subscales, Mann–Whitney tests were applied to com-pare sarcosine with placebo in these subcomponents and significance was assessed by comparing endpoint data while controlling for baseline data. Sarcosine was better than placebo in PANSS positive (z=x2.11, p=0.040), PANSS negative (z=x2.58, p=0.010), PANSS cognitive (z=x1.97, p=0.050), PANSS de-pression (z=x3.01, p=0.002), SANS affect (z=x2.30, p=0.023), SANS alogia (z=x2.36, p=0.021), SANS apathy (z=x2.65, p=0.010), and SANS anhedonia (z=x3.31, p=0.001), but not in PANSS excitement (z=x0.87, p=0.40) and SANS attention (z=x1.32, p=0.21). In contrast,D-serine treatment did not differ
significantly from placebo in any of the secondary measures.
With the analysis of treatment grouprtreatment duration interaction using the mixed-effects model,
Registered/eligible patients (n = 60) Baseline assessment and randomization Placebo + antipsychotics (n = 20) D-serine + antipsychotics (n = 20) Sarcosine + antipsychotics (n = 20)
Efficacy and side-effects assessed at weeks 2, 4, and 6 Withdrawn (n = 4) Non-adherence to protocol (n = 4) Completed trial (n = 16) Completed trial (n = 16) Completed trial (n = 19) Withdrawn (n = 4) Non-adherence to protocol (n = 4) Withdrawn (n = 1) Non-adherence to protocol (n = 1) Efficacy and side-effects
assessed at weeks 2, 4, and 6
Efficacy and side-effects assessed at weeks 2, 4, and 6
Fig. 1.Progress of 60 patients during the trial. There were nine dropouts, all due to non-adherence to protocol (see Results section).
sarcosine was better than placebo in the score-changing rates of all measures including PANSS total (p=0.0016), SANS total (p=0.0028), QOL scale (p= 0.0048), and GAF (p=0.0084) (Table 2).D-serine was
superior to placebo in PANSS total (p=0.0024) and QOL (p=0.021), but not in SANS total and GAF by the mixed-effects model.
For directly comparing the two active treatment groups, we also used the sarcosine group as the refer-ence group, with a single analysis of the mixed-effects model. Consistent with the findings with the placebo group as the reference group, the sarcosine group was numerically superior to theD-serine group in all
primary outcome domains but the differences did not reach statistical significance after Bonferroni correc-tion (results not shown).
Analysis of intra-group effect size between end-point and baseline showed that sarcosine treatment had the largest effect size, D-serine smaller, and
placebo the smallest : PANSS total (placebo 0.17,
D-serine 0.86, sarcosine 1.10), SANS total (placebo
0.19, D-serine 0.33, sarcosine 0.61), QOL scale
(pla-cebo 0.21,D-serine 0.38, sarcosine 0.69), GAF (placebo
0.17, D-serine 0.62, sarcosine 0.76) (effect sizes for
secondary outcomes had similar trends, results not shown). The more comprehensive efficacy of sarco-sine was not due to higher dropout rate of the
D-serine group since the reasons for dropping out
were protocol non-adherence, rather than changes of symptom severity that warranted discontinuation due to deterioration, or early graduation due to im-provement.
At endpoint, the sarcosine group had nine re-sponders, who had o20% reduction of the PANSS total score ; the D-serine group had seven ; and the
placebo group had none. Compared to the placebo group, both sarcosine (Fisher’s exact test, p=0.001) and D-serine (p=0.008) groups were more likely to
respond. Since no patient in the placebo group showed response, logistic regression to compare odds ratio of response rate with the other two groups was not attempted.
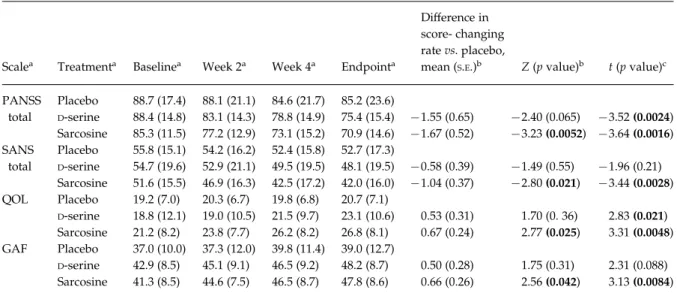
Table 2.Primary outcome measures for the 6-wk add-on sarcosine orD-serine treatment
Scalea Treatmenta Baselinea Week 2a Week 4a Endpointa
Difference in score- changing rate vs. placebo,
mean (S.E.)b Z (p value)b t (p value)c
PANSS total Placebo 88.7 (17.4) 88.1 (21.1) 84.6 (21.7) 85.2 (23.6) D-serine 88.4 (14.8) 83.1 (14.3) 78.8 (14.9) 75.4 (15.4) x1.55 (0.65) x2.40 (0.065) x3.52 (0.0024) Sarcosine 85.3 (11.5) 77.2 (12.9) 73.1 (15.2) 70.9 (14.6) x1.67 (0.52) x3.23 (0.0052) x3.64 (0.0016) SANS total Placebo 55.8 (15.1) 54.2 (16.2) 52.4 (15.8) 52.7 (17.3) D-serine 54.7 (19.6) 52.9 (21.1) 49.5 (19.5) 48.1 (19.5) x0.58 (0.39) x1.49 (0.55) x1.96 (0.21) Sarcosine 51.6 (15.5) 46.9 (16.3) 42.5 (17.2) 42.0 (16.0) x1.04 (0.37) x2.80 (0.021) x3.44 (0.0028) QOL Placebo 19.2 (7.0) 20.3 (6.7) 19.8 (6.8) 20.7 (7.1) D-serine 18.8 (12.1) 19.0 (10.5) 21.5 (9.7) 23.1 (10.6) 0.53 (0.31) 1.70 (0. 36) 2.83 (0.021) Sarcosine 21.2 (8.2) 23.8 (7.7) 26.2 (8.2) 26.8 (8.1) 0.67 (0.24) 2.77 (0.025) 3.31 (0.0048) GAF Placebo 37.0 (10.0) 37.3 (12.0) 39.8 (11.4) 39.0 (12.7) D-serine 42.9 (8.5) 45.1 (9.1) 46.5 (9.2) 48.2 (8.7) 0.50 (0.28) 1.75 (0.31) 2.31 (0.088) Sarcosine 41.3 (8.5) 44.6 (7.5) 46.5 (8.7) 47.8 (8.6) 0.66 (0.26) 2.56 (0.042) 3.13 (0.0084)
PANSS, Positive and Negative Syndrome Rating Scale ; SANS, Scales for the Assessment of Negative symptoms ; GAF, Global Assessment of Function.
Value at each visit, mean (S.D.) of raw data.
aClinical severity at baseline was similar in three treatment groups by ANOVA test (PANSS total, F=0.320, p=0.73 ; SANS total,
F=0.327, p=0.72 ; QOL, F=0.389, p=0.68 ; GAF, F=2.298, p=0.11 ; all d.f.=2).
bSee also Statistical Analyses and Clinical Outcome sections. Treatment grouprtreatment duration (week) interaction effects
betweenD-serine vs. placebo and between sarcosine vs. placebo using a single multiple linear regression analysis with the
generalized estimating equation (GEE) method controlling for baseline psychopathology. Y=baseline+treatment+time+ treatmentrtime (in week, as a continuous variable)+constant.
cTreatment grouprtreatment duration (week) interaction effects betweenD-serine vs. placebo and between sarcosine vs.
placebo using a single mixed-effects model controlling for baseline psychopathology (with all d.f. values=165). The difference in score-changing rate vs. placebo was similar to that with the GEE model (not shown).
Side-effects
All the three treatment groups had minimal EPS at the beginning of the study. The baseline scores of Simpson–Angus (sarcosine group 0.1¡0.3, D-serine
group 0.1¡0.4, placebo group 0.2¡0.5), AIMS (0.1¡ 0.3, 0.0¡0.0, 0.0¡0.0) and BAS (0.1¡0.4, 0.0¡0.0, 0.0¡0.0) were similar in the three groups (all p values=n.s.). At endpoint of the study, the severity of EPS remained minimal and did not have signifi-cant differences among the groups (Simpson–Angus, sarcosine group : 0.1¡0.3, D-serine group 0.1¡0.3,
placebo group 0.2¡0.4 ; AIMS : 0.1¡0.3, 0.0¡0.0, 0.0¡0.0 ; BAS : 0.1¡0.3, 0.0¡0.0, 0.0¡0.0) (all p values=n.s.).
Treatment-emergent adverse events other than extrapyramidal symptoms were also similar in the three groups (Table 3). These systemic side-effects were all mild, and did not warrant medical treatment. The routine blood cell count, chemistry, and EKG after treatment remained unchanged and were all within the normal ranges (data not shown). No dropout was due to side-effects.
Discussion
The efficacy profile of sarcosine is similar to that of the pilot study on the sarcosine add-on treatment for chronically stable patients (Tsai et al. 2004b), where sarcosine was better than placebo in all symptom pro-files. Importantly, the present study further indicates
that sarcosine can improve QOL and general func-tioning. D-serine’s efficacy does not appear evident
when analysed by the GEE model, the more stringent analysis (Table 2), this is consistent with the study in acute patients (Lane et al. 2005). However, it may re-quire more power to show efficacy ofD-serine
treat-ment. Similarly, the effect sizes ofD-serine treatment
are smaller than those of sarcosine treatment in all the measurements including negative symptoms. Con-sistent with the similar comparison study (Lane et al. 2005) in acutely ill patients, the present study suggests that the GlyT-1 inhibitor can be more efficacious than the NMDA/glycine site agonist for the treatment of schizophrenia, at the tested dosages (2 g/d, which is the only dose tested so far).
However, previousD-serine add-on trials
(Heresco-Levy et al. 2005 ; Tsai et al. 1998) of patients with chronically stable schizophrenia also showed com-prehensive symptom improvement. The discrepancy of findings inD-serine efficacy is probably due to the
difference in the concomitant antipsychotics ; patients were treated by an atypical antipsychotic in the pres-ent trial whereas the majority of patipres-ents were treated with conventional antipsychotics in our first trial (Tsai et al. 1998). Nevertheless, Heresco-Levy et al. (2005) reported significant effect sizes in multiple symptom domains, in whichD-serine was added on to
olanza-pine or risperidone. Moreover, this study is limited in sample size. Although the sarcosine group was nu-merically superior to theD-serine group in all outcome
domains, the differences did not reach statistical sig-nificance after Bonferroni correction. Therefore, the superior efficacy of sarcosine overD-serine should be
considered preliminary. The optimal doses for sarco-sine and D-serine can be different ; a higher dose of D-serine may be required to reach the same efficacy
as sarcosine.
To date, little data are available for comparisons between NMDA-enhancing agents (Heresco-Levy & Javitt, 2004 ; Lane et al. 2005). The results of the present study and the antecedent one (Lane et al. 2005) suggest that GlyT-1 may be a more effective target to enhance NMDA function than the NMDA/glycine site itself. This difference may due to the fact that sarcosine acts by blocking the re-uptake of released glycine whereas NMDA/glycine site agonists tonically stimulate the receptor. Further, transporter inhibitors may be more efficacious than the transmitter itself. Similarly, sero-tonin transporter inhibitors are superior to tryptophan (a neurotransmitter precursor, albeit not a neurotrans-mitter) for the treatment of depression (Shaw et al. 2002). It should be borne in mind that we only com-pared one dose ofD-serine and sarcosine. A detailed Table 3.Adverse events other than extrapyramidal
symptoms during the studya
Adverse event
Study groups (no. of patients)
Sarcosine D-serine Placebo Total
Weight gain 3 4 5 12 Insomnia 4 4 2 10 Fatigability 2 2 4 8 Sedation 2 1 3 6 Palpitations 2 3 1 6 Tension 2 1 2 5 Hypersomnia 1 1 2 4 Weight loss 2 2 0 4 Constipation 1 2 0 3 Others 1 3 3 7 Total 20 23 22 65
aAll p values are not significant for comparisons in three
study groups. Systemic side-effects of treatments were reviewed by applying the Udvalg for Kliniske Undersogelser (UKU) Side-effects Rating Scale.
parallel, fixed dose-finding study can resolve the issue clearly.
NMDA neurotransmission regulates synaptic
plasticity, memory, and cognition (Coyle, 1996). This cognition-enhancing effect is supported by the posi-tive finding in execuposi-tive function of ourD-serine study
(Tsai et al. 1998). Because cognitive deficiency in schizo-phrenia is increasingly viewed as a core factor for functional outcome (Green et al. 2004), the positive findings for the short-term QOL and general func-tioning of the present trial support the notion that NMDA-enhancing agents can improve functional out-come. Taken together, the findings from the trials of the NMDA-enhancing agent added to dopamine/ 5-HT receptor antagonists, sarcosine provides ad-ditional benefits not only for symptom reduction during both acute and chronic phases but also for the short-term functioning outcome. This novel approach represents a new avenue to improve the function and QOL of patients with schizophrenia who often suffer from lifelong functioning disability. Nevertheless, more meticulous research is required to test these agents before any conclusion can be drawn for the therapeutic effect of NMDA-enhancing agents on cognitive domains and long-term functional outcome in the community.
In earlier studies (Heresco-Levy et al. 2005 ; Lane et al. 2006 ; Tsai et al. 1998, 2004), sarcosine orD-serine
did not worsen the side-effects of other antipsychotics. The present study replicated these findings ; the few side-effects reported by the patients were minimal and did not differ significantly among groups, including the placebo group. Sarcosine is a naturally occurring amino acid in humans and food. Toxicological profiles of sarcosine have not been thoroughly examined. Supporting the safety of using sarcosine as a thera-peutic agent to enhance NMDA neurotransmission, sarcosinaemia due to defective sarcosine dehydro-genase is generally benign (Eschenbrenner & Jorns, 1999 ; Levy et al. 1984) and the phenotype of sarco-sine dehydrogenase mutant mice is unexceptional (Harding et al. 1992). However, Gly-T1 homozygous knockout mice cannot survive (Tsai et al. 2004). Complete blockade of Gly-T1 may be toxic for the rodent development due to the excessively inhibitory glycinergic drive to the respiratory neurons (Gomeza et al. 2003). A thorough human toxicology study, therefore, is necessary. After the present study was completed, sarcosine was identified as a differential metabolite that was detected as being greatly in-creased in urine during prostate cancer progression to metastasis (Sreekumar et al. 2009). Sarcosine is the major donor of the methyl group. Although this is
not a direct proof of the carcinogenicity of sarcosine, and possibly elevated levels of sarcosine can be the result rather than the cause of the tumour progression, it is important to monitor the risk of prostate cancer during the treatment of sarcosine. On the other hand, sarcosine has protective effects against hepatoma ; animals missing glycine N-methyltransferase that synthesizes sarcosine develop liver cancer whereas transgenic mice overexpressing glycine N-methyl-transferase are resistant to aflatoxin B1-induced liver cancer (Martı´nez-Chantar et al. 2008 ; Yen et al. 2009). In rodents, D-serine selectively damages renal
proxi-mal tubule cells (Williams & Lock, 2004). In humans, toxicological properties ofD-serine have not been fully
elucidated. However, D-serine at a dose of y2 g/d
was safe for the patients in all four trials (Heresco-Levy et al. 2005 ; Lane et al. 2005 ; Tsai et al. 1998, 1999). The present study was limited by the fixed-dose comparison without parallel, fixed dose-finding trials. The definitive difference of Gly-T1 inhibitors vs. NMDA/glycine site agonists and their clinical appli-cation requires further study. However, this study together with the one for acutely ill patients (Lane et al. 2005) and the single-agent study (Lane et al. 2008) in-dicate that sarcosine, a GlyT-1 inhibitor, represents a novel therapeutic approach that is worthy of further investigation (Javitt, 2008). Optimizing pharmaco-therapy for schizophrenia can be achieved by a com-bination treatment of atypical antipsychotics and a Gly-T1 inhibitor.
Acknowledgements
This research was funded by the National Science Council (Taiwan) 95-2314-B-006-118-MY3, NSC-97-2314-B-039-006-MY3, and NSC-98-2627-B-039-001, the National Health Research Institutes (Taiwan) NHRI-EX-97-9405PI and NHRI-EX-98-9405PI, China Medical University (Taiwan) DMR-98-096 (HYL). G.E.T. is supported in part by Los Angeles Biomedical Research Institute and an Independent Investigator Award from National Alliance of Research on Schizo-phrenia and Affective Disorder. [The trial was regis-tered with the name of ‘ Sarcosine or D-serine
add-on treatment for chradd-onic schizophrenia ’ at www. clinicaltrials.gov with the registration number of NCT00491569 (http://www.clinicaltrials.gov/ct/ show/NCT00491569?order=2).]
Statement of Interest
Sarcosine and D-serine are protected by US patent
6228875, 6667297, 6420351, 6974821 for which G.E.T. is an inventor.
References
Andreasen NC(1983). Scales for the Assessment of Negative symptoms (SANS). University of Iowa : Iowa City, IA. APA(1994). Diagnostic and Statistical Manual of Mental
Disorders, 4th edn. American Psychiatric Press : Washington, DC.
APA(1995). Structured Clinical Interview for DSM-IV. American Psychiatric Press : Washington, DC. Barnes TRE(1989). A rating scale for the drug-induced
akathesia. British Journal of Psychiatry 154, 672–676. Bergeron R, Meyer T, Coyle J, Greene R(1998). Modulation
of N-methyl-D-aspartate receptor function by glycine
transport. Proceedings of the National Academy of Sciences USA 95, 15730–15734.
Buchanan RW, Javitt DC, Marder SR, Schooler NR,et al. (2007). The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST) : the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. American Journal of Psychiatry 164, 1593–1602.
Chen L, Muhlhauser M, Yang CR(2003). Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. Journal of Neurophysiology 89, 691–703.
Coyle JT(1996). The glutamatergic dysfunction
hypothesis for schizophrenia. Harvard Review of Psychiatry 3, 241–253.
Deutsch SI, Mastropaolo J, Schwartz BL, Rosse RB,et al. (1989). A ‘ glutamatergic hypothesis’ of schizophrenia : rationale for pharmacotherapy with glycine. Clinical Neuropharmacology 12, 1–13.
Eschenbrenner M, Jorns MS(1999). Cloning and mapping of the cDNA for human sarcosine dehydrogenase, a flavoenzyme defective in patients with sarcosinemia. Genomics 59, 300–308.
Glorieux FH, Scriver CR, Delvin E, Mohyuddin F(1971). Transport and metabolism of sarcosine in
hypersarcosinemic and normal phenotypes. Journal of Clinical Investigation 50, 2313–2322.
Goff DC, Herz L, Posever T, Shih V,et al. (2005). A six month, placebo-controlled trial ofD-cycloserine
co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacology (Berlin) 179, 144–150.
Goff DC, Tsai G, Levitt J, Amico E,et al. (1999). A placebo-controlled trial ofD-cycloserine added to
conventional neuroleptics in patients with schizophrenia. Archives of General Psychiatry 56, 21–27.
Gomeza J, Hulsmann S, Ohno K, Eulenburg V,et al. (2003). Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40, 785–796.
Green MF, Nuechterlein KH, Gold JM, Barch DM,et al. (2004). Approaching a consensus cognitive battery for clinical trials in schizophrenia : the NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry 56, 301–307.
Guy W(1976). ECDEU Assessment Manual for
Psychopharmacology. US Department of Health, Education, and Welfare : Washington, DC.
Harding CO, Williams P, Pflanzer DM, Colwell RE,et al. (1992). Sar : a genetic mouse model for human
sarcosinemia generated by ethylnitrosourea mutagenesis. Proceedings of the National Academy of Sciences USA 89, 2644–2648.
Heinrichs DW, Hanlon TE, Carpenter WT(1984). The quality of life scale : an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin 10, 388–398.
Herdon HJ, Godfrey FM, Brown AM, Coulton S,et al. (2001). Pharmacological assessment of the role of the glycine transporter GlyT-1 in mediating high-affinity glycine uptake by rat cerebral cortex and cerebellum synaptosomes. Neuropharmacology 41, 88–96.
Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G,et al. (2004). High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biological Psychiatry 55, 165–171.
Heresco-Levy U, Ermilov M, Shimoni J, Shapira B,et al. (2002). Placebo-controlled trial ofD-cycloserine added
to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. American Journal of Psychiatry 159, 480–482. Heresco-Levy U, Javitt DC(2004). Comparative effects of
glycine and d-cycloserine on persistent negative symptoms in schizophrenia : a retrospective analysis. Schizophrenia Research 66, 89–96.
Heresco-Levy U, Javitt DC, Ebstein R, Vass A,et al. (2005).
D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biological Psychiatry 57, 577–585. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C,et al.
(1996). Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. British Journal of Psychiatry 169, 610–617. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C,et al.
(1999). Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Archives of General Psychiatry 56, 29–36.
Javitt DC(1987). Negative schizophrenic symptomatology and the phencyclidine (PCP) model of schizophrenia. Hillside Journal of Psychiatry 9, 12–35.
Javitt DC(2008). Glycine transport inhibitors and the treatment of schizophrenia. Biological Psychiatry 63, 6–8. Javitt DC, Balla A, Burch S, Suckow R,et al. (2004).
Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/ glycine-site agonists. Neuropsychopharmacology 29, 300–307. Javitt DC, Balla A, Sershen H, Lajtha A(1999). A. E. Bennett
Research Award. Reversal of phencyclidine-induced effects by glycine and glycine transport inhibitors. Biological Psychiatry 45, 668–679.
Johnson KW, Clemens-Smith A, Nomikos G, Davis R,et al. (2003). In vivo characterization of changes in glycine levels induced by GlyT1 inhibitors. Annals of the New York Academy of Sciences 1003, 412–414.
Karasawa J, Hashimoto K, Chaki S(2008).D-serine and a
glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behavioural Brain Research 186, 78–83.
Kay SR, Opler LA, Fiszbein A(1987). Positive and negative syndrome scale (PANSS) manual. Schizophrenia Bulletin 13, 261–276.
Lane HY, Chang YC, Chiu CC, Lee SH,et al. (2004). Fine-tuning risperidone dosage for acute schizophrenia : clinical determinants. Psychopharmacology 172, 393–399. Lane HY, Chang YC, Liu YC, Chiu CC,et al. (2005).
Sarcosine (N-methylglycine) orD-serine add-on treatment
for acute exacerbation of schizophrenia : a randomized, double-blind, placebo-controlled study. Archives of General Psychiatry 62, 1196–1024.
Lane HY, Chiu WC, Chou JCY, Wu ST,et al. (2000). Risperidone in acutely exacerbated schizophrenia : dosing strategies and plasma levels. Journal of Clinical Psychiatry 61, 209–214.
Lane HY, Huang CL, Wu PL, Liu YC,et al. (2006). Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biological Psychiatry 60, 645–649.
Lane HY, Liu YC, Huang CL, Chang YC,et al. (2008). Sarcosine (N-methylglycine) treatment for acute schizophrenia : a randomized, double-blind study. Biological Psychiatry 63, 9–12.
Lange N, Ryan L(1989). Assessing normality in random effects models. Annals of Statistics 17, 624–642.
Lindenmayer JP, Bernstein-Hyman R, Grochowski S(1994). Five factor model of schizophrenia. Initial validation. Journal of Nervous and Mental Disorder 182, 631–638. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ,et al. (1987).
The UKU Side Effect Rating Scale : a new comprehensive rating scale for psychotropic drugs and cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatrica Scandinavica 334 (Suppl.), 1–100.
Levy HL, Coulombe JT, Benjamin R(1984). Massachusetts metabolic disorders screening program : III. sarcosinemia. Pediatrics 74, 509–513.
Martı´nez-Chantar ML, Va´zquez-Chantada M, Ariz U, Martı´nez N,et al. (2008). Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology 47, 1191–1199.
McBain CJ, Kleckner NW, Wyrick S, Dingledine R(1989). Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed
in xenopus Oocytes. Molecular Pharmacology 36, 556–565.
Rosnow RL, Rosenthal R(1996). Computing contrasts, effect sizes, and counternulls on other people’s published data : general procedures for research consumers. Pyschological Methods 1, 331–340.
Shaw K, Turner J, Del Mar C(2002). Are tryptophan and 5-hydroxytryptophan effective treatments for depression? A meta-analysis. Australian and New Zealand Journal of Psychiatry 36, 488–491.
Simpson GM, Angus JWS(1970). A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 212(Suppl.), 11–19.
Smith KE, Borden LA, Hartig PR, Branchek T,et al. (1992). Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron 8, 927–935.
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP,et al. (2009). Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457, 910–914.
Tsai G, Coyle JT(2001). Glutamatergic mechanisms in schizophrenia. Annual Review of Pharmacology & Toxicology 42, 165–179.
Tsai G, Lane HY, Yang P, Chong MY,et al. (2004b). Glycine transporter I inhibitor, N-methylglycine (sarcosine) added to antipsychotics for the treatment of schizophrenia. Biological Psychiatry 55, 452–456.
Tsai G, Martina M, Bergeron R, Berger-Sweeney J,et al. (2004a). Gene knockout study of glycine transporter type 1. Proceedings of the National Academy of Sciences USA 101, 8485–8490.
Tsai G, Yang P, Chang Y, Chong M(2006).D-alanine added to antipsychotics for the treatment of schizophrenia. Biological Psychiatry 59, 230–234.
Tsai G, Yang P, Chung L, Lange N,et al. (1998).D-serine
added to antipsychotic for the treatment of schizophrenia. Biological Psychiatry 44, 1081–1089.
Tsai GE, Yang P, Chung LC, Tsai IC,et al. (1999).D-serine
added to clozapine for the treatment of schizophrenia. American Journal of Psychiatry 156, 1822–1825. Williams RE, Lock EA(2004).D-serine-induced
nephrotoxicity : possible interaction with tyrosine metabolism. Toxicology 201, 231–238.
Yen CH, Hung JH, Ueng YF, Liu SP,et al. (2009). Glycine N-methyltransferase affects the metabolism of aflatoxin B1 and blocks its carcinogenic effect. Toxicology and Applied Pharmacology 235, 296–304.
Zeger SL, Liang KY, Albert PS(1988). Models for longitudinal data : a generalized estimating equation approach. Biometrics 44, 1049–1060.