A Growth Check Deposited at Estuarine Arrival in Otoliths of Juvenile
Flathead Mullet (Mugil cephalus L.)
Chih-Chieh Hsu1, Chih-Wei Chang2, Yoshiyuki Iizuka3, and Wann-Nian Tzeng1,*
1Institute of Fisheries Science, College of Life Science, National Taiwan University, Taipei 106, Taiwan
2National Museum of Marine Biology and Aquarium, Pingtung 944, Taiwan
3Institute of Earth Science, Academic Sinica, Nankang, Taipei 115, Taiwan
(Accepted August 27, 2008)
Chih-Chieh Hsu, Chih-Wei Chang, Yoshiyuki Iizuka, and Wann-Nian Tzeng (2009) A growth check
deposited at estuarine arrival in otoliths of juvenile flathead mullet (Mugil cephalus L.). Zoological Studies 48(3):
315-324. An accessory primordium (AP) and secondary growth zone (SGZ) with unique microstructure and microchemistry were observed in otoliths of juvenile flathead mullet (Mugil cephalus L.) collected in estuaries. The microstructure, microchemistry, and crystalline structure of the mullet otoliths collected from 4 estuaries of western Taiwan from 1996 to 2004 were examined by optical and scanning electron microscopy, Raman spectroscopy, and an electron probe microanalyzer to evaluate the age at formation of the AP and whether the SGZ was deposited during a habitat transition from offshore to the estuary. The age was determined by the daily growth increments in mullet otoliths. Mean ages (32.9 ± 5.5 d) and lengths (28.3 ± 2.8 mm) of the fish at estuarine arrival did not significantly differ among years or estuaries (p = 0.82 and 0.31, respectively). The mean age when APs were deposited in juvenile mullet otoliths was 27.1 ± 2.5 d, which was consistent with the age of fish without an AP in the otolith (26.9 ± 3.0 d). The consistency in ages indicated that the AP was deposited very soon after estuarine arrival. The Raman shift and elemental composition indicated that the SGZ was a normal aragonite crystal without polymorphic vaterite or calcite inclusions, indicating that the formation of the SGZ was not due to a changing crystalline structure of CaCO3. Sr/Ca concentration ratios significantly decreased from
approximately 8.0 × 10-3 in the primordium of the otolith to < 4 × 10-3 at the AP when the juvenile mullet migrated
from highly saline offshore to fresh water in the estuary, during which time fish behavior changed from pelagic to benthic habits. This suggests that the AP can be used as a biological tracer to determine the age of the fish at recruitment and refine the reconstruction of the early life-stage environmental history of flathead mullet. http://zoolstud.sinica.edu.tw/Journals/48.3/315.pdf
Key words: Mugil cephalus, Otolith, Microstructure, Microchemistry.
* To whom correspondence and reprint requests should be addressed. Tel: 886-2-33662887. Fax: 886-2-23639570. E-mail:wnt@ntu.edu.tw
O
toliths (ear stones) are located in the inner ear sac of teleost fishes and function in balance and hearing of the fish (Lowenstein 1971). They are a bio-mineralized aragonite crystal, mainly composedof CaCO3 with a minor organic matrix and trace
elements (Calstrom 1963, Degens et al. 1969, Campana 1999). The daily growth increments (DGIs) in otoliths are deposited in approximately 24 h cycles which allow determination of the age of the fish on a daily schedule (Pannella 1971).
Thirty-one trace elements were found in otoliths (Campana 1999). Deposition of elements in otoliths is influenced by environmental factors such as the temperature, salinity, and water chemistry of the habitat and by ontogenetic changes and physiological conditions of the fish such as metamorphosis, growth, metabolism, and feeding habits (Mugiya et al. 1981, Gauldie and Nelson 1988, Buckel et al. 2004). Due to interactions of environmental factors and ontogenetic shifts
during habitat transitions, growth checks (GCs) with different microstructures and microchemical compositions have been found to be deposited in otoliths of many kinds of fishes (Bailey et al. 1977, Campana 1984, AlHossaini et al. 1989, Karakiri et al. 1989, Gartner 1991, Linkowski 1991, Sogard 1991, Volk et al. 1995, Zhang et al. 1995, Modin et al. 1996, Brown et al. 2001, Neuman et al. 2001, Plaza et al. 2001, Pontual et al. 2003, Xie et al. 2005). These checks can be used as landmarks to reconstruct the migratory environmental history (Chen and Tzeng 1996, Wang and Tzeng 1998 2000). Thus, the elemental composition of the increments accompanied by age determination has been widely used to reconstruct the past migratory environmental history of fishes (Tzeng 2003, Morales-Nin et al. 2005).
Formation of the GC is mainly attributed to an ontogenetic shift and/or habitat transition of a fish, such as checks deposited in otoliths of Japanese eel Anguilla japonica (Tzeng 1990 1996 2003, Tzeng and Tsai 1994, Arai et al. 1997, Tzeng et al. 2002), goby Sicyopterus japonicus (Shen and Tzeng 2002), and Pacific tarpon Megalops cyprinoides (Chen and Tzeng 2006) during metamorphosis from larvae to juveniles and when migrating from offshore to estuaries. Otoliths
may be comprised of different CaCO3 crystalline
polymorphs, i.e., aragonite, vaterite, and calcite, with different elemental compositions among polymorphs (Brown and Severin 1999, Melancon et al. 2005), which may also produce a GC (Tzeng et al. 2007).
The flathead mullet (Mugil cephalus L.) is a cosmopolitan euryhaline fish, distributed in coastal waters, lagoons, and estuaries between 42°N and 42°S (Thomson 1964). The mullet is an important fish for both capture fisheries and aquaculture in Taiwan. It spawns in northeastern and southwestern coastal waters of Taiwan in Dec. (Hsu et al. 2007). The larvae then passively drift with coastal currents to estuaries. During this passive dispersal process, they metamorphose from larvae to juveniles and actively migrate to estuarine nursery areas (Blaber 1987, Chang et al. 2000). During migration from offshore to estuarine nursery areas, the juvenile experiences both ontogenetic shifts and gradient changes in environmental factors. These changes may produce GCs in otoliths of juvenile mullet. In general, the GC is characterized as a band with narrow and discontinuous growth increments in the otolith which is deposited during ontogenetic changes
and habitat changes such as in the Japanese eel (Tzeng 2003) and goby (Shen and Tzeng 2002). However, the GC in the juvenile mullet otolith was composed of an accessory primordium (AP) and a secondary growth zone (SGZ) following the AP. The pattern of GCs in juvenile mullet otoliths differed from that reported in the past. Meanwhile, the timing and mechanism of the deposition of the AP in the mullet otolith are also not completely understood.
In this study, we attempted to (1) determine the age of juvenile flathead mullet at otolith AP deposition by counting otolith DGIs, (2) examine the crystalline structure of the SGZ by optical and scanning electron microscopy (SEM) and Raman spectroscopy, (3) measure temporal changes in the strontium (Sr) to calcium (Ca) ratio in otoliths of the mullet to understand the chemical compositions of the AP and SGZ using an electron probe microanalyzer (EPMA), and (4) integrate this information to evaluate whether deposition of the AP and SGZ results from a habitat transition and/ or ontogenetic shift of the fish.
MATERIALS AND METHODS Sampling design
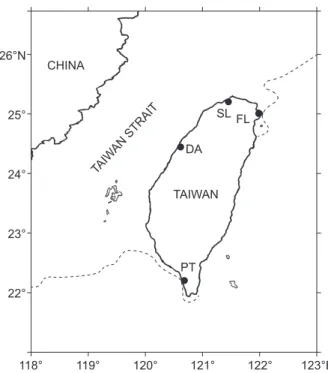
In total, 252 juvenile mullet were collected from the 4 estuaries of Fulong (FL), Salun (SL), Daian (DA), and Pingtung (PT) (Fig. 1) in Jan. of 2001 by a beach seine net with a mesh size of 1 mm. The mullet from SL were collected over 10 yrs, from 1996 to 2004. Fish total lengths (TLs) (± SD) were measured to the nearest 0.1 mm (Table 1), and the daily ages of all individuals were determined from otolith DGIs (Pannella 1971). The Sr/Ca ratios of otoliths from 32 randomly selected fish were examined by EPMA, and from 5 fish for otolith crystalline structure analysis by Raman spectroscopy.
Age determination
Sagittae, the largest of the 3 pairs of otoliths, were removed, ultrasonically cleaned with distilled water, air-dried, embedded in epoxy resin, ground, and polished along the sagittal plane until the primordium was exposed. Then the polished otolith was etched with 0.05 M HCl to enhance the DGIs. The ages of the fish at capture and at AP formation were respectively determined by DGI counts following Chang et al. (2000). Differences
in the mean ages of the fish at AP formation among months, years, and sampling areas were tested by analysis of variance (ANOVA, α = 0.05)
and then by multiple comparisons using Scheffe’s S method if significant differences existed. The growth rate (GR) of the fish before GC formation was estimated by the formula:
GR (μm/d) = PC/DAY;
where PC is the radius from the primordium (P) to the beginning of AP formation, and DAY is the age of fish at GC deposition in the otolith.
Identification of crystalline structures of otoliths by the Raman effect
The crystalline structure of the SGZ was compared with other otolith regions by both optical and SEM microscopy and microRaman spectrometry to determine if the SGZ consisted of aragonite and/or its polymorphs, vaterite and calcite. The spectra of unpolarized Raman signals under backscattering (180°) geometry were collected after otoliths were excited by the 514.5 nm line of an argon laser with an Olympus SLM Plan 20× microscope objective with 17 mW power focused on the sample at room temperature. The polymorphs were identified by the wave numbers of the Raman shift, which were accurate to ± 1 cm-1 as determined from plasma emission lines
(Melancon et al. 2005).
Table 1. Mean (± SD) total length (TL) and age of juvenile mullet at estuarine arrival and percentage of juveniles with an accessory primordium (AP) in their otoliths. Juveniles were collected from Fulong (FL), Salun (SL), Daian (DA), and Pingtung (PT) estuaries on the west coast of Taiwan
Sampling site Sampling date Sample size TL (mm) Age (d) Percent (%) with AP formation
FL 23 Jan. 2001 16 27.9 ± 2.7 32.6 ± 6.2 75.0 SL 5 Jan. 1996 18 23.8 ± 2.8 33.6 ± 5.4 50.0 10 Jan. 1997 21 26.5 ± 2.3 34.7 ± 6.2 85.7 28 Jan. 1998 18 29.4 ± 3.2 32.3 ± 5.9 66.7 25 Jan. 1999 21 31.3 ± 1.0 37.4 ± 3.7 85.7 6 Jan. 2000 18 28.1 ± 2.6 33.2 ± 5.9 61.1 7 Feb. 2001 18 28.8 ± 2.3 33.0 ± 5.6 72.2 12 Jan. 2002 14 27.5 ± 1.7 34.0 ± 4.6 78.6 2 Jan. 2003 12 25.9 ± 1.6 31.5 ± 4.0 50.0 24 Nov. 2003 19 28.5 ± 1.2 31.3 ± 3.9 68.4 25 Dec. 2003 18 27.4 ± 2.4 32.6 ± 4.2 88.9 19 Jan. 2004 20 28.6 ± 1.9 30.8 ± 6.4 45.0 19 Feb. 2004 17 31.3 ± 2.8 31.7 ± 6.7 58.8 DA 23 Jan. 2001 14 27.2 ± 2.3 32.4 ± 5.6 71.4 PT 28 Jan. 2001 8 31.2 ± 3.3 39.5 ± 2.1 100.0 Total 252 28.3 ± 2.8 32.9 ± 5.5 69.8
Fig. 1. Sampling sites of flathead mullet in the estuaries of
Taiwan. FL, Fulong; SL, Salun; DA, Daian; PT, Pingtung. The dotted line indicates the 200 m isobaric depth.
26°N 25° 24° 23° 22° 118° 119° 120° 121° 122° 123°E CHINA TAIWAN DA SL FL PT TAIW AN STRAIT
Analysis of Sr/Ca ratios in otoliths
After washing in 2% HNO3, polished otoliths
were air-dried in a class 100 clean room. Otolith Sr/Ca ratios were measured from the primordium to the postrostrum through the AP and SGZ by EPMA (JXA-8900R, JEOL, Japan). The EPMA beam conditions were an accelerating voltage of 15 keV, an accelerating current of 3 nA, and a beam size with a rectangular area of 5 × 4 μm. The wavelength dispersive strengths of Ca at the Kα shell and Sr at the Lα shell were evaluated for 20 and 80 s at the peak positions, and 10 and 20 s at the background positions, respectively.
Calcite (CaCO3, NMNH 136321) and strontianite
(SrCO3, NMNH R10065) were used as standards.
The quantitative data of Ca and Sr contents in the otoliths were calibrated by the ZAF method (Z, atomic number; A, absorption; F, fluorescence correction) (Tzeng et al. 2002).
RESULTS Microstructures of the AP and SGZ
The DGI in juvenile mullet otoliths is a bipartite structure, composed of a broad, light incremental zone and a narrow, dark discontinuous zone (Figs. 2a, b). The widths of DGIs from the primordium to the otolith edge varied among different radii. The SGZ was initially deposited with an AP in the postrostrum of the otolith. Most otoliths had a single AP and SGZ (Fig. 2c), but some had more than 2 (Figs. 2d-g). The DGIs in the SGZ seemed to be periodically and continuously deposited which allowed us to determine the duration of SGZ formation. However, the SGZ and non-SGZs were interrupted and discontinuous which led to difficulty in determining the age of the juvenile along the
growth axis with an SGZ.
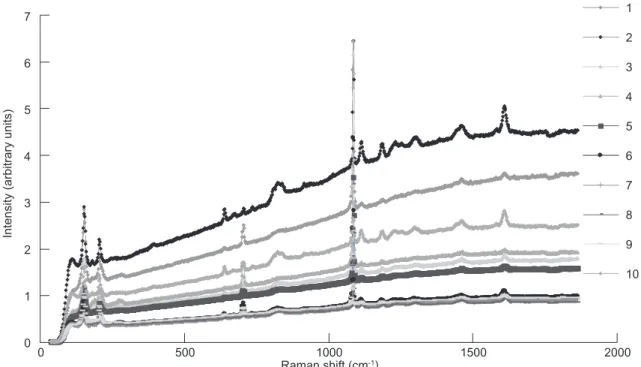
The 3 Raman shift characteristics, lattice mode, in-plane bending, and symmetric stretching, validated that the crystalline structure of the AP and SGZ in the 10 sampling regions of 2 selected juvenile mullet otoliths (Fig. 2) were all aragonite without the other polymorphs, i.e., vaterite or calcite (Fig. 3). The remaining 3 otoliths also had the same results but are not shown here. The lattice modes of the 10 regions were characterized
by peaks at 152 and 205 cm-1, in-plane bending
of C-O from CO32- bonded to Ca as characterized
by peaks at 702 and 705 cm-1, and a single peak
at 1084 cm-1 of symmetrical stretching. In other
words, the crystalline structures of CaCO3 in the
AP and SGZ were aragonite and were similar to those in other portions of the otolith.
Age and length at estuarine arrival and age at AP formation
A t e s t u a r i n e a r r i v a l , a p p r o x i m a t e l y 45.0%-100% (mean, 69.8%) of juvenile mullet were found to have an AP in the otolith. Neither the mean TLs nor ages of the juveniles at estuarine arrival significantly differed among estuaries or among years (Table 1, all p < 0.01). In addition, the selected juvenile mullets with and those without an AP in the otolith significantly differed in mean (± SD) TL (28.8 ± 2.8 vs. 27.3 ± 2.6 mm) at estuarine arrival (Table 2, p < 0.01). The age of juveniles with an AP in the otolith (35.7 ± 3.9 d) was approximately 9 d significantly older than those without an AP (26.9 ± 3.0 d) (Table 2, p < 0.01). However, the age of juveniles at AP formation was similar to that of juveniles without an AP at capture in the estuary (27.1 ± 2.5 d). This implies that juvenile mullet entering the estuary would develop an AP and SGZ shortly thereafter.
Table 2. Comparison of the mean (± SD) age and total length (TL) of juvenile mullets at estuarine arrival and the age at accessory primordium (AP) formation in otoliths between fish with and without an AP
Sample size Occurrence rate (%) Mean total length (mm) Mean age (d) at Estuarine arrival AP formation With an AP 176 69.8 % 28.8 ± 2.8 35.7 ± 3.9 27.1 ± 2.5 Without an AP 76 30.2 % 27.3 ± 2.6 26.9 ± 3.0
Comparison (p) 0.001 < 0.001 0.66a aComparison of the mean age at AP formation and estuarine arrival (without an AP) of juveniles mullet.
(a) D Po 4 1 2 (b) (b) (c) (d) A V DGI c P (e) (f) (g) SGZ AP Po A P Po A P 7 8 9 10 5 6 SGZ AP SGZ SGZ SGZ 3 (c)
Fig. 2. SEM microphotograph showing the primordium (P), daily growth increment (DGI) that consists of an incremental zone (dotted
circle) and discontinuous zone (solid circle), accessory primordium (AP), and secondary growth zone (SGZ). Otoliths of mullet were collected at Salun in Nov. (d) and Dec. (a, f) 2003 (total length (TL) = 27.7 mm and age = 35 d for (a); 27.7 mm and 34 d for (d) and 29.1 mm and 37 for (f). (b, c, e, g) are magnified from (a, d, f), respectively. A, anterior; Po, posterior; V, ventral; D, dorsal. Scale bars: a, d, f = 100 μm, b, c = 20 μm, e, g = 50 μm. The locations indicated by 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 were used for Raman spectrum analyses: 1, primordium; 2, non-SGZ area; 3, SGZ; 4, AP; 5, primordium; 6, non-SGZ area; 7-10, SGZ.
Effect of the growth rate on the age at AP formation
The mean (± SD) age of fish at AP formation was negatively correlated to the growth rate before AP formation (Fig. 4). In other words, if a fish grew faster, it would deposit an AP in the otolith at a younger age. For example, the faster-growing fish A and B deposited the AP approximately 5 d earlier than did the more-slowly growing fish C and D (Fig. 5). The growth rate was indicated by the otolith DGI width that was wider in fish A and B than in
fish C and D. Meanwhile, it also indicated that faster-growing mullet entered the estuary earlier than more-slowly growing fish.
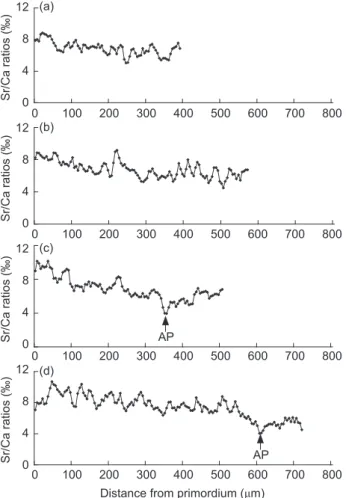
Temporal changes in Sr/Ca ratios in otoliths Temporal changes in Sr/Ca ratios from the primordium to the edge of the otolith were compared between juveniles with and those without AP and SGZ inclusions (Fig. 6). The Sr/Ca ratios in the primordium of otoliths were approximately 8 × 10-3 regardless of the existence
Fig. 4. Relationship between the age at accessory primordium
(AP) formation in otoliths of juvenile mullet and the growth rate before AP formation. 00 20 40 80 Y = -0.061X + 28.975 R2 = 0.065, n = 176, p < 0.001 20 40 Age of AP formation (d) 60 Growth rate (μm/d) 30 10
Fig. 5. Comparison of the daily growth increment widths
from the primordium to the age of accessory primordium (AP) appearance in otoliths between faster-growing groups (A: 27.69 mm in total length, B: 26.07 mm) and more-slowly growing groups (C: 27.99 mm, D: 28.88 mm). 01 6 11 21 26 A B C D 20 40 Increment width ( μm) 16 Age (d)
Fig. 3. Raman spectra of the crystalline structure of the 10 locations marked in otoliths as shown in figure 2.
7 5 0 0 500 1000 1500 2000 1 2 3 4 5 6 7 8 9 10 1 2 3 4 6 Raman shift (cm-1)
of a SGZ, then they gradually decreased (Figs. 6a, b) and dramatically decreased to a low level of < 4 × 10-3 in the new AP region (Figs. 6c, d).
Accordingly, the AP in otoliths of juveniles seemed to be a useful marker to delineate the timing of fish migration from more-saline offshore waters to the lower-saline, brackish-water estuary.
DISCUSSION
Otolith elemental signature changes with fish migration
The Sr/Ca ratios of otoliths of juvenile mullet decreased from approximately 8 × 10-3 in the core
region to < 4 × 10-3 in the AP. A rearing experiment
indicated that the mean Sr/Ca ratio in otoliths was
approximately 3.16 ± 0.36 × 10-3 when flathead
mullet were reared in fresh water but increased to
6.35 ± 0.70 × 10-3 in 5-35 psu seawater (Chang
et al. 2004). The drastic decrease in Sr/Ca ratios at the AP of otoliths implied that juvenile mullet migrated from highly saline offshore areas to low-salinity estuaries. Recovery of the Sr/Ca ratios after AP formation may indicate that juveniles migrated between brackish water and fresh water thereafter. Previous studies indicated that otolith elemental compositions changed with ambient water chemistry (Gillanders and Kingsford 1996 2000, Rooker et al. 2001, Gillanders 2002, Brazner et al. 2004, Patterson et al. 2004). These phenomena indicated that the elemental composition in otoliths of juvenile mullet significantly changed when the fish migrated from highly saline offshore areas to low-salinity estuaries and further validated that the AP was deposited when fish encountered fresh water. The timing and mechanism of AP and SGZ formation
The back-calculated mean age of flathead mullet at AP deposition was 27.1 ± 2.5 d, which was approximately 5-6 d earlier than the age at estuarine arrival of all mullet (32.9 ± 5.5 d, Table 1). The age at AP formation seemed to be flexible, because the age was negatively correlated with growth before recruitment into the estuary. This phenomenon supports the bigger-is-better hypothesis (Miller et al. 1988). The swimming ability of larvae is weak. If larvae drifted with the current to an area of lower food density, they might starve and have delayed metamorphosis and arrival at the estuarine nursery grounds. Faster-growing larvae can reduce the risk of exposure to predation. Factors such as food quality and quantity, and water temperature all have the potential to influence the early growth rate of fish and subsequently the variability of timing of AP formation. Variability in the age at AP formation might, in a future study, be linked to the effect of biological and oceanographic interactions on mullet recruitment so as to predict future stock abundances.
The mechanism of the formation of AP and SGZ in fish otoliths is not clear. The Raman shift and elemental composition indicated that the AP and SGZ in otoliths of juvenile flathead mullet were normal aragonite, not vaterite or calcite, although the AP and SGZ also appeared opaque and their Sr/Ca ratios were low, which is one of the characteristics used to identify vaterite in
Fig. 6. Temporal changes in Sr/Ca ratios in otoliths of mullet
collected at Salun in January 2003. (a) 22.3 and (b) 24.0 mm without an accessory primordium (AP), (c) 23.8 and (d) 26.7 mm with an AP. Arrows indicate the position of AP formation.
Distance from primordium (μm) 0 0 800 (a) 4 12 Sr/Ca ratios (‰) 8 700 600 500 400 300 200 100 0 0 800 (b) 4 12 Sr/Ca ratios (‰) 8 700 600 500 400 300 200 100 0 0 800 (c) 4 12 Sr/Ca ratios (‰) 8 700 600 500 400 300 200 100 0 0 800 (d) 4 12 Sr/Ca ratios (‰) 8 700 600 500 400 300 200 100 AP AP
other fishes (Brown and Severin 1999, Tzeng et al. 2007). Accordingly, the formation of the AP and SGZ in fish otoliths is not due to different crystalline structures. On the other hand, the ontogenetic shift from larvae to juvenile might be one of the reasons for AP formation, such as in Platichthys stellatus and Pleuronectes platessa (Campana 1984, AlHossaini et al. 1989, Karakiri et al. 1989), lantern fishes (Myctophidae) (Linkowski 1991), Trachurus japonicus (Plaza et al. 2001), and Sebastes inermis (Xie et al. 2005). However, only 69.8% of juvenile mullet were found to have an AP in their otoliths (Table 2). This may indicate that the AP in otoliths of juvenile mullet was not deposited during the ontogenetic shift from larval to juvenile stages before the juveniles were recruited to the estuary. Tung (1973) indicated that an ontogenetic shift in M. cephalus occurred from larvae to juveniles at a TL of 22-25 mm, which was close the mean TL of juvenile mullet with and those without AP deposition in this study (28.8 ± 2.8 and 27.3 ± 2.6 mm, respectively). This indicates that the ontogenetic shift may also have the potential to influence AP formation. The flathead mullet collected in this study were all at the late juvenile stage. The age of juveniles at AP formation was only 5 d before it was collected in the estuary, which was similar to that of juveniles without an AP in the their otoliths. The AP seemed to have been rapidly formed after recruitment into the estuary. Accordingly, formation of the AP in juvenile mullet otoliths might not have mainly resulted from the effects of an ontogenetic shift. In addition, the optical microstructure of the SGZ differed from those of other areas in the otolith. The formation of an AP and SGZ might not only have been due to the salinity gradient when the fish migrated from highly saline offshore areas into the low-salinity estuary, but also may have resulted from behavioral changes from a pelagic to a benthic environment similar to changes in otolith microstructures resulting from a habitat shift and change in feeding behavior in the amphidromous goby (Shen and Tzeng 2002).
In conclusion, the accessory primordium and secondary growth zone in the otoliths of juvenile flathead mullet are unique structures that are deposited during the habitat transition from highly saline offshore waters to low-salinity estuarine nursery grounds. The lower Sr/Ca ratios in the accessory primordium support this hypothesis. However, salinity changes might not be the sole cause, and accompanying changes in fish behavior may be more important. Identification of
the accessory primordium and secondary growth zone provides insights into the early life history of the flathead mullet which can help elucidate the recruitment dynamics and habitat transition rate of the fish in its early life stage.
Acknowledgments: This study was financially supported by the National Science Council of Taiwan (NSC93-2313-B-002-054, to WNT). The authors are grateful to Dr. Y.T. Wang, Mr. S.H. Lin, Ms. M.Y. Chang, and Ms. H.Y. Teng for specimen collection, and 2 anonymous reviewers for helpful comments on an early draft of the manuscript.
REFERENCES
AlHossaini M, Q Liu, TJ Pitcher. 1989. Otolith microstructure indicating growth and mortality among plaice,
Pleuronectes platessa L., post-larval sub-cohorts. J. Fish.
Biol. 35: 81-90.
Arai T, T Otake, K Tsukamoto. 1997. Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla
japonica. Mar. Ecol.-Prog. Ser. 161: 17-22.
Bailey RFJ, KW Able, WC Leggett. 1977. Evidence for the presence of a metamorphic check in capelin (Mallotus
villosus) otoliths and implications for age determination. J.
Fish. Res. B. Can. 34: 2008-2014.
Blaber SJM. 1987. Factors affecting recruitment and survival of mugilids in estuaries and coastal waters of southeastern Africa. Am. Fish. Soc. Symp. 1: 507-518.
Brazner JC, SE Campana, DK Tanner. 2004. Habitat fingerprints for Lake Superior coastal wetlands derived from elemental analysis of yellow perch otolith. Trans. Am. Fish. Soc. 133: 692-704.
Brown AL, MS Busby, KL Mier. 2001. Walleye pollock Theragra
chalcogramma during transformation from the larval to
juvenile stage: otolith and osteological development. Mar. Biol. 139: 845-851.
Brown R, KP Severin. 1999. Elemental distribution within polymorphic inconnu (Stenodus leucichthys) otoliths is affected by crystal structure. Can. J. Fish. Aquat. Sci. 56:
1898-1903.
Buckel JA, BL Sharack, VS Zdanowicz. 2004. Effect of diet on otolith composition in Pomatomus saltatrix, an estuarine piscivore. J. Fish. Biol. 64: 1469-1484.
Campana SE. 1984. Microstructural growth patterns in the otoliths of larval and juvenile starry flounder, Platichthys
stellatus. Can. J. Zool. 62: 1507-1512.
Campana SE. 1999. Chemistry and composition of fish otolith: pathways, mechanisms and application. Mar. Ecol.-Prog. Ser. 188: 263-297.
Carlstrom D. 1963. A crystallographic study of vertebrate otolith. Biol. Bull. 125: 441-463.
Chang CW, Y Iizuka, WN Tzeng. 2004. Migratory environ-mental history of the grey mullet Mugil cephalus as revealed by otolith Sr:Ca ratios. Mar. Ecol.-Prog. Ser.
269: 277-288.
Chang CW, WN Tzeng, YC Lee. 2000. Recruitment and hatching dates of grey mullet (Mugil cephalus L.) juveniles
in the Tanshui Estuary of northwest Taiwan. Zool. Stud.
39: 99-106.
Chen HL, WN Tzeng. 2006. Daily growth increment formation in otoliths of Pacific tarpon Megalops cyprinoides during metamorphosis. Mar. Ecol.-Prog. Ser. 312: 255-263.
Cheng PW, WN Tzeng. 1996. Timing of metamorphosis and estuarine arrival across the dispersal range of the Japanese eel Anguilla japonica. Mar. Ecol.-Prog. Ser.
131: 87-96.
Degens ET, WG Deuser, RL Haedrich. 1969. Molecular structure and composition of fish otoliths. Mar. Biol. 2:
105-113.
Gartner JV. 1991. Life histories of three species of lanternfishes (Pisces: Myctophidae) from the eastern Gulf of Mexico. I. Morphological and microstructural analysis of sagittal otoliths. Mar. Biol. 111: 11-20.
Gauldie RW, DG Nelson. 1988. Aragonite twinning and neuroprotein secretion are the cause of daily growth increment deposition in fish otoliths. Comp. Biochem. Physiol. 90: 501-509.
Gillanders BM. 2002. Temporal and spatial variability in elemental composition of otoliths: implication for determining stock identity and connectivity of populations. Can. J. Fish. Aquat. Sci. 59: 669-679.
Gillanders BM, MJ Kingsford. 1996. Elements in otoliths may elucidate the contribution of estuarine recruitment to sustaining coastal reef populations of a temperate reef fish. Mar. Ecol.-Prog. Ser. 141: 13-20.
Gillanders BM, MJ Kingsford. 2000. Element fingerprint of otoliths may distinguish estuarine “nursery” habitats. Mar. Ecol.-Prog. Ser. 201: 273-286.
Hsu CC, YS Han, WN Tzeng. 2007. Evidence of flathead mullet Mugil cephalus L. spawning in waters northeast of Taiwan. Zool. Stud. 46: 717-725.
Karakiri M, R Berghahn, H Von Westernhagen. 1989. Growth differences in 0-group plaice Pleuronectes platessa as revealed by otolith microstructure analysis. Mar. Ecol.-Prog. Ser. 55: 15-22.
Linkowski TB. 1991. Otolith microstructure and growth patterns during the early life history of lanternfishes (family Myctophidae). Can. J. Zool. 69: 1777-1792.
Lowenstein O. 1971. The labyrinth. In Hoar WS, DJ Randall eds. Fish physiology. New York: Academic Press, pp. 207-240.
Melancon S, BJ Fryer, SA Ludsin, JE Gagnon, Z Yang. 2005. Effects of crystal structure on the uptake of metals by lake trout (Salvelinus namaycush) otoliths. Can. J. Fish. Aquat. Sci. 62: 2609-2619.
Miller TJ, LB Crowder, JA Rice, EA Marschall. 1988. Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Can. J. Fish. Aquat. Sci. 45:
1657-1670.
Modin J, B Fagerholm, B Gunnarsson, L Pihl. 1996. Changes in otolith microstructure at metamorphosis of plaice,
Pleuronectes platessa L. ICES. J. Mar. Sci. 53: 745-748.
Morales-Nin B, SC Swan, JDM Gordon, M Palmer, AJ Geffen, T Shimmield, T Sawyer. 2005. Age-related trends in otolith chemistry of Merluccius merluccius from the north-eastern Atlantic Ocean and the western Mediterranean Sea. Mar. Freshw. Res. 56: 599-607.
Mugiya Y, N Watabe, J Yamada, JM Dean, DG Dunkelberger, M Shimuzu. 1981. Diurnal rhythm in otolith formation in the goldfish, Carassius auratus. Comp. Biochem. Physiol.
68: 659-662.
Neuman MJ, DA Witting, KW Able. 2001. Relationships between otolith microstructure, otolith growth, somatic growth and ontogenetic transitions in two cohorts of windowpane. J. Fish. Biol. 58: 967-984.
Pannella G. 1971. Fish otoliths: daily growth layers and periodical patterns. Science (NY) 173: 1124-1127.
Patterson HM, RS McBride, N Julien. 2004. Population structure of red drum (Sciaenops ocellatus) as determined by otolith chemistry. Mar. Biol. 144: 855-862.
Plaza G, S Katayama, M Omori. 2001. Otolith microstructure of the black rockfish, Sebastes inermis. Mar. Biol. 139:
797-805.
Pontual H, F Lagardère, R Amara, M Bohn, A Ogor. 2003. Influence of ontogenetic and environmental changes in the otolith microchemistry of juvenile sole (Solea solea). J. Sea. Res. 50: 199-210.
Rooker JR, DH Secor, VS Zdanowicz, T Itoh. 2001. Discrimination of northern bluefin tuna from nursery areas in the Pacific Ocean using otolith chemistry. Mar. Ecol.-Prog. Ser. 218: 275-282.
Shen KN, WN Tzeng. 2002. Formation of a metamorphosis check in otoliths of the amphidromous goby Sicyopterus
japonicus. Mar. Ecol.-Prog. Ser. 228: 205-211.
Sogard SM. 1991. Interpretation of otolith microstructure in juvenile winter flounder (Pseudopleuronectes
americanus): ontogenetic development, daily increment
validation, and somatic growth relationships. Can. J. Fish. Aquat. Sci. 48: 1862-1871.
Thomson JM. 1964. A bibliography of systematic references to the grey mullets (Mugilidae). Div. Fish. Oceanogr. CSIRO Tech. Pap. 16: 1-127.
Tung IH. 1973. On the egg development and larval stages of the grey mullet, Mugil cephalus Linnaeus. Rep. Inst. Fish. Biol. Ministry Econ. Aff. Natl. Taiwan. Univ. 3: 187-210.
Tzeng WN. 1990. Relationship between growth rate and age at recruitment of Anguilla japonica elvers in a Taiwan estuary as inferred from otolith growth increments. Mar. Biol. 107: 75-81.
Tzeng WN. 1996. Effects of salinity and ontogenetic movements on strontium: calcium ratios in the otoliths of the Japanese eel, Anguilla japonica. J. Exp. Mar. Biol. Ecol. 199: 111-122.
Tzeng WN. 2003. The processes of onshore migration of the Japanese eel Anguilla japonica as revealed by otolith microstructure. In Aida K, K Tsukamoto, K Yamauchi eds. Eel biology. Tokyo: Springer-Verlag, pp. 181-190. Tzeng WN, CW Chang, CH Wang, JC Shiao, Y Iizuka, YJ Yang,
CF You, L Ložys. 2007. Migratory history of anguillid eel might be misidentified by vateritic otolith. Mar. Ecol.-Prog. Ser. 348: 285-295.
Tzeng WN, JC Shiao, Y Iizuka. 2002. Use of otolith Sr: Ca ratios to study the riverine migratory behaviors of Japanese eel Anguilla japonica. Mar. Ecol.-Prog. Ser.
245: 213-221.
Tzeng WN, YC Tsai. 1994. Change in otolith microchemistry of young eel, Anguilla japonica, during its migration from the ocean to rivers of Taiwan. J. Fish. Biol. 45: 671-683.
Volk EC, DG Mortensen, AC Wertheimer. 1995. Nondaily otolith increments and seasonal changes in growth of a pink salmon (Oncorhynchus gorbuscha) population in Auke Bay, Alaska. In Secor DH, JM Dean, SE Campana eds. Recent developments in fish otolith research. Columbia, SC: Univ. of South Carolina Press, pp. 211-215. Wang CH, WN Tzeng. 1998. Interpretation of geographic
variation in size of American eel Anguilla roatrata elvers on the Atlantic coast of North American using their life history and otolith ageing. Mar. Ecol.-Prog. Ser. 168:
35-43.
Wang CH, WN Tzeng. 2000. The timing of metamorphosis and growth rates of American and European eel leptocephali: a mechanism of larval segregative migration. Fish. Res.
46: 191-205.
Xie S, Y Watanabe, T Saruwatari, R Masuda, Y Yamashita, C Sassa, Y Konishi. 2005. Growth and morphological development of sagittal otoliths of larval and early juvenile
Trachurus japonicus. J. Fish. Boil. 66: 1704-1719.
Zhang Z, RJ Beamish, BE Riddell. 1995. Differences in otolith microstructure between hatchery-reared and wild Chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 52: 344-352.