Study on the Reaction of CH
2
with H
2
at High Temperature
Pei-Fang Lee, Hiroyuki Matsui,* and Niann-Shiah Wang*
Department of Applied Chemistry, National Chiao Tung University, 1001, Ta Hsueh Road, Hsinchu 30010, Taiwan ABSTRACT: Thermal decomposition of CH2I2[sequential C−I
bond fission processes, CH2I2+ Ar→ CH2I + I + Ar (1a) and
CH2I + Ar→3CH
2+ I + Ar (1b)], and the reactions of3CH2+
H2→ CH3+ H (2) and1CH2+ H2→ CH3+ H (3) have been
studied by using atomic resonance absorption spectrometry (ARAS) of I and H atoms behind reflected shock waves. Highly diluted CH2I2(0.1−0.4 ppm) with/without excess H2(300 ppm) in Ar has been used so that the effect of the secondary reactions can be minimized. From the quantitative measurement of I atoms in the 0.1 ppm CH2I2 + Ar mixture over 1550−2010 K, it is
confirmed that two-step sequential C−I bond fission processes of CH2I2, (1a) and (1b), dominate over other product channels. The
decomposition step (1b) is confirmed to be the rate determining process to produce3CH
2and the least-squares analysis of the
measured rate gives, ln(k1b/cm3molecule−1s−1) = −(17.28 ± 0.79) − (30.17 ± 1.40) × 103/T. By utilizing this result, we
examine reactions 2 and 3 by monitoring evolution of H atoms in the 0.2−0.4 ppm CH2I2+ 300 ppm H2mixtures over 1850− 2040 K. By using a theoretical result on k2(Lu, K. W.; Matsui, H.; Huang, C.-L.; Raghunath, P.; Wang, N.-S.; Lin, M. C. J. Phys.
Chem. A 2010, 114, 5493), we determine the rate for (3) as k3/cm3molecule−1s−1= (1.27± 0.36) × 10−10. The upper limit of k 3
(k3max) is also evaluated by assuming k2= 0, i.e., k3max/cm3molecule−1s−1= (2.26± 0.59) × 10−10. The present experimental
results on k3and k3maxis found to agree very well with the previous frequency modulation spectroscopy study (Friedrichs, G.; Wagner, H. G. Z. Phys. Chem. 2001, 215, 1601); i.e., the importance of the contribution of1CH2in the reaction of CH2with H2
at elevated temperature range is reconfirmed.
1. INTRODUCTION
The methylene radical, CH2 (X̃3B
1 and a1̃A1, represented as 3CH
2 and 1CH2, respectively), is regarded as an important
reaction intermediate in hydrocarbon combustion. In the standard combustion conditions, CH2 radicals are supplied
mostly in the secondary reactions such as CH3+ OH→1CH 2+
H2O and sequential collisional quenching1CH
2+ M→3CH2+
M;1−3therefore, the higher the concentration of fuel species, the more important the role of CH2. Also, it is indicated that1CH2is
a direct product in the thermal decomposition of CH3OH.2,3In
this case, CH2radical plays an important role in the initial stage
of the chain branching processes even if the concentration of CH3OH is low.
Detection of CH2radical has been tried by using various
techniques (LMR spectrometers, mass spectrometers, infra-red diode laser absorption for 3CH2, and LIF and others for 1CH
2); a large amount of information about the rate
con-stants and reaction mechanism of CH2radical have been
ac-cumulated at low temperature range.4−21 Also, shock tube works combined with ARAS (atomic resonance absorption spectrometry) and the frequency modulation spectroscopy
have been conducted to explore the CH2 reactions above
1000 K.22−25,36
The main issue of this study is to obtain reliable kinetic information by monitoring evolution of H atoms produced in the reaction of CH2 + H2 at high temperature range. Highly sensitive detection of H atoms (1 × 1011/cm3) of this study
would be efficient to reduce the effects of the side reactions. In addition, excellent reproducibility of the experimental condition in this shock tube system enables comparative measurement to confirm the concentration of the minor component in the sample mixture, as well as to examine the contributions of the background H atoms.
In most of the previous experimental studies, photolysis or thermal decomposition of CH2CO has been used as a source of
supplying CH2. As CH2CO is relatively stable below 2000 K, it
is also the issue of the present study to search a clean source of producing CH2radical at lower temperature range. Therefore,
this study is divided into two main experimental parts. In the first part, evolution of I atoms has been monitored by using VUV absorption at 178.3 nm in the mixture of 0.1 ppm CH2I2+
Ar over 1550−2010 K to examine the thermal decomposition process (1), i.e., + → + + CH I2 2 Ar CH I2 I Ar (1a) and + → + + CH I2 Ar 3CH2 I Ar (1b) Received: October 5, 2011 Revised: January 16, 2012 Published: January 20, 2012 pubs.acs.org/JPCA
By using the result of thermal decomposition of CH2I2, we have examined the reactions
+ → + CH H CH H 3 2 2 3 (2) and + → + CH H CH H 1 2 2 3 (3)
by monitoring H atoms. Even though3CH2is expected to be the
main products in (1b), it is important to take into account (3) at elevated temperature condition, as the collisional energy transfer between1CH
2and3CH2, (4) is very fast:
+ = +
CH M CH M
1
2 3 2 (4)
2. EXPERIMENTAL SYSTEM
Experimental study has been conducted behind reflected shock waves in a diaphragmless shock tube apparatus (length 5.9 m and i.d. 7.6 cm). Details of experiments were described in previous studies.26,27 An atomic resonance absorption spec-trometry (ARAS) detection system has been used for the measurements of temporal profiles of [I] and [H]; i.e., the resonant atomic absorption of I atoms at 178.3 nm (corresponding to transition 2P1,3/2−2P0,3/2) and that of H
atoms at 121.6 nm are monitored by a microwave-discharge lamp filtered with a vacuum UV (VUV) monochromator and detected by a solar-blind photomultiplier tube (PMT). A gas mixture of about 1% I2 and H2 diluted in He of 10 Torr is
supplied in the microwave-discharge lamp. VUV light passes perpendicularly through the MgF2windows at 4 cm upstream
of the end plate of the shock tube. In the measurement of I atoms, a solid iodine pellet cooled at 281 K is used to supply I2.
Calibration curves for H and I atoms have been constructed by using decomposition of C2H5I,
+ → + + ϕ = ±
C H I2 5 M C H2 5 I M ( 0.90 0.05)
and sequential decomposition of C2H5,
→ +
C H2 5 C H2 4 H
Attention has been paid in the optical alignment to keep the sensitivity to be optimized; detection limit of 1011 atom/cm3 for I and H atoms has been attained. In compensation for the achievement of high sensitivity, the resolution time of the detection is not sufficiently short for the measurements of very rapid reaction phenomena. By monitoring evolutions of I and H atoms in the thermal decomposition of C2H5I at 1900−2000 K and 2 atm, we measured the response time of the detection system to be about 25μs.
However, the reliability of the observed evolutions of the signal intensities can be sufficiently retained (except for the initial 25 μs) if proper experimental conditions have been chosen; this is confirmed by analyzing evolutions of H atoms in the reaction of H + O2between 1700−2000 K in the mixtures
of 0.2−0.4 ppm C2H5I + 300−500 ppm O2.
The present experiment is conducted at very low concentration of sample mixtures, 0.1−0.4 ppm CH2I2 (and + 300 ppm H2) diluted in Ar so as to reduce the influence of
the side reactions; however, the sample mixtures are prepared simply by the measurement of pressure by using combination of Baratron pressure gauges.
He (99.9995%, AGA Specialty Gases), Ar (99.9995%, AGA Specialty Gases), and H2(99.9995%, AGA Specialty Gases) are used without further purification. CH2I2(99%, Sigma-Aldrich,
Reagent Plus grade) and C2H5I (99%, Sigma-Aldrich, Reagent Plus grade) are purified by repeating degassing by successive freezing and pumping cycles.
3. RESULTS AND DISCUSSIONS
3.1. Thermal Decomposition of CH2I2. Almost no kinetic
information is available in the past literatures for CH2I2
decomposition. In the study on thermal decomposition of CH2I2, evolution of I atoms in the 0.1 ppm CH2I2 in Ar is
monitored behind reflected shock waves over 1550−2010 K. Examples of the observed profile of [I] are demonstrated in Figure 1. As clearly shown, it is indicated that sequential two
decomposition steps of C−I bond fission dominate over other product channels because it is shown at high temperature that [I]∞/[CH2I2]0= 2 (where [I]∞and [CH2I2]0denote the con-centrations of final iodine atoms and initial CH2I2, respectively). Also, the first decomposition step, CH2I2+ Ar→ CH2I + I + Ar (1a) is found to be very fast, in comparison with the second step, CH2I + Ar→3CH2+ I + Ar (1b), exhibiting that the reaction
intermediate CH2I is stable even in the relatively high
tem-perature range (T > 1500 K).
Figure 1.Examples of the observed evolution of I atoms in 0.1 ppm CH2I2+ Ar: (A) T = 1556 K, P = 2.16 atm, [Ar] = 1.02× 1019/cm3;
(B) T = 1761 K, P = 2.12 atm, [Ar] = 8.82× 1018/cm3; (C) T = 1848 K, P = 2.08 atm, [Ar] = 8.26× 1018/cm3. Calculated profiles of I atoms by using eq III is shown by the dotted curve, where the initial rise is given for (A) by using k1a = 4.36× 10−9exp(−19858/T)/cm3
mol-ecule−1 s−1 (the rate for thermal decomposition of CH3I, which has
nearly equal dissociation energy with reaction 1a), and the rise rate given by the response time of the detection system was used for (B) and (C).
For the sequential decomposition of CH2I2, the profile of concentration of I atoms is analytically given by
= − − + − −
+ − −
R t F R t
F R t
[I]/[CH I ] [1 exp( )] [1 exp( )] [1 exp( )]
2 2 0 1 1 1
2 2 (I)
where R1= k1a(Ar), R2= k1b(Ar), F1= R2/(R2− R1), and F2=
R1/(R1− R2). Because R1≫ R2, the simple eq II is available in the analysis of the present experimental result,
= − −R t + − −R t [I]/[CH I ]2 2 0 [1 exp( 1)] [1 exp( 2)]
(II) The observed initial rise rate of [I] is found to be too fast to evaluate k1ain the temperature range above 1500 K. From the
profile of I atoms in the range 1 < [I]/[CH2I2]0< 2, the rate of
(1b) was evaluated by using an estimated rate for R1into eq II28
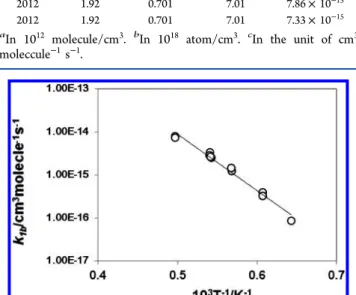
and the result is summarized in Table 1 as well as in Figure 2. Linear least-squares analysis of the data on k1bgives
= − ± − ± × − − k T ln( /cm molecule s ) (17.28 0.79) (30.17 1.40) 10 / 1b 3 1 1 3 (III)
over the temperature range T = 1500−2000 K. The heat of reaction is estimated asΔH0
298= 51.5 kcal mol−1
andΔH0298= 64.4 kcal mol−1for (1a) and (1b), respectively,
on the basis of the recent experimental data on the heat of formation of CH2I and the C− H bond fission energy.29The
heat of reaction for other possible 3-centered reactions such as CH2I2+ M→ CHI + HI + M, CH2I2+ M→1,3CH2+ I2+ M,
and CH2I + M → CH + HI + M, is estimated as ΔH0
298 =
72.5 kcal mol−1, 79.1 kcal mol−1(for the spin-forbidden3CH 2
formation), and 93.9 kcal mol−1, respectively.30−33 Therefore, dominance of (1) over other channels, as well as two-steps production behavior of I atoms observed in this study can be justified thermodynamically. The observed activation energy of 60 kcal/mol for (1b) seems to be consistent with the endothermicity of the reaction.
CH2I2is confirmed to be a clean and better source for supplying 3CH
2 than CH2CO for the shock tube study below 2000 K;
however, about 1800 K is indicated to be the lower limit for the purpose of quick supply. As (1b) is the rate determining step to produce3CH
2, result III is used as the production rate for3CH2in
the analysis in the CH2 + H2 reaction in the following section.
More detailed study on thermal decomposition of CH2I2is now under way and will be presented elsewhere.34
3.2. Reaction of CH2 with H2. Evolution of [H] in the
mixtures of 0.2−0.4 ppm CH2I2 + 300 ppm H2 in Ar is
monitored over 1850−2040 K. In conducting an experiment with very low concentration of sample gas such as employed in this study, it is especially important to examine the validity of the prepared concentration of minor component, as well as to confirm that the reaction system is free from the effect of impurities: these requirements may not be generally so easy to achieve when the concentration of sample gas is extremely low. As described above, the validity of the nominal concentration of CH2I2 prepared by pressure measurement has been confirmed here (because the measured yield of I atoms is equal to 2 times of the nominal concentration of CH2I2for T > 1800 K). In addition, measurement
of [H] in the CH2I2+ (excess H2), as described below is useful to confirm this. These evidence ensure that the loss of CH2I2should
be negligible even for such low concentration samples.
The experimental condition of the present study is sum-marized in Table 2. All the data shown in the table are the averages
of 2 data points conducted at the same shock wave condition; shot-by-shot fluctuation of the temperature shown in this table is less than ±5 K. Averaged values are shown for T and P. Repetition of the measurement at the same condition is useful to improve the S/N ratio (by signal averaging), as well as to confirm that reasonable reproducibility of the profiles of H atoms has been attained.
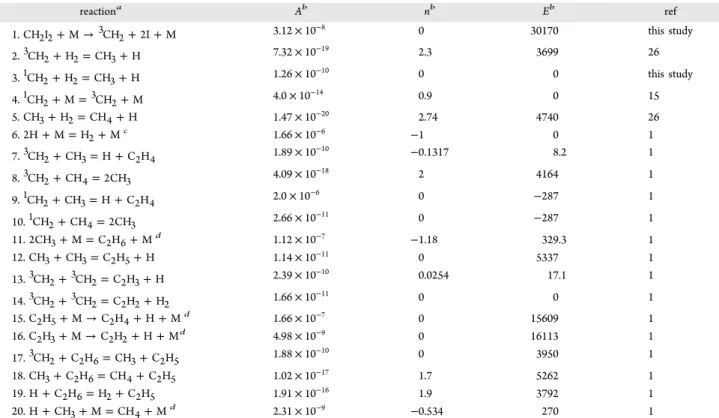
Table 1. Summary of the Experimental Condition for Thermal Decomposition of CH2I2and the Rate Constantk1b
T/K P/atm [CH2I2]0a [Ar]b k1bc 1556 2.16 1.02 10.2 8.64× 10−17 1648 1.36 0.607 6.07 3.93× 10−16 1648 1.36 0.607 6.07 3.28× 10−16 1761 2.12 0.882 8.82 1.23× 10−15 1763 2.12 0.883 8.83 1.46× 10−15 1843 2.07 0.825 8.25 2.52× 10−15 1849 2.08 0.826 8.26 3.33× 10−15 1852 2.09 0.827 8.27 2.84× 10−15 1848 2.08 0.826 8.26 2.66× 10−15 2012 1.92 0.701 7.01 7.86× 10−15 2012 1.92 0.701 7.01 7.33× 10−15
aIn 1012 molecule/cm3. bIn 1018 atom/cm3. cIn the unit of cm3
moleccule−1s−1.
Figure 2.Arrhenius plot of the reaction rate for CH2I + Ar→3CH2+
I + Ar (1b). Linear least-squares-analysis of the data is given by k1b/cm3molecule−1s−1= 3.12× 10−8exp(−30170/T) and expressed
by the straight solid line.
Table 2. Summary of the Experimental Conditions for CH2+
H2Reaction and the Measured Rate Constantsk3andk3max T/K P/atm [Ar]a [CH2I2]0b 1010k3c 1010k3maxc
0.4 ppm CH2I2+ 300 ppm H2+ Ar 2044 1.75 6.29 0.252 1.03± 0.33 2.14± 0.83 1958 1.99 7.45 0.298 1.44± 0.20 2.31± 0.73 1852 2.09 8.27 0.313 1.15± 0.31 2.16± 0.18 0.2 ppm CH2I2+ 300 ppm H2+ Ar 2041 1.86 6.69 0.134 1.40± 0.15 2.66± 0.47 2002 1.88 6.9 0.138 1.36± 0.47 2.39± 0.93 1938 2.01 7.62 0.152 1.17± 0.64 2.16± 0.30 1902 2.01 7.77 0.155 1.40± 0.19 2.21± 0.45 1855 2.08 8.23 0.165 1.21± 0.55 2.06± 0.83
aIn 1018 atom cm−3. bIn 1013 molecule cm−3. cIn the unit of cm3
Also all the data shown in Table 2 are associated with blank
tests using pure Ar and 300 ppm H2 + Ar (both sample
mixtures are prepared in the same condition with the mixture of 0.2−0.4 ppm CH2I2 + 300 ppm H2 + Ar) to confirm that
H atom is not supplied by impurities in Ar, H2, nor the shock tube wall. Background H atom produced in the pure Ar sample was confirmed to be below the detection limit (1× 1011 atom/cm3)
in all the experimental conditions, but a small amount of H atom production is observed (up to 5× 1011atom/cm3) in the
300 ppm H2+ Ar mixture at the highest temperature of this study:
this is not from the impurities but it can be attributed to thermal decomposition of H2.1,35
+ → +
H2 Ar 2H Ar (6)
An example of the observed profile of H atom produced in the mixtures of 0.2 ppm CH2I2+ 300 ppm H2is shown in Figure 3A.
Numerical simulations have been conducted to analyze the reaction rates of (2) and (3), however, it is practically impossible to evaluate k2 and k3 independently, because the collisional energy
transfer from3CH
2to1CH2(−4) is very fast and quasi-equilibrium
between the two electronic states of CH2is maintained; two kinds
of analyses have been tried in this study to estimate k3by assuming
the magnitude of k2, as described below.
The first approach of the analysis on k3here is to employ the
result of theoretical calculation (ab initio molecular orbital and
transition state theory, including Eckert correction) for the reaction (2) expressed as27 = × − = − − − − k T T T /cm molecule s 7.32 10 exp( 3699/ ) 200 3000 K 2 3 1 1 19 2.3 (IV) Using (IV) may be justified by the fact that (IV) agrees very well with the semiempirical data on k2 based on the
mea-surement of Gesser and Steacie12for the relative rate constant
Figure 3.Example of the observed evolution of H atoms in the highly diluted CH2I2+ 300 ppm H2in Ar and comparisons with simulations.
(A) Experimental result shown by the black solid line. Sample gas: 0.2 ppm CH2I2+ 300 ppm H2in Ar, T = 2002 K, P = 1.88 atm, [Ar] =
6.90× 1018/cm3, [CH
2I2]0 = 1.38 × 1012/cm3. Kinetic simulations
using the mechanism of Table 2 are shown by the red solid curve and the values for ±30% of k3 are shown by the red broken curves,
respectively. The solution with the best fit of k3max(maximum value for
k3assuming k2= 0) is shown by the black open circle. (B) Sensitivity
coefficient (nondimension) for H atoms; SHj, evaluated for the
experimental condition of (A). The number indicated in this figure corresponds to the reaction number in the text and Table 3. The reactions in Table 3 not shown in this figure do not show any sensitivity.
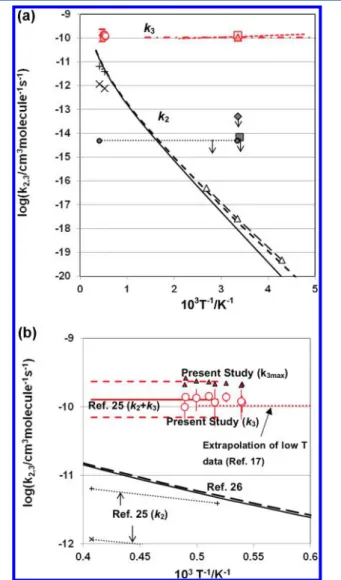
Figure 4.Summary of the reaction rates for3CH
2+ H2→ CH3+ H
(2) and1CH
2+ H2→ CH3+ H (3). (A) k2(expressed by the black
symbols): black solid line and black dashed line, TST calculation without Eckert correction and TST calculation with Eckert correction, respectively (ref 26); Δ connected by the black broken line, semiempirical result obtained by the relative measurement of k2
combined with TST/quantum chemical calculation (refs 12 and 26);
■, ref 13;◆, ref 14; dotted line, ref 16;× and +, ref 25. k3(expressed
by the red symbols): red circle, present study; red solid line and red dashed line, ref 25 (k2+ k3); red dashed-dotted line, ref 17; red dashed
line, ref 18; red square, ref 20; red triangle, ref 21. (B) k2(expressed by
the black symbols): black solid line and black dashed line, TST calculation without Eckert correction and with Eckert correction, respectively (ref 21);× and + with dotted line, ref 25. k3(expressed by
the red symbols): red circle, present study for k3; red triangle, present
study for the maximum value for k3, i.e., k3max(see text); red solid line,
ref 25 (k2+ k3), where the upper and lower limits of ref 25 are shown
by the red dashed lines; red dotted line, extrapolation of the low temperature study of ref 17.
for the reaction of3CH 2with H2(2) against + → + CH CH CO C H CO (reference reaction) 3 2 2 3 2 4
combined with computed rate constant for3CH
2+ CH2CO 27
for the temperature range 230−370 K.
By using a reaction scheme shown in Table 3, we con-ducted fitting the numerical simulation to the observed profile for the evolution of H atoms in the range of initial fast rise for t = 25−150 μs, where the profile of H atoms is sensitive to the reactions 3CH
2 + H2→ CH3 + H (2) and
1CH
2+ H2→ CH3+ H (3). The optimized solutions for k3,
which give the best fit to the experimental profiles, are summarized in Table 2, and an example is shown by the red solid curve in Figure 3A.
The second approach of the present analysis is the limiting case evaluating the upper limit of k3, i.e., k3max, with an
as-sumption k2= 0. As shown by the black circle in Figure 3A, it is possible to achieve good agreement of the numerical simulation to the experimental profile of H atoms even for neglecting the contribution of (2). The results of the analyses on k3max are
summarized also in Table 2.
An example of the computed sensitivity coefficients (non-dimensional) are shown in Figure 3B. Contributions of the reactions in Table 3 other than (1)−(6) are in fact negligibly small, as the concentration of CH2I2used in this study is very low. Contribution for the delay of producing 3CH2 in the
thermal decomposition of CH2I2(1) is significant at the initial stage of the reaction, CH3+ H2→ CH4+ H2(5) dominates for
large t, and the reaction (6) has some sensitivity at high temperature range, T > 2000 K, nevertheless, it is demonstrated that (2) and (3) are sufficiently sensitive to evaluate kinetic rate constant. It is also worthwhile to mention that the numerical
simulation can reproduce very well the observed profile of H atoms with using the nominal value of the initial concentra-tion of CH2I2 for all the experimental data; computation to estimate the accuracy for [CH2I2]0 has been also performed
and the nominal value is concluded to be reliable with±10% error limit.
The present experimental result for (3) by employing theoretical result for k2can be expressed as
= ± ×
− − −
k /cm molecule3 3 1s 1 (1.27 0.36) 10 10 (V) and the upper limit of k3(k3max) by assuming k2= 0 can be
ex-pressed as
= ± ×
− − −
k3max/cm molecule3 1s 1 (2.26 0.59) 10 10
(VI) for the temperature range of T = 1850−2050 K, here, the error limit is given by 2σ.
The result of the present study on k3 is compared with
previous works on k2and k3in Figure 4A (summary of the data for a wide temperature range), and in Figure 4B (high tem-perature data including k3max).
As shown in Figure 4A,B, the rate for (3) evaluated in this study is found to agree very well with that of previous shock tube work,15 as well as with the experimental works below 1000 K.17−20Agreement of the high temperature data on k3 with the experimental result by Gannon et al. conducted between 195−798 K17 implies that k3 shows almost no
tem-perature dependence. Although the result by Friedrichs and Wagner25was indicated to be k2+ k3, it should be approximately equal to k3, because the contribution of k2 is indicated to be
Table 3. Reaction Mechanism for Analyzing the Observed Profile of the H Atom
reactiona Ab nb Eb ref 1.CH I2 2+M→3CH2+2I+M 3.12× 10 −8 0 30170 this study 2.3CH2+H2=CH3+H 7.32× 10−19 2.3 3699 26 3.1CH2+H2=CH3+H 1.26× 10−10 0 0 this study 4.1CH2+M=3CH2+M 4.0× 10 −14 0.9 0 15 5.CH3+H2=CH4+H 1.47× 10−20 2.74 4740 26 6.2H+M=H2+Mc 1.66× 10−6 −1 0 1 7.3CH2+CH3=H+C H2 4 1.89× 10−10 −0.1317 8.2 1 8.3CH2+CH4=2CH3 4.09× 10−18 2 4164 1 9.1CH2+CH3=H+C H2 4 2.0× 10−6 0 −287 1 10.1CH2+CH4=2CH3 2.66× 10 −11 0 −287 1 11.2CH3+M=C H2 6+Md 1.12× 10−7 −1.18 329.3 1 12.CH3+CH3=C H2 5+H 1.14× 10−11 0 5337 1 13.3CH2+3CH2=C H2 3+H 2.39× 10−10 0.0254 17.1 1 14.3CH2+3CH2=C H2 2+H2 1.66× 10−11 0 0 1 15.C H2 5+M→C H2 4+H+Md 1.66× 10−7 0 15609 1 16.C H2 3+M→C H2 2+H+Md 4.98× 10−9 0 16113 1 17.3CH2+C H2 6=CH3+C H2 5 1.88× 10 −10 0 3950 1 18.CH3+C H2 6=CH4+C H2 5 1.02× 10−17 1.7 5262 1 19.H+C H2 6=H2+C H2 5 1.91× 10−16 1.9 3792 1 20.H+CH3+M=CH4+Md 2.31× 10−9 −0.534 270 1
aForward and reverse reactions are considered when connected by“=”.bk = ATnexp(−E
a/RT) [molecule, cm3, K, cal]. cThird-body collision
efficiency for M (=Ar) is taken from ref 1.dOnly parameters for the high pressure limit are shown but the rate for the falloff region is evaluated by using parameters given in ref 1.
minor. Their estimated upper limit for k2+ k3also agrees very well with k3maxof this study.
As for reaction (2), it is difficult to examine k2precisely only from the present experimental information. The results on k2
evaluated by Friedrichs and Wagner25are also shown in Figure 4, but it seems difficult to extract a reliable estimate because it should be very sensitive to the uncertainty of the magnitude of measured k3.
The main conclusion of this study, in agreement with Friedrichs and Wagner,25is that the importance of1CH2in the
reaction of CH2+ H2has been confirmed; i.e., the reaction at elevated temperature can proceed through collisional excitation
from 3CH
2 to 1CH2 if the reaction rate of 3CH2 is not
ex-tremely large. The same scenario may hold for the reactions of CH2with other molecules. Gannon et al.17demonstrated in the study of1CH2+ D2reaction that an insertion reaction can be
competitive to the direct abstraction reaction, because they confirmed that two-thirds of the products of this reaction was H atom. For the molecules other than H2, insertion reaction can still be a part of the main channels for the thermal reactions of CH2 at elevated temperature. Examination of the relative contributions of3CH2and1CH2in many of the key reactions
in combustion system seems to be still a challenging task.
■
AUTHOR INFORMATIONCorresponding Author
*E-mail: N.S.W., nswang@nctu.edu.tw; H.M., matsui@tut.ac.jp.
Notes
The authors declare no competing financial interest.
■
ACKNOWLEDGMENTSThis work was supported by National Science Council of Taiwan under grant no. NSC 99-2113-M-009-014. H.M. deeply acknowledges the supports by NSC and National Chiao Tung University for a distinguished visiting professorship.
■
REFERENCES(1) Smith, G. P.; Golden, D. M.; Frenklach, M.; Moriarty, N. W.; Eiteneer, B.; Goldenberg, M.; Bowman, C. T.; Hanson, R. K.; Song, S.; Gardiner; et al. http://www.me.berkeley.edu/gri mech.
(2) Xia, W. S.; Zhu, R. S.; Lin, M. C.; Mebel, A. M. Faraday Discuss. 2002, 119, 191−205.
(3) Srinivasan, N. K.; Su, M.-C.; Michael, J. V. J. Phys. Chem. A 2007, 111, 3951−3958.
(4) Böhland, T.; Temps, F.; Wagner, H. G. Ber. Bunsen-Ges. Phys. Chem. 1984, 88, 455−458.
(5) Seidler, V.; Temps, F.; Wagner, H. G.; Wolf, M. J. Phys. Chem. 1989, 93, 1070−1073.
(6) Böhland, T.; Temps, F.; Wagner, H. G. Proceedings of the 2Ist Symposium (International) on Combustion; The Combustion Institute: Washington, DC, 1988; pp 841−850.
(7) Kraus, H.; Oehlers, C.; Temps, F.; Wagner, H. G.; Wolf, M. Ber. Bunsen-Ges. Phys. Chem. 1989, 97, 545−553.
(8) Böhland, T.; Heberger, K.; Temps, F.; Wagner, H. G. Ber. Bunsen-Ges. Phys. Chem. 1989, 93, 80−87.
(9) Heberger, K.; Temps, F.; Volker, S.; Wolf, M.; Wagner, H. G. Proceedings of the 23rd Symposium (International) on Combustion; The Combustion Institute: Washington, DC, 1991; pp 29−35.
(10) Goldbach, A; Temps, F.; Wagner, H. G. Ber. Bunsen-Ges. Phys. Chem. 1990, 94, 104−110.
(11) Darwin, D. C.; Young, A. T.; Johnston, H. S.; Moore, C. B. J. Phys. Chem. 1989, 93, 1074−1078.
(12) Gesser, H.; Steacie, E. W. R. Can. J. Chem. 1956, 34, 113−122. (13) Darwin, D. C.; Moore, C. B. J. Phys. Chem. 1995, 99, 13467− 13470.
(14) Pilling, M. J.; Robertson, J. A. J. Chem. Soc., Faraday Trans. 1 1977, 73, 968−984.
(15) Brown, W.; Bass, A. M.; Pilling, M. J. Chem. Phys. 1970, 52, 5131−5143.
(16) Tsang, W.; Hampson, R. F. Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds. J. Phys. Chem. Ref. Data 1986, 15, 1087−1279.
(17) Gannon, K. L.; Blitz, M. A.; Pilling, M. J.; Seakins, P. W.; Klippenstein, S. J.; Harding, L. B. J. Phys. Chem. A 2008, 112, 9575− 9583.
(18) Böhland, T.; Temps, F.; Wagner, H. G. J. Phys. Chem. 1987, 91, 1205−1209.
(19) Sosa, C.; Schlegel, H. B. J. Am. Chem. Soc. 1984, 106, 5847− 5852.
(20) Ashfold, M. N. R.; Fullstone, M. A.; Hancock, G.; Ketley, G. W. Chem. Phys. 1981, 55, 245−257.
(21) Langford, A. O.; Petek, H.; Moore, C. B. J. Chem. Phys. 1983, 78, 6650−6659.
(22) Dombrowsky, Ch.; Wagner, H. G. Ber. Bunsen-Ges. Phys. Chem. 1992, 96, 1048−1056.
(23) Dombrowsky, Ch.; Hwang, S. M.; Rohrig, M.; Wagner, H. G. Ber. Bunsen-Ges. Phys. Chem. 1992, 96, 194−198.
(24) Frank, P.; Bhaskaran, K. A.; Just, Th. J. Phys. Chem. 1986, 90, 2226−2231.
(25) Friedrichs, G.; Wagner, H. G. Z. Phys. Chem. 2001, 215, 1601− 1623.
(26) Lu, K. W.; Matsui, H.; Huang, C.-L.; Raghunath, P.; Wang, N.-S.; Lin, M. C. J. Phys. Chem. A 2010, 114, 5493−5502.
(27) Wu, C.-W.; Matsui, H.; Wang, N.-S.; Lin, M. C. J. Phys. Chem. A 2011, 115, 8086−8092.
(28) Kumaran, S. S.; Su, M.-C.; Michael, J. V. Int. J. Chem. Kinet. 1997, 29, 535−543.
(29) Seetula, J. A. Phys. Chem. Chem. Phys. 2002, 4, 455−460. (30) Lias, S. G.; Bartmess, J. E.; Liebman, J. F.; Holmes, J. L.; Levin, R. D.; Mallard, W. G. J. Phys. Chem. Ref. Data 1988, 17 (Suppl. 1), 1− 861.
(31) Kudchadker, S. A.; Kudchadker, A. P. J. Phys. Chem. Ref. Data 1976, 5, 529−530.
(32) Furuyama, S.; Golden, D. M.; Benson, S. W. J. Phys. Chem. 1968, 72, 4713−4715.
(33) Carson, A. S.; Laye, P. G.; Pedley, J. B.; Welsby, A. M. J. Chem. Thermodyn. 1993, 25, 261−269.
(34) Lee, P.-F.; Matsui, H.; Chen, W.-Y.; Wang, N.-S. Manuscript under preparation.
(35) Warnatz, J. In Combustion Chemistry; Gardiner, W. C., Ed.; Springer-Verlag: Berlin, 1984; Chapter 5.
![Figure 1. Examples of the observed evolution of I atoms in 0.1 ppm CH 2 I 2 + Ar: (A) T = 1556 K, P = 2.16 atm, [Ar] = 1.02 × 10 19 /cm 3 ;](https://thumb-ap.123doks.com/thumbv2/9libinfo/7926214.156989/2.938.506.819.315.828/figure-examples-observed-evolution-atoms-ppm-ch-atm.webp)