727 Bulletin of Marine Science
© 2006 Rosenstiel School of Marine and Atmospheric Science of the University of Miami
SEX RATIOS, SIZE AT SEXUAL MATURITY, AND
SPAWNING SEASONALITY OF SAILFISH IStIophoruS
platypteruS FROM EASTERN TAIWAN
Wei-Chuan Chiang, Chi-lu Sun, Su-Zan yeh,
Wei-Cheng Su, Don-Chung liu, and Wen-yie Chen
ABSTRACT
Lower jaw fork length (lJFl), round weight (rW) data, and 246 female gonads from 6279 sailfish ranging in size from 78 to 239 cm lJFl (or 1–60 kg rW) were randomly sampled at the Shinkang Fishing Port in southeastern Taiwan from July 1998 to December 2002. Sixty-nine percent of these sailfish were males. The sex ratio defined as the proportion of females in the sample increased as the lJFl in-creased beyond 145 cm and reached 98.6% at sizes > 227 cm lJFl (or 46 kg) (sex ratio = 3.07 × 10−7 lJFl3.9782). Female gonads were classified into seven stages of maturity
based on histological structures. Sexually mature individuals were defined as fe-males with ripe ovaries or advanced oocytes. Estimated mean lJFl at sexual matu-rity (l50) was 166 cm for females, and the smallest mature female was 162 cm lJFl. Monthly variations in gonadosomatic indices of 313 mature sailfish peaked during April–September, which is coincident with the histological findings that females were in spawning and recently-spawned stages during this time, indicating this to be the spawning period off eastern Taiwan.
Sailfish, Istiophorus platypterus (Shaw in Shaw and Nodder, 1792), are widely
dis-tributed in tropical and temperate surface waters of the world’s oceans (Nakamura,
1985). Off Taiwan’s eastern coast, sailfish are of substantial economic importance
and are seasonally abundant from April to October (peaking from May to July). This
species is taken primarily by drift gill nets, although they are also caught by set nets,
harpoons, and as incidental by-catch in inshore longline fisheries (Chiang, 2004). For
the past 10 yrs, annual landings of sailfish off Taiwan have fluctuated from 500 to
1000 metric tons (mt), of which > 50% are from waters off Taitung (eastern Taiwan).
The size and age at sexual maturity and sex ratios are fundamental biological
pa-rameters used in stock assessments (Wang et al., 2003). Estimates of body size, or age
at sexual maturity, are necessary parameters for calculating spawning stock biomass
in size- and age-structured models (Deriso et al., 1985; Gabriel et al., 1989; Quin II et
al., 1990; Foale and Day, 1997). They are also required in estimating biological
refer-ence points used in determining the status of fish populations (Hilborn and Walters,
1992).
Several studies on the reproductive characteristics of sailfish have been published
for the eastern Pacific Ocean. Kume and Joseph (1969a), Shingu et al. (1974), Miyabe
and Bayliff (1987), Nakano and Bayliff (1992), and Uosaki and Bayliff (1999) used
gonad index data to estimate the body size at sexual maturity for female sailfish and
described the geographic distribution of mature sailfish in the eastern Pacific.
El-dridge and Wares (1974) used gonad index data and the number of modes in the
oo-cyte diameter distribution to infer the sexual maturity and spawning seasonality for
female sailfish. Using histology, Hernández-Herrera and Ramírez-Rodriguez (1998)
estimated the spawning seasonality and length at maturity of sailfish caught by the
BULLETIN OF MARINE SCIENCE, VOL. 79, NO. 3, 2006 728
Mexico’s recreational fishery. In contrast, there has been no histological analysis of
the reproductive biology of sailfish in the western Pacific.
The objective of this study was to estimate sex ratios and length at sexual maturity
for sailfish in the waters off eastern Taiwan. To evaluate maturity, gonadal
develop-ment was determined through histological examination in addition to the
measure-ment of the most advanced group of oocytes and estimates of a gonad index. The
results of this study will be used as biological input parameters for stock assessment
of the sailfish population in the northwestern Pacific Ocean.
Materials and Methods
Collection of Samples and General Biological Data.—Sailfish biological data were collected monthly at Shinkang fishing port (Fig. 1) from July 1998 to December 2002. Speci-mens were selected at random from landings, and length/weight data and gonad samples were collected. The sex of each specimen was identified based on the appearance of the gonads. Specimens were measured to the nearest centimeter for length (EFL = eye fork length, lJFl = lower jaw to fork length), to the nearest kilogram for weight (rW) and to the nearest 0.1 g for ovarian weight (oW). Gonadosomatic index (GSI) was calculated as in Uchiyama and Shomura (1974). Sex ratio was expressed as proportion of females to total numbers of females and males by length class (5-cm length intervals) and by quarter intervals (3 mo).
Preparation for Histological Examination.—Gonads were preserved in 10% buff-ered formalin for histological examination. The developmental stages of oocytes were catego-rized following several authors (Hunter et al., 1992; Arocha, 2002). Microscopic slides were Figure 1. The fishing port in Taitung (eastern Taiwan) where gonad samples of sailfish
(Istiopho-rus platypte(Istiopho-rus) and measurements for the sex ratio analysis were collected. Oblique and cross
line areas indicated the fishing grounds of longline and gillnet fisheries based at the Shinkang fishing port.
examined with a Leica DM LS compound microscope at a magnification of 40–1000×. Histo-logical classification of gonadal developmental stages was based on the criteria of DeMartini et al. (2000). Stages 4–6 are reproductively active stages, and stages 1–3 and 7 are reproduc-tively inactive stages (Wang et al., 2003).
Oocyte Measurement.—Oocyte size was obtained by measuring the diameter of oo-cytes on histological slides using the Image-Pro Image analysis software package (Media Cybernetics, Silver Spring MD, 1997) in combination with a dissecting microscope (model: Leica-MZ6) equipped with a charged coupled device (CCD) camera (model: Toshiba IK-630) and a computer with high-resolution monitor. However, histological sectioning deforms the oocyte from its sphere-like shape and three different measurements were made following Arocha (2002): (1) early developed oocytes were measured using the major axis crossing the nucleus, (2) maturing oocytes were measured across the nucleus from well-formed spheres, and (3) fully mature oocytes diameter (D) was calculated from D = p π−1, where p is the
cir-cumference of the oocyte. Samples of 200–350 oocytes were measured at various stages of maturity.
The relationship between oocyte size and the probability of reproductive activity was rep-resented by a logistic model (DeMartini et al., 2000; Wang et al., 2003) described as:
ln
c
1 -
p
p
m
=
a
+ #
b
OD
where oD = whole oocyte diameter (µm); and p = probability of an egg of size oD being de-fined as in the reproductive activity stage 4–6.
Sexual Maturity.—Sexually mature individuals were defined as females with ovaries in the ripening or more advanced stages. The length at which 50% of all individuals were sexu-ally mature (l50) was estimated from a logistic model (King, 1995) described as:
exp
P
r
L
L
1
1
50=
+
6
-
#
]
-
g
@
where p = the proportion of mature individuals within length class l (5 cm length interval);
r = the slope of the curve describing the rate of changes in p from 0 to 1; and l50 = the length
(lJFl) at 50% sexual maturity. l50 and r were estimated using the nonlinear least square pro-cedure (Gauss-Newton method, NLIN of SAS Institute, 1990).
Results
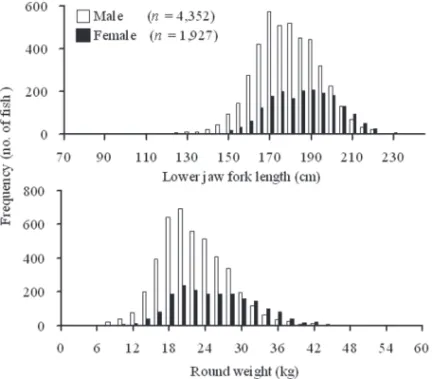
Size Distribution and Sex Ratio.—The lJFl of 1927 females and 4352 males
was measured for the size-specific sex ratio analysis. The range of the lJFl was 80–
239 cm for females and 78–227 cm for males, and the range of rW was 2–60 kg for
females and 1–46 kg for males (Fig. 2).
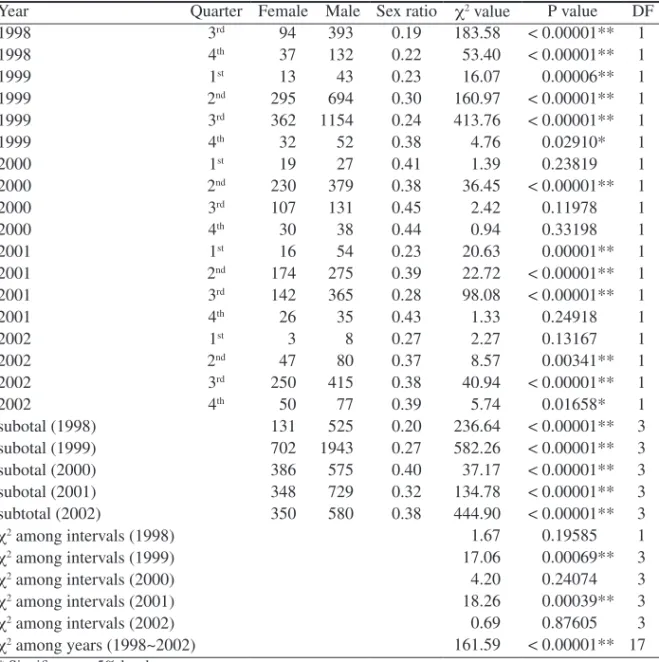
The estimated sex ratio for all samples was 0.31 which differed significantly (χ
2=
936.55; P < 0.01) from the expected value of 0.5 or 1:1. The proportion of males was
higher than the proportion of females in each quarter and year. Sex ratios differed
significantly from the expected value of 0.5 during the following periods: 1
stQ 2000,
3
rdQ to 4
thQ 2000, 4
thQ 2001, and 1
stQ 2002 (Table 1). However, it should be noted
that the sample size in a quarter was small.
The sex ratio fluctuated without significant pattern for fish with a lJFl of < 145 cm.
The sex ratio increased for specimens with a lJFl > 145 cm, and all specimens with
a lJFl > 230 cm were females. The relationship between the sex ratio and lJFl over
the range from 145 to 230 cm (Fig. 3) was given by
BULLETIN OF MARINE SCIENCE, VOL. 79, NO. 3, 2006 730
.
Sex ratio
3 07
10
10LJFL
3 9782.=
#
-(r
2= 0.933; n = 18; 5-cm classes; P < 0.05)
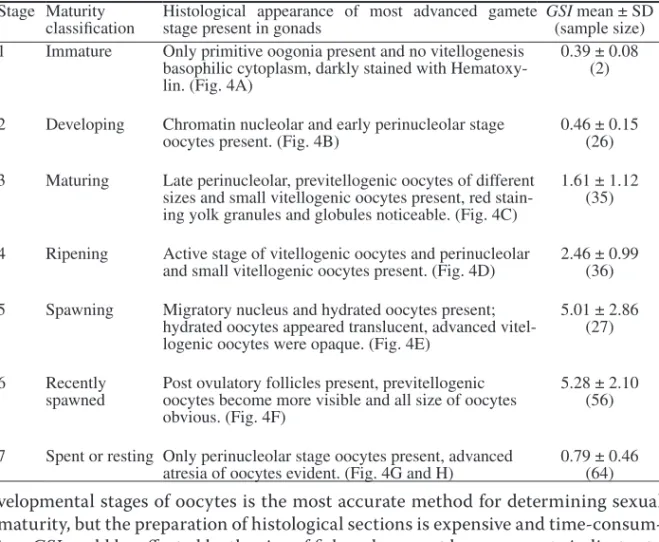
Sexual Maturity.—Size at maturity was estimated based on the ovarian
exami-nation of 246 females (98–225 lJFl) randomly collected during January
1999–Sep-tember 2000. Of these 246 ovarian samples, 183 (74%) were designated as mature
(developmental stage ≥ stage 4) (Fig. 4; Table 2). The minimum size at maturity was
162 cm lJFl.
The proportion of mature females for each length class (5-cm intervals) was fitted
to the logistic curve to estimate the l
50:
.
.
exp
P
LJFL
1
0 2277
166 376
1
=
+
6
#
^-
h@
(r
2= 0.987; n = 22; 5-cm classes).
The 95% confidence interval (C.I.) for l
50was 166.4 ± 1.3 cm (Fig. 5), corresponding
to an age at maturity of about 5 yrs (Chiang et al., 2004).
Oocyte Composition During Ovarian Maturation.—As the largest oocytes
reached the tertiary yolk stage, a clutch consisting of oocytes ~500 µm in size was
found. In the spawning stage ovary, the oocytes formed an advanced batch, which
was distinct from adjacent groups of smaller oocyte cohorts. In the mature ovary,
only the oocytes in the advanced batch increased in size, while those in the less
ad-vanced oocyte cohorts remained < 500 µm.
The logistic regression equation of reproductive activity on the diameter of
oo-cytes (OD) was estimated as:
Figure 2. The size-frequency distributions by (A) 5-cm intervals and (B) 2-kg intervals for male and female sailfish (Istiophorus platypterus) collected from the waters off eastern Taiwan.
ln
c
1 - p
p
m =-5.8829 + 0.0157 # OD
(r
2= 0.921; n = 24; 50-µm classes).
Accordingly, 50% of oocytes were active when the oocyte diameter was about 348
µm, 90% of oocytes were active when the oocyte diameter was 515 µm, and 99% of
oocytes were active when the oocyte diameter was 667 µm.
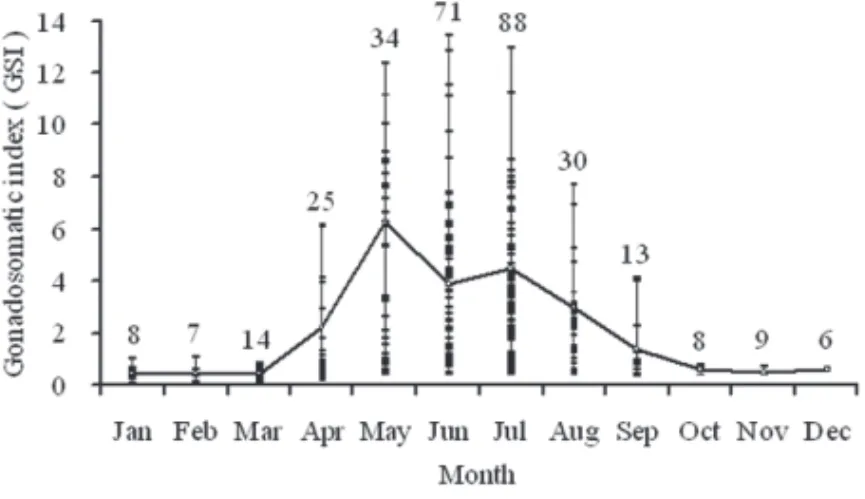
Spawning Seasonality.—Females with maturing stage ovaries occurred from
March to August. Females with spawning stage or recently spawned stage ovaries
occurred from April to September. The ovary samples were all in spent or resting
stage between October and December (Fig. 6). The mean GSI began to increase in
April and remained high until August, then decreased gradually in September and
remained low from November to March (Fig. 7). All these coincident findings
in-dicated that the spawning season of sailfish off eastern Taiwan was from April to
September.
Table 1. Numbers of male and female sailfish (Istiophorus platypterus) grouped by annual quar-ter inquar-tervals with χ2 values assuming a 1:1 sex ratio in each interval.
Year Quarter Female Male Sex ratio χ2 value P value DF
1998 3rd 94 393 0.19 183.58 < 0.00001** 1 1998 4th 37 132 0.22 53.40 < 0.00001** 1 1999 1st 13 43 0.23 16.07 0.00006** 1 1999 2nd 295 694 0.30 160.97 < 0.00001** 1 1999 3rd 362 1154 0.24 413.76 < 0.00001** 1 1999 4th 32 52 0.38 4.76 0.02910* 1 2000 1st 19 27 0.41 1.39 0.23819 1 2000 2nd 230 379 0.38 36.45 < 0.00001** 1 2000 3rd 107 131 0.45 2.42 0.11978 1 2000 4th 30 38 0.44 0.94 0.33198 1 2001 1st 16 54 0.23 20.63 0.00001** 1 2001 2nd 174 275 0.39 22.72 < 0.00001** 1 2001 3rd 142 365 0.28 98.08 < 0.00001** 1 2001 4th 26 35 0.43 1.33 0.24918 1 2002 1st 3 8 0.27 2.27 0.13167 1 2002 2nd 47 80 0.37 8.57 0.00341** 1 2002 3rd 250 415 0.38 40.94 < 0.00001** 1 2002 4th 50 77 0.39 5.74 0.01658* 1 subotal (1998) 131 525 0.20 236.64 < 0.00001** 3 subotal (1999) 702 1943 0.27 582.26 < 0.00001** 3 subotal (2000) 386 575 0.40 37.17 < 0.00001** 3 subotal (2001) 348 729 0.32 134.78 < 0.00001** 3 subtotal (2002) 350 580 0.38 444.90 < 0.00001** 3 χ2 among intervals (1998) 1.67 0.19585 1 χ2 among intervals (1999) 17.06 0.00069** 3 χ2 among intervals (2000) 4.20 0.24074 3 χ2 among intervals (2001) 18.26 0.00039** 3 χ2 among intervals (2002) 0.69 0.87605 3 χ2 among years (1998~2002) 161.59 < 0.00001** 17 * Significant at 5% level. ** Significant at 1% level.
BULLETIN OF MARINE SCIENCE, VOL. 79, NO. 3, 2006 732
Discussion
The proportion of female sailfish off eastern Taiwan increased gradually with fish
length at 145–205 cm lJFl. As fish length increased beyond 205 cm lJFl, the
pro-portion of males declined sharply, and no males > 230 cm lJFl were caught. The sex
ratio remained at < 0.5 in every season, in contrast to that found in the eastern Pacific
(Hernández-Herrera and Ramírez-Rodriguez, 1998) where sex ratios tended to be
larger than 0.5 during spawning season, but did not differ from 1:1 at other times. Off
eastern Taiwan waters, the sex ratio followed an increasing power function between
145 and 230 cm lJFl. Our results are consistent with other observations of sex ratios
in blue marlin (Tzeng, 2002) and swordfish (Wang et al., 2003) in the same study
area. Billfishes have a clear sexual dimorphism, which may result from growth rate
differences between males and females (Sun et al., 2002, Chiang et al., 2004) or from
sex-specific fishing and natural mortality rates (Sun et al., 2005). The relationship
between the sex ratio and body size can provide effective information to reconstruct
the sex composition from catch data.
Ovaries were examined for reproductive activity using three different methods:
histology, largest oocyte size, or GSI (West, 1990). All three techniques can be used
as proxies for each other (Young et al., 2003). Histological examination of the
de-Figure 3. Relationship between proportion of females and lower jaw fork length (LJFL, 5-cm classes) for the sailfish (Istiophorus platypterus) collected from the waters off eastern Taiwan. Closed circles, values used for estimating the relationship; open circles, values not used.Figure 4. Photomicrographs of ovaries at different maturity stages in sailfish (Istiophorus
platyp-terus): (A) immature; (B) developing; (C) maturing; (D) ripening; (E), spawning; (F) recently spawned; (G) spent; and (H) resting. POG: primitive oogonia; PN: perinucleolar oocytes; VT: early vitellogenic oocytes; VO: vitellogenic oocytes; MN: migratory nucleus oocyte; OD: oil droplet; HY: hydrated oocyte; N: nucleus; POF: post ovulatory follicle; α atre: α atretic oocytes. Scale bar = 100 µm.
velopmental stages of oocytes is the most accurate method for determining sexual
maturity, but the preparation of histological sections is expensive and
time-consum-ing. GSI could be affected by the size of fish and may not be an accurate indicator to
separate between mature but reproductively inactive females (stage 7) and immature
females (stage 1–3) during nonspawning seasons (Mejuto and Garcia, 1997;
Hernán-dez-Herrera and Ramírez-Rodriguez, 1998; Wang et al., 2003). Generally, maximum
oocyte diameter, calibrated by histology, is a more accurate indicator of the
tempo-ral and geographical extent of spawning in scombrids (Schaefer, 2001). In order to
Table 2. Histological criteria for classification of gonadal developmental stages and maturation in female sailfish (Istiophorus platypterus) collected from the waters off eastern Taiwan. Maturity stages were based on the criteria of DeMartini et al. (2000). Gonadosomatic index were shown for each stage.Stage Maturity
classification Histological appearance of most advanced gamete stage present in gonads GSI(sample size) mean ± SD 1 Immature Only primitive oogonia present and no vitellogenesis
basophilic cytoplasm, darkly stained with Hematoxy-lin. (Fig. 4A)
0.39 ± 0.08 (2) 2 Developing Chromatin nucleolar and early perinucleolar stage
oocytes present. (Fig. 4B) 0.46 ± 0.15 (26) 3 Maturing Late perinucleolar, previtellogenic oocytes of different
sizes and small vitellogenic oocytes present, red stain-ing yolk granules and globules noticeable. (Fig. 4C)
1.61 ± 1.12 (35) 4 Ripening Active stage of vitellogenic oocytes and perinucleolar
and small vitellogenic oocytes present. (Fig. 4D) 2.46 ± 0.99 (36) 5 Spawning Migratory nucleus and hydrated oocytes present;
hydrated oocytes appeared translucent, advanced vitel-logenic oocytes were opaque. (Fig. 4E)
5.01 ± 2.86 (27) 6 Recently
spawned Post ovulatory follicles present, previtellogenic oocytes become more visible and all size of oocytes obvious. (Fig. 4F)
5.28 ± 2.10 (56) 7 Spent or resting Only perinucleolar stage oocytes present, advanced
atresia of oocytes evident. (Fig. 4G and H) 0.79 ± 0.46 (64)
Figure 5. Relationship between percentage of mature female sailfish (Istiophorus platypterus) and lower jaw fork length (5 cm class) in the waters off eastern Taiwan. 50% maturity is attained at 166.37 cm LJFL.
BULLETIN OF MARINE SCIENCE, VOL. 79, NO. 3, 2006 734
determine reproductive activity, female samples were classified using the criterion
of an oocyte diameter of > 348 µm (i.e., the probability of sexual maturity, P > 50%).
Similar results were obtained using histological observation and the criterion of
oo-cyte diameter. Only two of the 56 ripening samples were determined to be inactive.
Therefore, the “reproductively active oocyte diameter” predicted in this study should
be more accurate than the reproductively active diameter estimated by Eldridge and
Wares (1974). They used the number of modes in the oocyte diameter distribution
(0.3 mm) to infer the sexual maturity and spawning seasonality for female sailfish in
the eastern Pacific Ocean.
Nakano and Bayliff (1992) and Uosaki and Bayliff (1999) assumed that eastern
Pa-cific female sailfish were about to spawn when the gonad index was > 3, and the body
length was about 121–140 cm EFL. In this study, a l
50of 146 cm EFL (or 167 cm
lJFl, according to the relationship between lJFl and EFL; Chiang et al., 2004) was
estimated based on histological examination for female sailfish caught in the eastern
waters of Taiwan. Hernández-Herrera and Ramírez-Rodriguez (1998) estimated l
50values to be 175 cm EFL for female sailfish caught by the Mexico’s recreational
fish-ery based on histological examination. The difference between the eastern and
west-ern Pacific might be a result of geographical isolation, stock structuring, different
environmental conditions, or sampling errors resulting from sample size and timing.
The different fisheries are also a source of variation. Further studies are needed to
evaluate and identify the factors causing differences between the eastern and
west-ern Pacific populations.
Sailfish are distributed from the coast of Ecuador to Mexico in the eastern Pacific
Ocean and their migration pattern is related to the 28 °C surface isotherm
(Ovchin-nikov, 1966; Kume and Joseph, 1969b). Hernández-Herrera and Ramírez-Rodriguez
(1998) found that the sailfish spawning season in the eastern Pacific is protracted
during summer and autumn when the surface isotherm was 27–30 °C. Similarly, we
found that sailfish spawned from April to September in the waters off eastern
Tai-wan when the surface isotherm was 26–29 °C (IGOSS, 1999). Beardsley et al. (1975)
inferred that spawning of sailfish might occur throughout warm tropical waters.
We believe that, while sailfish are migrating, they reproduce wherever they can find
optimal water temperatures and suitable oceanic conditions. It is clear that sailfish
spawn extensively in eastern Taiwan waters. Additional research (including tagging
Figure 6. Monthly changes in the frequency of occurrence of various maturity stages of ovaries of sailfish (Istiophorus platypterus) in the waters off eastern Taiwan.experiments) is necessary to better understand the migration route after spawning.
Furthermore, international cooperative research and management are necessary to
maintain optimal harvest levels for this species.
A
cknowledgments
We thank Y. Chen of the School of Marine Science, University of Maine, ME, and the two anonymous referees for variable comments on the manuscript. This study was partially finan-cially supported by the Fisheries Agency, Council of Agriculture, Taiwan, through the grant 92AS-9.1.1-FA-F1(20) to C. -L. Sun.
Literature Cited
Arocha, F. 2002. Oocyte development and maturity classification of swordfish from the north-western Atlantic. J. Fish Biol. 60: 13–27.
Beardsley, Jr G. L., N. R. Merrett, and W. J. Richards. 1975. Synopsis of the biology of the sailfish, Istiophorus platypterus (Shaw and Nodder, 1791). Pages 95–120 in R. S. Shomura and F. Williams, eds. Proc. Int. Billfish Symp. Part 3. Species Synopses. US Dept. Comm., NOAA Tech. Rep. NMFS SSRF-675. U.S. Government Printing Office, Washington, D.C. 159 p.
Chiang, W. C. 2004. Population dynamics and stock assessment of the sailfish (Istiophorus
platypterus) in waters off eastern Taiwan. Ph.D. Diss., National Taiwan University, Taipei.
171 p.
Chiang, W. C., C. L. Sun, S. Z. Yeh, and W. C. Su. 2004. Age and growth of sailfish (Istiophorus
platypterus) in waters off eastern Taiwan. Fish. Bull. 102: 251–263.
DeMartini, E. E., J. H. Uchiyama, and H. A. Williams. 2000. Sexual maturity, sex ratio, and size composition of swordfish, Xiphias gladius, caught by the Hawaii-based pelagic longline fishery. Fish. Bull. 98: 489–506.
Deriso, R. B., T. J. Quinn II, and P. R. Neal. 1985. Catch-age analysis with auxiliary information. Can. J. Fish. Aquat. Sci. 42: 815–824.
Eldridge, M. B. and P. G. Wares. 1974. Some biological observations of billfish taken in the east-ern Pacific Ocean 1967–1970. Pages 89–101 in R. S. Shomura and F. Williams, eds. Proc. Int. Billfish Symp. Part 2. Review and contributed papers. US Dept. Comm., NOAA Tech. Rep. NMFS SSRF-675. U.S. Government Printing Office, Washington, D.C. 336 p.
Figure 7. Monthly changes in mean gonadosomatic index (GSI) of female sailfish (Istiophorus
platypterus) in the waters off eastern Taiwan (vertical bars, standard error; numbers above verti-cal bars, sample size).
BULLETIN OF MARINE SCIENCE, VOL. 79, NO. 3, 2006 736
Foale, S. and R. Day. 1997. Stock assessment of trochus (trochus niloticus) (Gastropoda: Tro-chidae) fisheries at West Nggela, Solomon Islands. Fish. Res. 33: 1–16.
Gabriel, W. L., M. P. Sissenwine, and W. J. Overholtz. 1989. Analysis of spawning stock bio-mass per recruit: an example for Georges Bank haddock. North Am. J. Fish. Manage. 9: 383–391.
Hernández-Herrera, A. and M. Ramírez-Rodríguez. 1998. Spawning seasonality and length at maturity of sailfish (Istiophorus platypterus) off the Pacific coast of Mexico. Bull. Mar. Sci. 63: 459–467.
Hilborn, R. and C. J. Walters. 1992. Quantitative fisheries stock assessment: choice, dynamics and uncertainty. Chapman and Hall, New York. 570 p.
Hunter, J. R., B. J. Macewicz, N. C. Lo, and C. A. Kimbrell. 1992. Fecundity, spawning, and ma-turity of female Dover sole Microstomus pacificus, with and evaluation of assumptions and precision. Fish. Bull. 90: 101–128.
IGOSS. 1999. Integrated Global Ocean Services System Products Bulletin. Available from: http://ingrid.ldeo.columbia.edu/SOURCES/IGOSS/. Accessed: December 2003.
King, M. 1995. Reproduction and recruitment. Pages 151–165 in M. King, ed. Fisheries biology, assessment, and management. Fishing News Books Press, Oxford.
Kume, S. and J. Joseph. 1969a. The Japanese longline fishery for tunas and billfishes in the eastern Pacific Ocean, East of 130°W, 1964–1966. Bull. Inter-Am. Trop. Tuna Comm. 13: 277–418.
________ and ________. 1969b. Size composition and sexual maturity of billfish caught by the Japanese longline fishery in the Pacific Ocean east of 130°W. Bull. Far Seas Fish. Res. Lab. 2: 115–162.
Media Cybernetics, 1997. Image-Pro Plus, version 3.0 for Windows, MD. Media Cybernetics, Silver Spring. 480 p.
Mejuto, J. and B. Garcia. 1997. A preliminary analysis of gonadal indices of the swordfish
(Xi-phias gladius L.) in the Atlantic Ocean. Int. Comm. Conserv. Atl. Tunas Coll. Vol. Sci.
Pa-pers 46: 336–344.
Miyabe, N. and W. H. Bayliff. 1987. A review of the Japanese longline fishery for tunas and billfishes in the eastern Pacific Ocean, 1971–1980. Bull. Inter-Am. Trop. Tuna Comm. 19: 1–163.
Nakamura, I. 1985. FAO species catalogue. Billfishes of the world. An annotated and illustrated catalogue of marlins, sailfishes, spearfishes, and swordfishes known to date. FAO Fish. Syn-op., No. 125, vol. 5. FAO, Rome. 65 p.
Nakano, N. and W. H. Bayliff. 1992. A review of the Japanese longline fishery for tunas and billfishes in the eastern Pacific Ocean, 1981–1987. Bull. Inter-Am. Trop. Tuna Comm. 20: 187–355.
Ovichinnikov, V. V. 1966. The effect of oceanographic conditions on distribution of the sailfish,
Istiophorous americanus, off the west African coast. Oceanography 6: 566–567.
Quinn II, T. J., R. Fagen, and J. Zheng. 1990. Threshold management policies for exploited populations. Can. J. Fish. Aquat. Sci. 47: 2016–2029.
SAS Institute. 1990. SAS/STAT user’s guide, Ver. 6. 4th ed. Pages 1135–1194. SAS Institute.
Cary, NC. 1686 p.
Schaefer, K. M. 2001. Reproductive biology of tunas. Pages 142–148 in B. A. Block and E. D. Stevens, eds. Tuna physiology, ecology, and evolution. Fish Physiology. Academic Press, New York. 468 p.
Shingu, C., P. K. Tomlinso, and C. L. Peterson. 1974. A review of the Japanese longline fishery for tunas and billfishes in the eastern Pacific Ocean, 1967–1970. Bull. Inter-Am. Trop. Tuna Comm. 16: 65–230.
Sun, C. L., S. P. Wang, and S. Z. Yeh. 2002. Age and growth of the swordfish (Xiphias gladius L.) in the waters around Taiwan determined from anal-fin rays. Fish. Bull. 100: 822–835.
________, ________, C. E. Porch, and S. Z. Yeh. 2005. Sex-specific yield per recruit and spawn-ing stock biomass per recruit for the swordfish, Xiphias gladius, in the waters around Tai-wan. Fish. Res. 71: 61–69.
Tseng, C. C., 2002. Reproductive biology of blue marlin Makaira mazara in the western Pa-cific. M.S. Thesis, National Taiwan University, Taipei. 86 p.
Uchiyama, J. H. and R. S. Shomura. 1974. Maturation and fecundity of swordfish, Xiphias
gla-dius, from Hawaiian waters. Pages 142–148 in R. S. Shomura and F. Williams, eds. Proc. Int.
Billfish Symp. Part 2. Review and contributed papers. US Dept. Comm., NOAA Tech. Rep. NMFS SSRF-675. U.S. Government Printing Office, Washington, D.C. 336 p.
Uosaki, K. and W. H. Bayliff. 1999. A review of the Japanese longline fishery for tunas and billfishes in the eastern Pacific Ocean, 1988–1992. Bull. Inter-Am. Trop. Tuna Comm. 21: 275–439.
Wang, S. P., C. L. Sun, and S. Z. Yeh. 2003. Sex ratios and sexual maturity of swordfish (Xiphias
gladius L.) in the waters of Taiwan. Zool. Stud. 42: 529–539.
West, G. 1990. Method of assessing ovarian development in fishes: a review. Aust. J. Mar. Freshw. Res. 41: 199–222.
Young, J., A. Drake, M. Brickhill, J. Farley, and T. Carter. 2003. Reproductive dynamics of broad-bill swordfish, Xiphias gladius, in the domestic longline fishery off eastern Australia. Aust. J. Mar. Freshw. Res. 54: 315–332.
Addresses: (C.-L.S., S.-Z.Y.) Institute of oceanography, National taiwan university, No.1,
Sec. 4, roosevelt rd., taipei 106, taiwan. (W.-C.C., W.-Y.C.) eastern Marine Biology research Center of Fisheries research Institute, Council of agriculture, executive yuan, taitung 961, taiwan. (W.-C.S., D.-C.L.) Fisheries research Institute, Council of agriculture, executive yuan, No. 199, hoyee rd., Keelung 202, taiwan. Corresponding Author: (C.-L.S.) e-mail: <chilu@ntu.edu.tw>.