中 國 醫 藥 大 學
專題研究計畫成果報告
計畫名稱:醬油粕之抗氧化活性物質之鑑定
計畫編號:CMU 99- COL – 23
執行期限:100 年 5 月 1 日至 101 年 4 月 30 日
單位名稱:營養學系
主持人:徐國強
中 華 民 國 101 年 7 月 31 日
附件一、前言
醬油中的重要成分為胜肽、游離胺基酸、有機酸、還原醣及鹽等。過去研究中多半 以日式醬油之機能性及其成分為主 (Kataoka, 2005),結果發現日式醬油中 nicotianamine 具有降血壓(Kinoshita et al., 1993)、p-hydroxy-butyl benzoate 具有抗菌 (Akiba et al., 1957)、4-hydroxy-5-methyl-3(2H)-furanone (HMF),4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF) 及 4-hydroxy-2(or5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF) 具有抗癌 (anticarcinogenic) (Nagahara et al., 1992),抗白內障 (anticataract) 等功能。而醬油經研究 發現具有良好之抗氧化特性,陳年醬油 (亦稱老抽,dark soy sauce) 中之抗氧化物質目 前被證實的只有 3-hydroxy-2-methyl-4H-pyran-4-one (maltol) (Long et al., 2000; Wang et al., 2007);魚醬油及日式醬油具有良好清除 DPPH 自由基清除能力 (Aoshima and Ooshima, 2009),但作者並未測定其主要之抗氧化物質為何。
過去研究發現,發酵大豆產品之脂質較未發酵大豆產品更不易發生氧化 (Esaki et al. 1994)。游離異黃酮 (free isoflavones) 如 daizein (7,4’-dihydroxyisoflavone) 及 genistein (5,7,4’-trihydroxyisoflavone) 均為經微生物發酵後所產生的抗氧化物質。若將大豆以不同 微生物進行發酵,則會產生不同型態及抗氧化能力之異黃酮,如大豆以 Aspergillus saitoi 發酵後,則會產生 8-hydroxydaidzein (8-OHD) 及 8-hydroxygenistein (8-OHG),此二種 物質是在發酵過程中進行 hydroxylation 作用而產生,且較 daizein 及 genistein 之抗氧 化力為佳 (Esaki et al., 1998);另天貝 (tempeh) 之製程中若將大豆 Rhizopus 菌屬發酵 後更可得到具有高抗氧化力之 6-hydroxydaidzein (6-OHD),但若以純粹培養後之
Rhizopus oligosporus 進行發酵,則發現並未能形成 6-OHD,顯示以大豆以不同微生物
進行發酵,異黃酮成分之型式亦有不同,其所具有之抗氧化力也隨之有異 (Esaki et al., 1999)。Ito et al. (1993) 認為醬油中之異黃酮成分具有抗腫瘤的功效,但亦有其他研究認 為醬油中不具有多量水溶性之異黃酮,因為如 genistin 及 daidzin 在發酵時會轉變成水 難溶性之 genistein 及 daidzein (Kataoka, 2005)。醬油粕中含有豐富的膳食纖維外,亦含 有水難溶性之 genistein 及 daidzein (Yeh et al., 2005),其總含量約為 1200-1480
μmol/100 g,因此若善加利用,亦可開發成保健食品。HDMF 及 HEMF 具有甜味及焦 糖之風味,一般認為是日式醬油發酵過程中由 Zygosaccharomyces rouxii 合成 (Sasaki et al., 1991; Hecquet et al., 1996),醬油中的這二種 4-hydroxyfuranones 主要是因還原醣及 胺基酸之梅納反應 (Maillard reaction) 而產生,而醬油在加熱殺菌過程中此反應會更加 快速。梅納反應產物在醬油中除具有增色及增加風味等功能外,亦具有清除 DPPH 及 ABTS 等自由基之抗氧化能力 (Nagahara et al., 1992; Kataoka et al., 1997; Kim and Lee, 2009)。 因此本研究之目的為測定醬油粕之抗氧化活性並分析並純化其抗氧化物質,包含異 黃酮及梅納反應產物等。 二、材料與方法 (一) 材料 醬油粕由屏東縣之醬油工廠提供,置於 -20℃ 下運送至實驗室,並直接於同溫度 下凍藏備用。 (二) 方法 1. 樣品處理
實驗進行前,冷凍之醬油粕進行冷凍乾燥後以粉碎機粉碎成粉末狀。
2. DPPH 自由基清除能力 (DPPH radical-scavenging capacity) 之測定
取反應物 (3 mL) 含 0.5 mL 0.5 M acetic acid buffer (pH 5.5),1 mL 0.2 mM DPPH 之 ethanol 溶液及 1.5 mL 經 50% ethanol 適當稀釋之樣品,快速振盪混合之 (Aoshima et al., 2004),在室溫下靜置 30 min,測定其在 517 nm 下之吸光值,對照組則以去離子 水取代樣品行之。
DPPH radical-scavenging capacity (%) = [1-(OD517 sample/OD517 control)]×100
OD517 sample:樣品之吸光值
OD517 control:以去離子水取代樣品之吸光值
3. 亞鐵離子螯合能力 (Decker and Welch, 1990)
取 0.25 mL 經適當稀釋之樣品加入 0.025 mL 之 2 mM FeCl2˙4 H2O 及 0.925
mL 甲醇,30 s 後再加入 0.05 mL 之 ferrozine 反應 10 min,測定其在 562 nm 之吸光 值。亞鐵離子 (Fe2+
) 與 ferrozine 所形成之複合物於波長 562 nm 下有強的吸收,吸光 值越強,表示樣品螯合亞鐵離子的能力越強。
亞鐵離子螯合能力 (%) = [1-(OD562 sample / OD562 control)] × 100
OD562 sample:樣品之吸光值
OD562 control:以去離子水取代樣品之吸光值
4. 抗氧化活性物質分析及鑑定 (1) 異黃酮分析
a. 異黃酮之萃取
參考 Genovese and Lajolo (2002) 之方法,取 1 g 樣品 以 80% methanol 水溶液 (1:20 w/v) 在 4℃ 下攪拌 2 h,萃取物以 Whatman No. 6 過濾,此步驟重覆 3 次,以 ≦ 40℃ 進行減壓濃縮至 methanol 脫除為止,萃取物總體積以去離子水調整至 30 mL,再注入預先以 20 mL methanol 及 60 mL 去離子水調整之 polyamide column,以 20 mL 去離子水沖洗不純物,再以 50 mL methanol/ammonia (99.5:0.5) 沖提。生醬油及 醬油則直接取 5 mL 直接注入 polyamide column,所得之沖提液則以減壓濃縮法將體積 濃縮至 0.2-0.4 mL,再加入 LC 級 methanol 調整體積至 2 mL,以 0.22 μm PTFE 濾 膜 (tetrafluoroethylene) 過濾,並進行 HPLC 分析。
b. HPLC 分析---Daidzein, Genistein, Daidzin, Genistin
異黃酮之 HPLC 分析參考 Song et al. (1998) 之方法,使用 C18 NovaPak 管柱 (30 cm × 4.6 mm i.d.),取 20 μL 萃取物注入,移動相組成包含 0.1% glacial acetic acid in water 及 0.1% glacial acetic acid in acetonitrile 以線性梯度為之,實驗條件為:15% 之 0.1% glacial acetic acid in acetonitrile 維持 5 min,在之後 31 min 增加至 29%,之後 8 min 後增加至 35%,之後 6 min 再減低為 15%,流速:前 5 min 為 1 mL/min,之後 0.5 min 增加至 1.5 mL/min 並維持 39 min,之後再調整至 1 mL/min,photodiode array detector 記錄 200-350 nm 下之吸光值。
c. HPLC 分析---8-OHD, 8-OHG, 6-OHD
為鑑定在醬油發酵期間是否產生 8-OHD、8-OHG 甚至 6-OHD,因此參考 Esaki et 12
al. (1999) 之方法,取樣品 10 g 加入 15 mL methanol (含 0.1 % trifluoroacetic acid; TFA) 在室溫下萃取 20 min,再以 Amberlite XAD-4 column chromatography 分劃,經
methanol-water (60:40 v/v) 沖提後以 fluorescent TLC plate 與 toluene:ethyl
formate:formic acid (10:8:2 v/v/v) 展開,若沖提液中含有 8-OHD 及 8-OHG 者,會與 tocopherol-stripped soybean oil 分別在 Rf 0.45 及 0.60 產生抗氧化點,在 Rf 0.52 處有
抗氧化點時則為 6-OHD。若在其他處產生抗氧化點時,即代表有其他抗氧化物質,則 再進行 HPLC、FAB-MS 或 NMR 純化及鑑定。
(2) 梅納反應產物之分析 a. 樣品之萃取
參考 Kataoka et al. (1997) 之方法,取醬油粕乾燥粉末 20 g,生醬油及醬油各 300 mL,以 400 mL ethyl acetate 萃取 2 次,在有機層中加入 anhydrous sodium sulfate 脫 水,以 Whatman No. 4 過濾後將濾液進行減壓濃縮,最終濃縮物則溶解於 300 mL 去 離子水中,以 0.45 mm 膜過濾滅菌後貯藏於 4℃ 下備用。
b. HPLC 分析
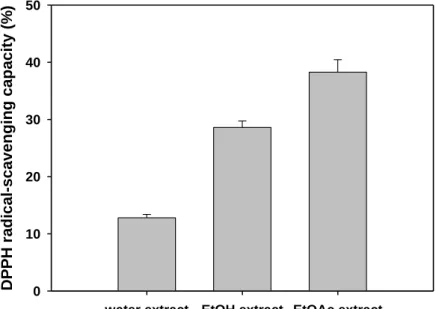
以 C-18 RP column (4.6 × 250 mm) 為管柱,分析條件如下:acetonitrile/0.1 M acetate buffer (pH 3.7) 為移動相,1% for 15 min, 1-40% linear gradient over 80 min, 40-90% linear gradient over 30 min,流速為 1 mL/min,在室溫下以 285 nm 檢測之。若有其他物質, 則再進行 HPLC、FAB-MS 或 NMR 純化及鑑定。 三、結果與討論 (一) DPPH 自由基清除能力及亞鐵離子螯合能力 本研究利用不同溶劑萃取其抗氧化成分,結果發現 DPPH 自由基清除能力中 (Figure 1),水萃取物約為 12.8%、甲醇萃取物為 28.6% 及乙酸乙酯萃取物為 38.3%。 而亞鐵離子螯合能力方面 (Figure 2),水萃取物約為 8.7%、甲醇萃取物為 33.2% 及乙 酸乙酯萃取物為 35.7%。此結果顯示水萃取物中,應多為可溶性有機酸、胺基酸等物質, 因此其抗氧化能力較弱;而甲醇萃取物中可將異黃酮萃取出來,而異黃酮具有相當之抗 氧化力;乙酸乙酯萃取物則含有異黃酮物質及梅納反應產物,因此具有更佳之抗氧化力 (Kataoka et al., 1997; Esaki et al., 2004)。
(二) 抗氧化物質鑑定 本研究將各種萃取物進行鑑定其抗氧化物質,結果發現在水萃取物中具有低含量之 異黃酮物質,如 8-OHD、8-OHG、daidzein 及 genistein,且其含量分別為 0.28、0.32、 0.46 及 0.32 mg/g (Table 1)。而甲醇萃取物中含有較高含量之異黃酮,8-OHD、8-OHG、 daidzein 及 genistein 之含量分別為 1.6、1.9、13.4 及 15.8 mg/g;而乙酸乙酯不僅含 有異黃酮,另含有梅納反應產物,8-OHD、8-OHG、daidzein、genistein、HDMF 及 HEMF, 且其含量分別為 3.5、3.7、25.6、33.2、0.24 及 0.23 mg/g。由此結果發現,醬油粕中 各萃取物含量與之前研究相似 (Esaki et al., 2004),而此結果也可印證與前述抗氧化能 力,乙酸乙酯萃取物因含有較高之異黃酮及梅納反應產物,因此所具有之抗氧化能力最 高。 四、結論 本研究結果證實醬油粕中確實含有良好之抗氧化物質,其中包含異黃酮類物質及梅 納反應產物,而且以乙酸乙酯萃取物中含量最高,因此若能將醬油粕回收加以利用,則
可以開發為保健食品,並且避免環境污染及增加收益。
water extract EtOH extract EtOAc extract
DPPH rad ical -s cav en g in g cap acit y ( % ) 0 10 20 30 40 50
Figure 1 DPPH radical-scavenging capacity of each fraction prepared from soy sauce cake.
water extract EtOH extract EtOAc extract
Ch elat in g act ivit y ( % ) 0 10 20 30 40
Figure 2 Chelating activity of each fraction prepared from soy sauce cake.
Table 1 Amounts of antioxidative compounds in each fraction prepared from soy sauce cake (mg/g).
8-OHD 8-OHG Daidzein Genistein HDMF HEMF Water extract 0.28 0.32 0.46 0.32 nd nd EtOH extract 1.6 1.9 13.4 15.8 nd nd
EtOAC extract
3.5 3.7 25.6 33.2 0.24 0.23
nd: not determined
五、參考文獻
Akiba, C., Ujiie, T., and Yokoyama, S. 1957. The sterilize effects of soy sauce and sauce on the pathogens in the digestive tract. Chomi Kagaku (in Japanese), 9:10-12.
Ames, B. N. 1989. Endogenous DNA damage as related to cancer and aging. Mutation
Research, 214:41-46.
Aoshima, H. and Ayabe, S. 2007. Prevention of the deterioration of polyphenol-rich beverages.
Food Chemistry, 100:350-355.
Aoshima, H. and Ooshima, S. 2009. Anti-hydrogen peroxide activity of fish and soy sauce.
Food Chemistry, 112:339-343.
Aoshima, H., Tswunoue, H., Koda, H., and Kiso, Y. 2004. Aging of whiskey increases 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity. Journal of Agricultural and
Food Chemistry, 52:1025-1031.
Becker, G. L. 1993. Preserving food and health: antioxidants make functional, nutritious preservatives. Food Process, 12:54-56.
Chen, H. M., Murumoto, K., and Yamauchi, F. 1995. Structural analysis of antioxidative peptides from soybean β-conglycinin. Journal of Agricultural and Food Chemistry, 43:574-578.
Chen, H. M., Muramoto, K., Yamauchi, F., and Nokihara, K. 1996. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. Journal of Agricultural and Food Chemistry, 44:2619-2623.
Decker, E. A. and Welch, B. 1990. Role of ferritin as a lipid oxidation catalyst in muscle food.
Journal of Agricultural and Food Chemistry, 38:674-677.
Ebine, H., Kimura, E., Fukuzaki, K., Furuta, T., Maeda, H., and Yokotsuka, T. 1976. Burean of Foods: Standard Production and Circulation of Shoyu. Tokyo, Japan. p. 97.
Esaki, H., Kawakishi, S., Morimitsu, Y., and Osawa, T. 1999. New potent antioxidative
ο-dihydroxyisoflavones in fermented Japanese soybean products. Bioscience, and Biotechnology, Biochemistry, 63:1637-1639.
Esaki, H., Onozaki, H., Morimitsu, Y., Kawakishi, S., and Osawa, T. 1998. Potent antioxidative isoflavones isolated from soybeans fermented with Aspertillus saitoi.
Bioscience, and Biotechnology, Biochemistry, 62:740-746.
Esaki, H., Onozaki, H., and Osawa, T. 1994. Antioxidative activity of fermented soybean products. In “Food Phytochemicals for Cancer Prevention I,” ed. By Huang M. T., Osawa, T., Ho, C. T., and Rosen, R. T., American Chemical Society, Washington DC, pp. 353-360.
Frlich, I. and Riederer, P. 1995. Free radical mechanisms in dementia of Alzheimer type and 7
the potential for antioxidative treatment. Drug Research, 45:443-449.
Genovese, M. I. and Lajolo, F. M. 2002. Isoflavones in soy-based food consumed in Brazil: levels, distribution, and estimated intake. Journal of Agricultural and Food Chemistry, 50:5987-5993.
Halliwell, B., Aeschbach, R., Löliger, J., and Aruoma, O. I. 1995. The characterization of antioxidants. Food and Chemical Toxicology, 33:601-617.
Halliwell, B. and Gutteridge, J. M. C. 1990. Role of free radicals and catalytic metal ions in human disease. Methods in Enzymology, 186:1-85.
Hecquet, L., Sancelme, M., Bolte, J., and Demuynck, C. 1996. Biosynthesis of
4-hydroxy-2,5-dimethyl-3(2H)-furanone by Zygo-saccharomyces rouxii. Journal of
Agricultural and Food Chemistry, 44:1357-1360.
Imaida, K., Fukushima, S., Shivai, T., Ohtani, M., Nakanishi, K., and Ito, N. 1983. Promoting activities of butylated hydroxyanisole and butylated hydroxytoluene of 2-stage urinary bladder carcinogenesis and inhibition of γ-glutamyl transpeptidase-positive foci development in the liver of rats. Carcinogenesis, 4:885-889.
Ito, A., Watanabe, H., and Basaran, N. 1993. Effects of soy products in reducing risk of spontaneous and neutron-induced liver tumors in mice. International Journal of
Oncology, 2:773-776.
Kataoka, S. 2005. Functional effects of Japanese style fermented soy sauce (shoyu) and its components. Journal of Bioscience and Bioengineering, 100, 227-234.
Kataoka, S., Liu, W., Albright, K., Storkson, J., and Pariza, M. 1997. Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia and reduction of H2O2
concentration in human polymorphonuclear leucocytes by flavour components of Japanese-style fermented soy sauce. Food and Chemical Toxicology, 35:449-457. Kim, J. S. and Lee, Y. S. 2009. Antioxidant activity of Maillard reaction products derived
from aqueous glucose/glycine, diglycine, and triglycine model systems as a function of heating time. Food Chemistry, 116:227-232.
Kim, S. K., Kim, Y. T., Byun, H. G., Nam, K. S., Joo, D. S., and Shahidi, F. 2001. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. Journal of Agricultural and Food Chemistry, 49:1984-1989.
Kinoshita, E., Yamakoshi, J., and Kikuchi, M. 1993. Purification and identification of an angiotensin I-converting enzyme inhibitor from soy sauce. Bioscience, Biotechnology,
and Biochemistry, 57:1107-1110.
Long, L. H., Kwee, D. C., Halliwell, B. 2000. The antioxidant activities of seasonings used in Asian cooking. Powerful antioxidant activity of dark soy sauce revealed using the ABTS assay. Free Radical Research, 32:181-186.
Mendis, E., Rajapakse, N., and Kim, S. K. 2005. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin 8
hydrolysate. Journal of Agricultural and Food Chemistry, 53:581-587.
Moore, S. and Stein, W. H. 1963. Chromatographic determination of amino acids by the use of automatic recording equipment. Methods in Enzymology, vol 6, p. 819 Academic press, Inc., New York.
Murakami, H., Asakawa, T., Terao, J., and Matsushita, S. 1984. Antioxidative stability of tempeh and liberation of isoflavones by fermentation. Agricultural and Biological
Chemistry, 48:2971-2975.
Nagahara, A., Benjamin, H., Storkson, J., Krewson, J., Sheng, K., Liu, E., and Pariza, M. W.
1992. Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia by a principal
flavor component of Japanese-style fermented soy sauce. Cancer Research, 52:1754-1756.
Qian, Z-J., Jung, W-K., Kim, S-K. 2008. Free radical scavenging activity of a novel Antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana
Shaw. Bioresource Technology, 99:1690-1698.
Rajapakse, N., Mendis, E., Byun, H. G., and Kim, S. K. 2005. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. Journal of Nutritional Biochemistry, 16:562-569.
Sasaki, M., Nunomura, N., and Matsudo, T. 1991. Biosynthesis of 4-hydroxy-2(or
5)-ethyl-5(or 2)-methyl-3(2H)-furanone by yeasts. Journal of Agricultural and Food
Chemistry, 39:934-938.
Shi, X. and Dalal, N. S. 1991. Antioxidant behavior of caffeine: efficient scavenging of hydroxyl radicals. Food and Chemical Toxicology, 29:1-6.
Song, T., Barua, K., Buseman, G., and Murphy, P. A. 1998. Soy isoflavones analysis: quality control and a new internal standard. The American Journal of Clinical Nutrition,
68:1474S-1479S.
Wang, H. S., Jenner, A. M., Lee, C. Y., Shui, G. G., Tang, S. Y., Whiteman, M., Wenk, M. R., and Halliwell, B. 2007. The identification of antioxidants in dark soy sauce. Free
Radical Research, 41:479-488.
Wu, H-C., Chen, H-M., Shiau, C-Y. 2003. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food
Research International, 36:949-957.
Yeh, H. Y., Su, N. W., and Lee, M. H. 2005. Chemical compositions and physicochemical properties of the fiber-rich materials prepared from shoyu mash residue. Journal of
Agricultural and Food Chemistry, 53:4361-4366.
9