國 立 交 通 大 學
材料科學與工程學系
博 士 論 文
聚亞醯胺發光二極體及奈米複合材料之
合成與特性研究
Synthesis and Characterization of Polyimide Light-
Emitting Diodes and Nanocomposites
研 究 生: 徐守謙
指導教授: 黃華宗 博士
To my family and ray-ray
with gratitude………
聚亞醯胺發光二極體及奈米複合材料之合成與特性研究
摘要
顯示和奈米科技是近年來不管在基礎學科或高科技產業中相當熱門的兩個 課題。本論文將分成兩個部分,共七個章節,針對聚亞醯胺之發光二極體及奈米 複合材料的特性 (2–5 章),和一維奈米材料的製備兩個主題做深入的研究(6,7 章)。 首先在第二章為BAO 系列聚亞醯胺發光二極體特性研究。所有合成的 BAO 系列聚亞醯胺玻璃轉化溫度皆大於 250℃,5 wt.-% 熱裂解溫度也大於 510℃, 顯示良好的熱安定性及機械性質。這些聚亞醯胺也均有螢光特性,而且螢光強度 跟分子鏈的排列有密切的關係。進一步將合成之聚亞醯胺製作成單層發光二極體 元件,只有 BAO-ODPA 和 BAO-6FDA 兩種聚亞醯胺觀察到電致發光性質, BAO-PMDA 和 BAO-BPADA 兩種聚亞醯胺可能因薄膜均勻性太差導致原件短 路。另外,BAO-ODPA 在雙層發光二極體元件中(ITO/PPV-PVA/PI/Al)也具有 良好的電子傳輸及電洞阻障的功能,可將PPV-PVA 發光效率提高兩個級數。 第三章為利用真空蒸鍍聚合製備以BAO-6FDA 和 BAPF-6FDA 兩種聚亞醯胺 為發光層之單層發光二極體。利用真空蒸鍍聚合,聚亞醯胺薄膜的厚度可降低至 150 Å,兩種聚亞醯胺二極體元件也都表現出 4.5V 和 6.5V 相當低的啟動電壓。 經由原子力顯微鏡的分析,BAO-6FDA 和 BAPF-6FDA 兩種聚亞醯胺薄膜皆有良 好的表面平整度,分別為8.8 Å 和 4.7 Å。BAO-6FDA 發光二極體具有較寬的電 致發光頻譜,其範圍從400 nm 到 700 nm。而 BAO-BAPF 發光二極體則表現出 較佳的發光效率,這可能是因為較平衡的電子/電洞注入及較強的分子間電荷轉 移作用。 第 四 章 敘 述 聚 亞 醯 胺/ZnO 奈米混成膜的製備與特性。PMDA-ODA 和 BTDA-ODA 兩種不同柔軟度的聚亞醯胺作為高分子基材進行研究。經由 FTIR 和XPS 的分析,推斷 ZnO 表面的 OH 基和聚亞醯胺的 C=O 官能基會形成交鏈, 進而提升混成膜的熱和機械性質。另外,穿透式電子顯微照片說明,ZnO 奈米粒 子分散在較剛硬的 PMDA-ODA 聚亞醯胺,粒子尺寸會大於分散在較柔軟的 BTDA-ODA 聚亞醯胺中。 第五章為利用蒸鍍/氧化二段法大量製備ZnO 奈米單晶粒子於石英及聚亞醯胺基材上。蒸鍍後的Zn 金屬在 350℃熱風循環機進行氧化 2 小時,可完全轉變
成透明的ZnO。經由高解析度 TEM 觀察,製備的 ZnO 奈米粒子為晶格規則排列
的單晶結構,並無晶格缺陷,並顯示出一395 nm 的紫外光發光特性。
第 六 章 敘 述oleic acid/1-decanol/ammonium hydroxide 三 相 系 統 的 inverse hexagonal (HII) 液晶相的製備與特性。HII相位於此三相圖的中央,由 21/55/24,
28/27/45 和 62/5/33 (oleic acid/1-decanol/ammonium hydroxide) 三點組成的三角形
區域。在此HII相區域中,隨著 1-decanol含量的減少液晶消失溫度(isotropic temperature)變化從 55℃到 142℃。經由XRD分析,推斷製備的HII相的圓柱直徑 為4−4.4 nm,內部水相直徑為 1−1.4 nm。在此三相系統中,ammonium hydroxide 的含量提高至45 wt.-%,及摻入多種金屬離子,如Ag+, Cu2+, Ni2+, Co2+, Zn2+, 和 Cd2+,皆不會破壞原本規則的HII相。 本論文第七章敘述利用先合成的管狀銀離子先驅物,在室溫下即時還原製備 銀奈米電纜(nanocable)。經由FTIR 分析,推論配位的銀離子錯合物形成交鏈, 自身聚集,進而促成管狀先驅物的形成。此銀離子管狀先驅物長度達數微米,外 徑為155−200 nm,徑/長比,管壁厚度為 60−70 nm。經甲醛還原後,原本管狀 先驅物的中空部份,均勻的被直徑30−45 nm 的銀奈米線所填充,形成奈米電纜 結構。還原條件,如還原劑濃度和還原方法,對於最後產物的型態有很大的影響。
Synthesis and Characterization of Polyimide Light- Emitting
Diodes and Nanocomposites
Abstract
Display and nano technologies are two hottest topics in recent years not only in academic research but in high-tech industry. This thesis is divided into two parts to investigate the characterization of polyimide (PI)-based light emitting diodes (LED) and nanocomposites (chapter 2−5), and the preparation of one-dimensional nanostructures (chapter 6, 7).
Chapter 2 describes the characteristics of a single layer and a double layer 2,5-Bis(4-aminophenyl)-1,3,4-oxadiazole (BAO)-based LED. All the resultant PIs possess high glass transition temperatures ( >250 ℃ ) and high decomposition temperatures of 5 wt.-% weight loss (Td, >510℃). They also show obviously
fluorescent characteristic, and the intensity is related to the arrangement of the molecular chains. Electroluminescent (EL) spectra were detected by using BAO-ODPA and BAO-4,4’-(hexafluoroisopropylidene)diphthalic anhydrid (6FDA) as an emitting layer in a single LED device. In addition, in the double layer LED device, ITO/PPV-PVA/BAO-ODPA/Al, BAO-ODPA can be used as an excellent electron transport and electron/hole blocking layer, a significant improvement in the EL efficiency by two order of magnitude.
In chapter 3 presents that BAO and 4,4’-(9-Fluorenylidene)dianiline ( BAPF ) reacting with 6FDA were carried out by using vapor deposition polymerization (VDP) for single layer LED devices. The thickness of the PI thin film can be reduced to 150Å, and both PI-LEDs show low threshold voltages, 4.5V and 6.5V for BAO-6FDA and BAPF-6FDA LEDs, respectively. The root mean square of the surface roughnesses of the BAO-6FDA and BAPF-6FDA thin films are 8.8Å and 4.7Å, respectively, which are far smaller than that of wet coating process. The BAO-6FDA LED emits a broader EL band, covering the full range of visible light (400 nm to 700 nm), than the BAPF-6FDA LED. While the electroluminescent efficiency of BAPF-6FDA LED is higher than BAO-6FDA LED, it may suggest the better balance on holes and electrons injection in the former and better intermolecular charge transfer.
Chapter 4 reports the study of a series of PI/ZnO nanohybrid films with different ZnO contents, which prepared by a rigid pyromellitic dianhydride (PMDA)-4,4’-diaminodiphenylether (ODA) and a flexible 3,3’,4,4’-benzophenonetetracarboxylic acid dianhydride (BTDA)-ODA PI matrixes. Analyses of Fourier transform infrared (FTIR) and X-ray photoelectron spectroscopy depict that the ZnO nanoparticles function as a physical crosslinking agent with PI chains through hydrogen bonding between the OH on the ZnO surfaces and the C=O of the imide groups. This crosslink causes the enhancement of thermal and mechanical properties of the hybrid films. Transmission electron microscopy (TEM) images reveal that the rigid matrix induces larger ZnO particle size (30−40 nm) compared the flexible matrix (10−15 nm).
In chapter 5 describes an efficient evaporation/oxidation two-step approach to massive prepare ZnO nanocrystals on quartz and flexible PI film. Evaporated metallic Zn can quickly transform into transparent ZnO via low temperature oxidization at 350 ℃ for 2h in air-circulating. TEM images show that the singular ZnO nanocrystals without stacking faults were obtained. Deposited ZnO on PI film substrates can obtain individual and well distribution nanocrystals with average crystal size is 20-30 nm. In photoluminescence measurement, the produced ZnO nanocrystals exhibit strong UV emission at 395 nm, and no visible emission was detected.
Chapter 6 presents the ternary system oleic acid/1-decanol/ammonium hydroxide exhibiting an inverse hexagonal (HII) liquid crystalline phase, which exists between
the compositions 21/55/24, 28/27/45, and 62/5/33 (oleic acid/1-decanol/ammonium hydroxide). The isotropic temperature increases from 55 °C to 142 °C with decreasing 1-decanol content. X-ray diffraction reveals interdigitated columns of 4−4.4 nm diameter with an internal water channel of 1−1.4 nm diameter. The system can tolerate up to 45 wt-% of ammonium hydroxide before the hexagonal phase collapses and can be doped with up to 0.1 mM concentrations of metals such as Ag+, Cu2+, Ni2+, Co2+, Zn2+, and Cd2+.
Chapter 7 describes a simple and efficient method to in situ fabricate silver nanocables at room temperature from a self-assembling tubular silver precursor. FTIR spectroscopy revealed that the organic sheath to be crosslinked via bridging-type coordination to the silver ions, which helps in the formation of the tubular aggregation. The length of these tubular precursors is in the order of several microns, with outer diameters of 155−200 nm. The wall thickness is 60−70 nm. After reduction, a sharp contrast between sheath and core can be clearly observed in the TEM micrograph.
The cores are straight and uniform with diameter of 30−45 nm. In addition, properly control of the reaction condition, such as reagent concentration and method of reduction, is important to obtain well-defined nanocables.
誌 謝
指導教授 . 黃華宗 教授 除了研究上的教導,黃老師更是一位理想的傾聽者,六年多的循循善誘,學 生由衷感激。 口試委員 周卓煇 教授、韋光華 教授、楊長謀 教授、許聯崇 副教授 感謝各位老師在學生論文口試的指導與建議。 交大光電所 許根玉 教授 感謝許老師在推薦信上的美言,讓學生得以獲得2004 三明治計畫獎學金, 順利赴德研究。DWI an der RWTH Aachen
Germany Prof. Dr. Martin Möller, Dr. Oliver Weichold
PD Dr. Uwe Beginn, Prof. Dr. Dimitri A. Ivanov, Dr. Ahmed Mourran, Dr. Kim-Hô Phan, Dr. Xiaomin Zhu
Yvonne Noppeney, Heidrun Keul, Linglong Yan, Irene Colicchio, Stephan Rütten
Thomas, Pooja, Britta
I appreciate your assistance during my study in DWI. A special thanks goes to my supervisor Dr. Oliver Weichold for his guidance, understanding and patience, for his encouragement and support at all levels.
駐德科技組 胡昌智組長、彭雙俊組長、淑娟姐、程芝姐、張秘書 你們就像我在德國的家人,一年半的國外生活,有你們的照料及精神上的鼓 勵與支持,讓我得以堅強。 交大學長姐 慶勳學長、美慧學姐、金賢學長、男哥、佩君學姐、吳聲昌學長、旭 .昌、泰翔、施文慈學姐、田運宜學長、良佑、Mickey、小猪、小嘉學 . ..長、德富學長、惠晶學姐、蔡欣瑩小姐、黃昆平學長 你們在研究領域的專業,學弟仰之彌高;與老闆過招的種種情節,學弟百聽 不厭。感謝你們一路上的經驗分享,讓學弟不致重蹈覆轍。
交大同學 孝蔚、黃俊清、阿家、淑慧、汪立亨、邱麗娟 每每想起交大生活,最難忘的就是那段一起當菜鳥日子,這麼多年過去,始 終如此。 學弟學妹 治明、博仁、國倫、柏霖、育生、佩盈、玉芳、彥文、國容、惟升、 .育銓、雅婷、昭業、中彬、老周、耀德、阿茂 學長需要你們的時候,你們都在。感謝你們給我充分的尊重與實驗上適時的 協助。實驗室有你們,變的好好笑………^_^ 三明治同學 日新、敏綺、宏宜、名瑩、立鼎、衍志、小敏、其璜、美瑤、美怡、 醇鴻、正恩、文威、Céline、至玟、騰合 一個意外的緣分讓大家在德國相識,那段同甘共苦的日子,每天想都不會膩。 教會朋友 鄭姐、簡哥、焜哥 你們對信仰的執著及對我們的用心,實在令我感動。謝謝你們每週給實驗室 所有成員及我們家人的祝福。
Content
摘要………..………...i Abstract……….iii 誌謝………...vi Content………viii Figure list………..xi Table list……….………..xv Preface…………..………....…...…...1Chapter 1 Literature Review: Preparation and Characterization of Polyimides………5
1.1 Preparation………....6
1.1.1 Two-step method………..6
1.1.2 One-step method………...13
1.1.3 Vapor deposition polymerization………....13
1.2 Characterization………..17
1.2.1 General charge transfer theory………....17
1.2.2 Polyimide properties influenced by CT interactions………..20
References………..25
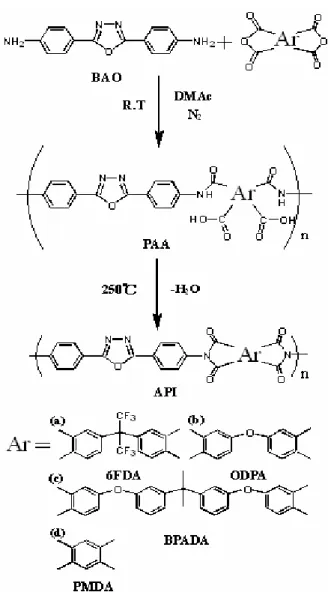
Chapter 2 Electroluminescence and Electron Transport Characteristics of Aromatic Polyimides....28 2.1 Introduction………29 2.2 Experimental section………..30 2.2.1 Materials……….………...….30 2.2.2 Preparation of PI films………...……...30 2.2.3 Devices fabrication……….30 2.2.4 Characterization………..31
2.3 Results and discussion...………...…..32
2.4 Conclusions………...………..41
References………..42
Chapter 3 Vapor Deposition Polymerization of Aromatic Polyimides for Electroluminescent Devices………...44
3.2 Experimental section………..46
3.2.1 Materials……….………...….46
3.2.2 Vapor deposition polymerization………...……... 47
3.2.3 Characterization………..47
3.3 Results and discussion...………...………48
3.4 Conclusions………...………...55
References………..56
Chapter 4 Characterization of Polyimide/ZnO Nanohybrid Films………....57
4.1 Introduction……….58
4.2 Experimental section………...59
4.2.1 Materials……….………...….59
4.2.2 Synthesis of ZnO-TPM nanoparticles………59
4.2.3 Preparation of PI/ZnO nanohybrid films………....59
4.2.4 Characterization………..61
4.3 Results and discussion.………...…………61
4.4 Conclusions………...………...70
References……….72
Chapter 5 Fabrication of ZnO Nanocrystals by Evaporating Oxidizing Two-step Approach………..73
5.1 Introduction……….74
5.2 Experimental section………...74
5.3 Results and discussion.………...………….75
5.4 Conclusions………...………...79
References………..81
Chapter 6 Formation of Inverted Hexagonal Liquid Crystal in Mixtures of Amine-metal Hydroxides……….………....82
6.1 Introduction……….83
6.2 Experimental section………...84
6.2.1 Materials……….………...….84
6.2.2 Preparation of HII phase liquid crystals…. ………84
6.2.3 Characterization………..85
6.3 Results and discussion.………...…………85
6.4 Conclusions………...………...91
Chapter 7 Preparation of Silver Nanocables from Self-assembling
Tubular Silver ion Precursors...94
7.1 Introduction……….95
7.2 Experimental section………...95
7.2.1 Materials……….………...….95
7.2.2 Preparation of silver nanocables……….96
7.2.3 Characterization………..96
7.3 Results and discussion.………...…………97
7.4 Conclusions………...………....104
References………105
Summary………106
Publication……….109
Figure list
Preface
Figure 1 Chemical structure of Kapton H………...1
Chapter 1 Literature Review: Preparation and Characterization of Polyimides Figure 1.1 Chemical reaction of polyimide formation………...…...6
Figure 1.2 Formation mechanism of poly(amic acid)………..…………...7
Figure 1.3 Mechanism of thermal imidization………..12
Figure 1.4 Schematic setup of vapor deposition system………...14
Figure 1.5 Reaction mechanism for the formation of VDP polyimides………....16
Figure 1.6 Orbital charge density for HOMO and LUMO in PMDA–ODA…....18
Figure.1.7 Schematic diagrams for: (a) HOMO–LUMO and HOMO–second LUMO transitions; (b) CT emission in PMDA–ODA…….…...19
Figure 1.8 . Torsional angle ω and the intramolecular donor/acceptor nature of the polyimide………....20
Figure 1.9 Fluorescence spectra of several commercial PI films (50 μm thick) upon excitation at 300 nm………....21
Figure 1.10 .Photocurrent responses as a function of applied electric field for ..7.5 μm thick Kapton film and DMA–loaded Kapton film upon ..excitation at 480 nm………....….22
Chapter 2 Electroluminescence and Electron Transport Characteristics of Aromatic Polyimides Figure 2.1 Monomer structures and the synthesis route of poly(amic acid) and . polyimides………...31
Figure 2.2 Absorption spectra of the API films with 20 μm thickness…….…....34
Figure 2.3 Fluorescence spectra of the APIs films with 20 μm thickness………35
Figure 2.4 WAXD patterns of the APIs films with 20 μm thickness…………...35
Figure 2.5 Polyimide chain–chain interaction (a)CT interaction and (b)crystal..36
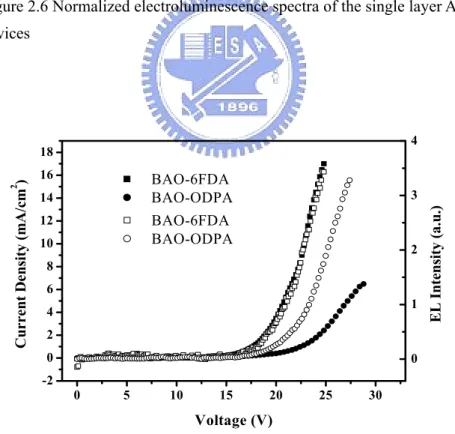
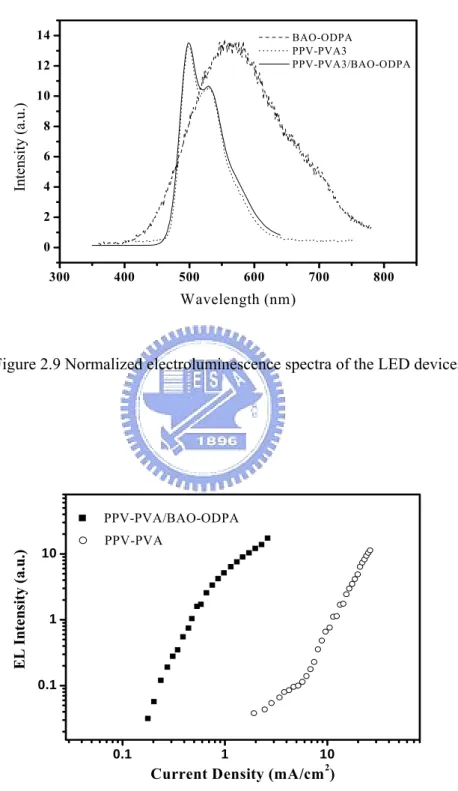
Figure 2.6 Normalized electroluminescence spectra of the single layer API– LED device………..37
Figure 2.7 ..Current density–voltage characteristic (closed mark) and EL intensity –voltage (open mark) for the single layer API–LED devices………..37
Figure 2.8 EL efficiency of single layer API–LED devices……….38
Figure 2.10 .EL efficiency of the LED devices: ITO/PPV–PVA/Al and
ITO/PPV–PVA/BAO–ODPA/Al...39
Figure 2.11 .Band diagram of ITO/PPV–PVA/BAO–ODPA/Al LED device... 40
Chapter 3 Vapor Deposition Polymerization of Aromatic Polyimides for Electroluminescent Devices
Figure 3.1 Monomer structures and the preparation route of PIs……….………46 Figure 3.2 FT-IR spectra of PI thin films (a) BAO–6FDA and (b) BAPF–6FDA before and after imidization……….48 Figure 3.3 Surface morphologies of the PI thin films (800Ǻ) (a) BAO–6FDA and (b) BAPF–6FDA by vapor deposition; (c) BAO–6FDA by wet
coating………..49
Figure 3.4 UV–Vis absorption spectra of BAO–6FDA and BAPF–6FDA PI thin films………..50 Figure 3.5 Electroluminescent spectra of PI–LED devices: ITO/BAO–6FDA/Al and ITO/BAPF–6FDA/Al...51 Figure 3.6 (a) Current density-voltage and (b) brightness-voltage characteristics
of PI-LED devices ITO/BAO-6FDA/Al with different PI thickness: 150Å, 300Å and 600 Å………...………...52 Figure 3.7 (a)Current density-voltage and (b)brightness-voltage characteristics
of PI-LED devices ITO/BAPF-6FDA/Al with different PI thickness: 150Å, 300Å and 600Å….……….…..52
Figure 3.8 Brightness-current density characteristics of PI-LED devices with different PI thickness…….…………...………..53 Figure 3.9 Cyclic voltammograms of polyimides (a) oxidation curves (b)
.reduction curves………..54
Figure 3.10 .Band diagrams of ITO/BAO-6FDA/Al (left) and ITO/BAPF-6FDA/ Al (right)………..54
Chapter 4 Characterization of Polyimide/ZnO Nanohybrid Films
Figure 4.1 Monomer structures and the synthetic route of PI/ZnO hybrid
.films……….………...……60
Figure 4.2 FT-IR spectra of BTDA–ODA/ZnO hybrid films………...62 Figure 4.3 XPS wide-scan spectrum of BTDA–ODA/ZnO 5 wt.-% film………62 Figure 4.4 Thermal gravimetric profiles of unmodified ZnO nanoparticles and TPM–stabilized ZnO nanoparticles……….63 Figure 4.5 Zn 2p core-level spectra of various BTDA–ODA/ZnO hybrid films With different ZnO content……….64
Figure 4.6 Illustration of the interaction between ZnO nanoparticles and PI
chains……….………..64 Figure 4.7 Dynamic mechanical storage moduli of BTDA–ODA/ZnO and
PMDA–ODA/ZnO hybrid films………..66 Figure 4.8 Dynamic mechanical tan δ curves of the PI/ZnO hybrid films BTDA– ODA/ZnO and PMDA–ODA/ZnO………66
Figure 4.9 Deconvolution of tan δ curves of BTDA–ODA/ZnO nanohybrid films…67 Figure 4.10 Dynamic thermal gravimetric profiles of BTDA–ODA/ZnO and
.PMDA–ODA/ZnO nanohybrid films………...……..…..68
Figure 4.11 TEM images of (a) TPM–stabilized ZnO nanoparticles (b) BTDA– .ODA/ZnO–5 wt/.-%, and (c) PMDA–ODA/ZnO–5 wt.-% hybrid
films……….69
Chapter 5 Fabrication of ZnO Nanocrystals by Evaporating Oxidizing Two-step Approach
Figure 5.1 XRD pattern of ZnO samples as-deposited and after oxidized at
350 ℃ for 2h………..76
Figure 5.2 Top view (a–c) and side view (d–e) FE-SEM images of the ZnO samples fabricated on quartz substrate at three different deposition time 2 min, 5 min and 10 min, respectively, and then oxidized at 350 ℃ for 2 h……….…77 Figure 5.3 (a) Low magnification TEM image of the ZnO nanocrystals and
high magnification TEM images (inset); (b) HRTEM image and the SAED patten (inset)………...78 Figure 5.4 (a) FE-SEM images of the ZnO samples fabricated on PI substrates; (b) TEM image of the same sample……….78 Figure 5.5 Room temperature PL spectra of the ZnO nanocrystals for deposited 10 min on quartz substrates……….………….79
Chapter 6 Formation of Inverted Hexagonal Liquid Crystal in Mixtures of Amine-metal Hydroxides
Figure 6.1 Phase diagram for the ternary mixture oleic acid/1-decanol/
ammonium hydroxide system at 25 °C………86 Figure 6.2 POM textures of oleic acid/1-decanol/ ammonium hydroxide
(35/38/27) at (a) 70 °C and (b) 25 °C recorded during the cooling
from Ti (95 °C)……….87
Figure 6.3 X-ray diffraction patterns of oleic acid/1-decanol/ ammonium
Figure 6.4 Schematic representation of a three-dimensional network of the inverted hexagonal phase……….………..89 Chapter 7 Preparation of Silver Nanocables from Self-assembling
Tubular Silver ion Precursors
Figure 7.1 Images of the dark-field optical microscopy for a diamminesilver-(І) oleate gel at a molar ratio of 30 before (a, b) and after (c) dispersion
in ethanol……….……….97
Figure 7.2 TEM micrographs (unstained) of a diamminesilver-(І) oleate gel at a molar ratio oleic acid/Ag+ of 30 (a) and 50 (b-d)…….……….98
Figure 7.3 Bright-field TEM micrographs of nanocables after reduction of a gel with a molar ration oleic acid/Ag+ of 50 (a-c) and a dark-field TEM micrographs (d, top) and the corresponding silver profile across the nanocable (d, bottom)………..99 Figure 7.4 TEM micrographs of the reduction products made by fast addition
of KOH (a-c) and substituting NaBH4 for formaldehyde (d)…...…..100
Figure 7.5 Schematic structure of the oleic acid/Ag+ nanotube………..101
Figure 7.6 FT-IR spectra of oleic acid, the nanotubes, and silver nanocables....103
Table list
Chapter 1 Literature Review: Preparation and Characterization of Polyimides
Table 1.1 Electron affinity of aromatic dianhydridesa………..9
Table 1.2 Basicity pKa of diamines and their reactivityes toward PMDA….…...9
Chapter 2 Electroluminescence and Electron Transport Characteristics of Aromatic Polyimides
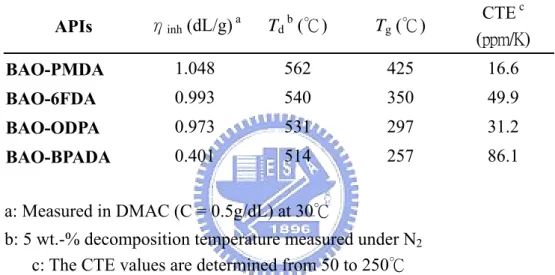
Table 2.1 Inherent viscosity of the PAAs and thermal properties of the APIs....33
Chapter 3 Vapor Deposition Polymerization of Aromatic Polyimides for Electroluminescent Devices
Table 3.1 Electrochemical potentials and energy levels of the polyimides……55
Chapter 4 Characterization of Polyimide/ZnO Nanohybrid Films
Table 4.1 Coefficient of thermal expansion of pure PI and PI/ZnO nanohybrid films……….65 Table 4.2 Glass transition temperaturesa of pure PI and PI/ZnO nanohybrid
films……….68 Table 4.3 Thermogravimetric analysis of pure PI and PI/ZnO nanohybrid
films……….69
Chapter 6 Formation of Inverted Hexagonal Liquid Crystal in Mixtures of Amine-metal Hydroxides
Table 6.1 Phase behaviour of the ternary mixtures………..……...87
Preface
Polyimide (PI) is a class of representative high-performance polymer possessing cyclic imides and aromatic groups in the main chain. The most familiar PI is widely known as Kapton H® (Figure 1.1), which was first commercialized in early 1960s. Thereafter, a number of PIs have been synthesized and investigated extensively in aspects of structure and property relationships and applications [1,2].
N N O O O O O
Kapton H
Figure 1.1 Chemical structure of Kapton H.
In general, PI is known to be thermally stable due to its heterocyclic imide rings on the backbone, and the thermal stability is further significantly improved by incorporating aromatic rings on the backbone and/or side groups. In addition to such high thermal stability, the nature of the chemical structure consisting rigid imide and aromatic rings always provides excellent mechanical and dielectric properties as well as high chemical resistance. Beyond these advantageous properties, a variety of functionalities, for instance, photoreactivity, molecular recognition ability, nonlinear optical responsibility, light-emitting ability and so on, can be added into the backbone and/or side groups of PIs, depending on their demands in applications [3]. Due to these advantageous properties and the functionalities, PIs have found diverse applications in the microelectronics, flat panel display, aerospace, and chemical and environmental
industries as flexible circuitry carriers, stress buffers, interdielectric layers, passivation layers, liquid crystal alignment layers, varnishing resins, fibers, matrix materials, and gas and chemical separation membranes [4-7].
Display and nano technology are two hottest topics no matter in academic research or high-tech industries in recently years. To further understand the potential application of PI in these two fields, the first five chapters of this thesis work aims at the studies as shown below:
1. PI-based polymer light-emitting diodes 2. PI/ZnO nanocomposites
In Chapter 1, the state of the literature is summarized regarding the objective of this thesis. This chapter first highlights in different preparation methods of PIs, including two-step and one -step wet-chemical approach, vapor deposition polymerization, thermal and chemical imidization. The reaction mechanisms and the effect of monomers and experimental conditions are also described. The second part of this chapter focuses on the charge transfer in PIs and the influence on PI properties.
Chapter 2 describes the newly synthesized 2,5-Bis(4-aminophenyl)- 1,3,4-oxadiazole (BAO) based PIs. The relationship between the different dianhydride structures and molecular aggregation were studied by X-ray diffraction analysis, and the leading charge transfer interaction in PIs and the fluorescence behavior were also discussed. Furthermore, single layer and double layer PI based light-emitting diodes were prepared. The electron transport characteristics of the PIs and the device characteristics were further investigated.
In Chapter 3 is focus on using vapor deposition polymerization to fabricate BAO/4,4 ′ -(hexafluoro-iso-propylidene) diphthalic anhydride (6FDA) and 4,4’-(9-Fluorenylidene)dianiline ( BAPF )/6FDA single layer light-emitting diode. A series of the device character are presented. The morphology and quality of PI films were observed by atomic force microscopy and compared with that prepared by wet chemical route. In addition, the effect of the diamine structure on device efficiency was discussed.
Chapter 4 presents the study of the incorporation of ZnO nanocrystals in PI matrix in formation of PI/ZnO nanohybrid films. Two PIs with different rigidity, 3,3’,4,4’-benzophenonetetracarboxylic acid dianhydride (BTDA)/
4,4’-diaminodiphenylether (ODA) and pyromellitic dianhydride (PMDA)/ODA, were used in this study. The thermal, mechanical and morphology characteristics of the hybrid films were established. Also the effect of PI structures on the variation of ZnO particle size, and the structural change of ZnO nanoparticles before and after thermal imidization were reported.
Chapter 5 describes using a convenient thermal coater and an air-circulating to carry out thermal evaporation and oxidization processes, respectively, in formation of ZnO nanocrystals at low temperature on quartz and flexible PI film substrates. The structure and photoluminescence properties of the resultant ZnO nanocrystals are studied.
In addition, an additional research subject, which is about preparation of one-dimension nanomaterials, is incorporated in chapter 6 and 7. This is the research results when I gained the scholarship of 2004 NSC-DAAD Sandwich Program and worked in DWI an der RWTH Aachen, Germany, supervised by Oliver Weichold, in the last one and a half year of my PhD program.
Chapter 6 presents the lyotropic liquid crystal that preferentially forms the inverted hexagonal phase by using ternary system consisting of oleic acid/1-decanol/ammonium hydroxide solution. Oleic acid was further replaced by stearic acid and elaidic acid in order to understand the effect of monomer structures on liquid crystalline phase. Besides, various metal ions, such as Ag+, Cu2+, Ni2+, Co2+, Zn2+, and Cd2+, were doped in the prepared the inverted hexagonal liquid crystalline phase. This inverted hexagonal phase system is suggested that may serve an easy route in preparation of nanorods and nanowires.
In Chapter 7 describes a simple and efficient method to fabricate silver/oleic acid nanocables. The tubular diamminesilver (І) oleate which acted as template was prior prepared. And then directly reduced the tubular precursor by formaldehyde, the silver/oleic acid nanocables can be obtained. The reducing conditions and the molar ratio of oleic acid/silver ion show great influence on the morphology of the final products.
References
[1] M. K. Ghosh and K. L. Mittal, Polyimides: Fundamentals and Applications, Marcel Dekker: New York, 1996.
[2] D. Wilson and P. Hergenrothe (Ed:H. D. Stenzenberger), Polyimides, Chapmam & Hall, London, 1990.
[3] Z. Ahmad, J. E. Mark. Chem. Mater. 2001, 13, 3320.
[4] M. J.M. Abadie, V. Y. Voytekunas, A. L. Rusanov. Iranian Polym. J. 2006. 15. 65.
[5] M. Ree, T. J. Shin, S. W. Lee. Korea Polym. J. 2001, 9, 1. [6] M. Ree. Macromol. Res. 2006, 14, 1.
Chapter 1
Literature Review:
1.1 Preparation
Polyimides (PIs) are generally classified as soluble and nonsoluble polymers depending on their solubilities [1-3]. A few soluble aliphatic and aromatic PIs have been reported so far [4,5]. Most aromatic polyimides cannot be processed because they are insoluble and have a high glass transition temperature. Thus, they are first synthesized in a soluble precursor form and then processed in various ways, before finally being converted to PIs. Poly(amic acid) (PAA) and poly(amic dialkyl ester), which are representative soluble precursors, are usually processed first as a specific form depending on applications and then imidized in formation of PIs.
1.1.1 Two-step method
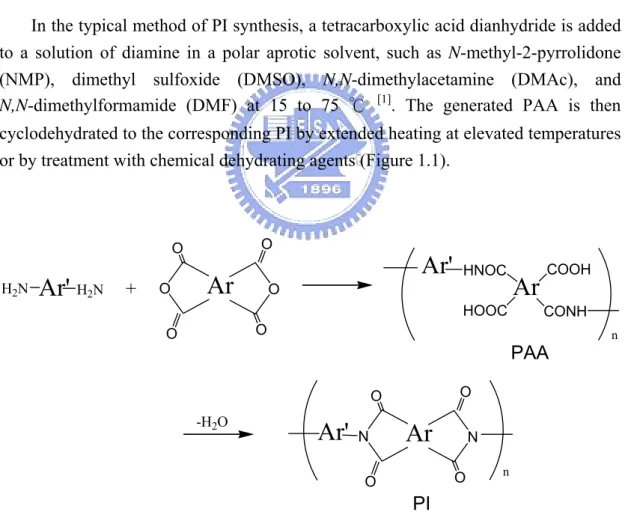
In the typical method of PI synthesis, a tetracarboxylic acid dianhydride is added to a solution of diamine in a polar aprotic solvent, such as N-methyl-2-pyrrolidone (NMP), dimethyl sulfoxide (DMSO), N,N-dimethylacetamine (DMAc), and N,N-dimethylformamide (DMF) at 15 to 75 ℃ [1]. The generated PAA is then cyclodehydrated to the corresponding PI by extended heating at elevated temperatures or by treatment with chemical dehydrating agents (Figure 1.1).
H2N
Ar'
H2NAr
O O O O O O +Ar
COOH CONH HNOC HOOCAr'
-H2OAr
N O O N O OAr'
PAA PI n nPoly(amic acid)
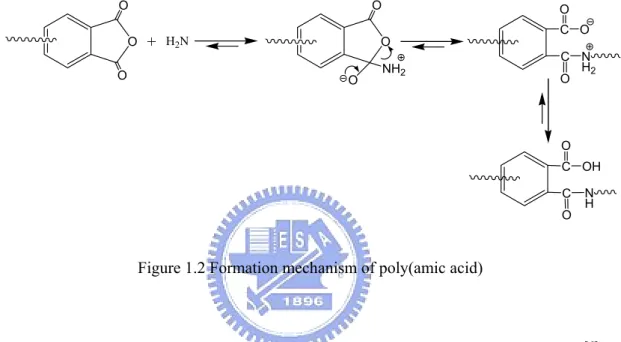
The molecular weight of the resultant PAA has great influence on final PI’s physical properties; simply speaking, higher PAA molecular weight possesses better PI physical properties [2]. The reaction mechanism of PAA formation involves the nucleophilic attack of the amino group on the carbonyl carbon of the anhydride group, followed by the opening of the anhydride ring to form amic acid group (Figure 1.2).
O O O + H2N O O NH2 O C C O O O N H2 C C O O OH N H
Figure 1.2 Formation mechanism of poly(amic acid)
The most important aspect of this process is that it is an equilibrium reaction [6]. Often it appears to be an irreversible reaction because a high molecular weight PAA is readily formed in most cases as long as pure reagents are used. This is because the forward reaction is much faster than the reverse reaction, often by several orders of magnitude. If the large reaction rate difference is not met, the high molecular weight PAA is not formed. Therefore, it is important to examine the driving forces that favor the forward reaction over the reverse reaction. It should be note that the acylation reaction of amines is an exothermic reaction and that the equilibrium is favored at lower temperatures. The forward reaction in dipolar solvents is a second order reaction and the reverse reaction is a first order reaction. Therefore, the equilibrium is favored at high monomer concentrations to form higher molecular weight PAAs.
Solvent effect
increases as the solvent becomes more polar and more basic [7,8]. For instance, the results of one model compound study showed that the rate of acylation increases with solvent in the order tetrahydrofuran (THF)<acetonitrile<DMAc<m-cresol [1]. The large rate constant obtained in m-cresol suggests that the solvent also functions as an acid catalyst.
Monomer reactivity
As mentioned above the mechanism of PAA formation is a nucleophilic substitution reaction at one of the carbonyl carbon atoms of the dianhydride with diamine. Therefore, it is expected that the reaction rate is primarily governed by the electrophilicity of the carbonyl groups of dianhydride and the nucleophilicity of the amino nitrogen of the diamine. The two carbonyl groups on a dianhydride are situated at the ortho position to each other and their strong electron-withdrawing effect activates each other towards nucleophilic reaction. The effect is particularly enhanced by the preferred carbonyl conformation locked in the coplanar aromatic ring.
Pyromellitic dianhydride (PMDA) displays the most reactive among the commercially available dianhydrides. It possesses four carbonyl groups attached to the same benzene ring in a coplanar conformation and, therefore, shows the strongest tendency to accept an electron. The electrophilicity of carbonyl carbons of each dianhydride can be measured in terms of the electron affinity (Ea), a tendency to
accept an electron, of the molecule (Table 1.1). For dianhydrides with the bridged bisphthalic anhydride structure, the bridge groups strongly influence Ea of
dianhydrides. Compared with 3,3’4,4’-biphenyltetracarboxylic dianhydride (BPDA), which lacks a bridge group, the electron-withdrawing bridge group such as SO2 and
C=O increase the Ea values substantially, while electron-donating group such as
ethers decrease it. The reactivity of ether-containing dianhydrides is significantly reduced so that they are practically not affected by the atmospheric moisture [6] while PMDA and 3,3’4,4’-benzophenonetetracarboxylic dianhydride (BTDA) must be handle under strictly moisture-free conditions at all times.
Unlike the Ea value of dianhydrides, the ability of diamines to give off an electron,
the ionization potential, does not seem to correlate well in a simple manner, although the reaction rates of diamines towards a given dianhydride generally increase with increase in their ionization potential. The reactivity of diamines instead correlates well with its basicity (pka) in Hammett’s relation [7]. The reaction rates (k) of various
diamines toward PMDA are shown in Table 1.2 in relation to their pka. The structure
of diamines seems to influence the rate of acylation reaction more than the variation in dianhydrides structure. It should be noted that the rate constants differ by four orders of magnitude between amines with electron donating substituents and those with electron withdrawing ones. If reactive dianhydrides such as ether containing dianhydride are reacted with 4,4’-diaminodiphenylsulfone or 4,4’-diaminobenzophenone, it is expected that the molecular weight of the resulting PAAs would be lower.
diamines toward PMDA are shown in Table 1.2 in relation to their pka. The structure
of diamines seems to influence the rate of acylation reaction more than the variation in dianhydrides structure. It should be noted that the rate constants differ by four orders of magnitude between amines with electron donating substituents and those with electron withdrawing ones. If reactive dianhydrides such as ether containing dianhydride are reacted with 4,4’-diaminodiphenylsulfone or 4,4’-diaminobenzophenone, it is expected that the molecular weight of the resulting PAAs would be lower.
Table 1.1 Electron affinity of aromatic dianhydrides [1]
Table 1.2 Basicity pKa of diamines and their reactivityes toward
Effect of reaction conditions
Early workers in the field of PI chemistry found that a higher molecular weight PAA could be produced by using higher concentrations of monomers [2]. It was also discovered that the molecular weight of the product was influenced by the order and mode of monomer addition, with the highest molecular weight being obtained when solid dianhydride was added to a solution of diamine. In fact, a slight stoichiometric excess of dianhydride was used to enhance the molecular weight. Temperature was also found to be critical, with the best result being obtained when polymerization was run between –20℃ and 70℃ [9,10].
The fact that the highest molecular weight PAA was obtained when solid dianhydride was added to a diamine solution was first thought to be due entirely to the avoidance of side reactions [11]. Aromatic dianhydrides were known to react with water and other impurities in amide solvents. However, since it was known that their reactions with diamines were considerably faster, it was correctly reasoned that competing reactions could be minimized by adding the dianhydride as a solid.
The solubility of dianhydrides is another important factor that influences the molecular weight of PAA. The solid dianhydride does not dissolve immediately upon its addition to a diamine solution [10]. In fact, its rate of dissolution is slow and dependent upon the concentration of monomers. Above, a certain critical concentration, which depends on the solubility of the dianhydride in the solvent and reactivity of the monomer, the rate of dissolution becomes slower than the rate of polymerization. The consequence of this is that some very high molecular weight product is formed almost immediately, long before all the dianhydride has dissolved and a stoichiometric balance of monomers has been achieved [12]. Some low molecular weight product is also formed in regions where the monomer stoichiometric is out of balance. Thus, the final polymer has a broad molecular weight distribution (MWD, >2) that is often bimodal. Its weight-average molecular (Mw)
and intrinsic viscosity are also considerably higher than those that could be obtained in a reaction-controlled, step-growth process [13].
Another early observation was that the viscosities of PAAs rapidly decreased when they were stored after preparation [9,11]. This was most often attributed to the sensitivity of amic acid to hydrolysis. Later work, however, showed that the phenomenon is associated with the reversibility of the propagation reaction and the fact that the polymers were produced by interfacial polymerizations [14]. The rate
constant for the reverse reaction is quite small, which means that only a few reactions take place during the first few hours and days of storage. These reactions, however, have a dramatic effect on the Mw of the polymer. The longest chains, which are a
result of the interfacial nature of the polymerization and are the most significant in the determination of Mw, are the ones most affected by a single chain cleavage. PAAs will
re-equilibrate in solution leading to the Mw decrease. The decrease in Mw can be
slowed and often prevented by storing the solutions below 10℃. Of course, if the temperature is allowed to increase, the Mw will immediately begin to decrease. The
most effective way to maintain the artificially high Mw values is to prevent the reverse
reaction completely. This can be accomplished by neutralizing the carboxylic acid groups with bases, such as tertiary amines, so that there are no protons available to initiate the reverse reaction. Similar reasoning can be used to explain why esterified PAAs decrease in molecular weight upon storage [15].
Thermal imidization
The most widely used second step in the two-step method of PI synthesis involves heating the PAA in the solid state to 250−400℃. This is usually preceded by a processing operation, whereby a PAA solution is used to cast a film, form a coating, or spin a fiber. Molding powders are also prepared by the precipitation of PAAs from various solutions [11]. Too rapid a heating may cause the formation of bubbles in the sample. One of the most popular heating cycles consists of one hour at 100℃, followed by one hour at 200℃, and followed by one hour at 300℃ [1,2]. However, in one study on thin films, the maximum degree of imidization that could be obtained with such a stepwise cycle was 85%. Much higher degree of imidization, i.e. >99% was obtained by constant heating at 230−250℃ for 10 minutes [16].
The imidization proceeds rapidly at the initial stage and tapers off at plateau, which is a typical diffusion-limited kinetic process. As the degree of imidization increases, the Tg or stiffness of the polymer chain increases. When the Tg approaches
the reaction temperature, the imidization rate slows down markedly [1].
Possible reaction pathways for the imidization process are shown in Figure 1.3 [17]. The first point to note is that the time at which the proton attached to the nitrogen is lost is different in the two pathways. In the first, the proton is lost after cyclization; while in the second, it is removed prior or during the ring closure. Since the conjugate base of the amide is a considerably stronger nucleophile than the amide, ring
cyclization should occur considerably faster in the latter case. C C O O NH OH N O O O H H N O O HO H N O O H2O C C O O NH OH H C C O O NH O C C O O N OH N O O O H H H H2O N O O Pathway 1 Pathway 2
Figure 1.3 Mechanism of thermal imidization
The most commonly used method to determine the degree of chemical transformations in PIs is Fourier-transform infrared (FTIR) spectroscopy. Several characteristic absorption bands are used for the quantification of five-membered aromatic imides. The strongest absorption occurs at 1720 cm-1 (C=O) symmetrical stretching). However, the band partially overlaps with strong carboxylic acid band (1700 cm-1, C=O) of PAA. The more useful bands of imide groups are 1780 cm-1 (C=O asymmetrical stretching), 1380 cm-1 (C–N stretching), and 725 cm-1 (C=O bending). The absorption of anhydrides also occurs at 1780 and around 720 cm-1. When a mixture of imide and anhydride groups is analyzed, these bands may partially overlap with those of imide group, requiring proper correction. The carboxylic acid bands, 1700 cm-1 (C=O) and 2800−3200 cm-1 (OH), and amide bands, 1600 cm-1 (C=O, amide І), 1550 cm-1 (C–NH, amide Ⅱ) and bands at 3200−3300 cm-1 (NH) often appear as broad peaks, particularly when they are strongly associated with hydrogen bonds [1].
Chemical imidization
PAAs can be converted to the corresponding PIs at ambient temperature by treatment with mixture of aliphatic carboxylic acid dianhydrides and tertiary amines
[18,19]. Acetic anhydride and pyridine or triethyl amine are normal used. Although this
chemical method does not have the energy requirement of thermal imidization, it is generally not used commercially because of the problems associated with handling the reagents. The one exception is in the manufacture of molding powders, which are often prepared by this technique.
1.1.2 One-step method
PIs that are soluble in organic solvents are often prepared by the so called one-step or single stage method [1,2]. In this procedure, the dianhydride and diamine are stirred in a high-boiling organic solvent at 180−220 ℃. Under these conditions, chain growth and imidization essentially occur spontaneously. The water generated by imidization is usually allowed to distill from the reaction mixture. Nitrobenzene, α-chloronapthalene and m-cresol containing isoquinoline are the most widely used solvents.
The one-step method is especially useful in the polymerization of unreactive dianhydrides and diamines. For example, phenylated dianhydrides cannot be used to prepare high molecular weight PAAs. These arterially hindered monomers, however, react rapidly with diamines at elevated temperature to give high molecular weight PIs
[20,21]. Another interesting feature of the one-step method is that it can yield a material
with a higher degree of crystallinity than can be obtained from the two-step method. The excellent salvation of the polymer at high temperature may allow it to obtain a more favorable conformation for packing.
1.1.3 Vapor deposition polymerization
Vapor deposition polymerization (VDP) is an alternative to the industrially important spin-coating method to prepare PI films [22, 23]. In the VDP process the diamine and the dianhydride are co-evaporated onto a substrate where they react to form a PI precursor, which converts to PI upon thermal curing. VDP is a dry process and does not require the use of solvents. This can be advantageous in some
applications, where the use of solvents can have deleterious effects or where contaminations have to be strictly avoided. VDP can be used to prepared thin PI films in nonplanar substrates, which is not possible using the spin-coating method. However, VDP is a slower and more expensive process compared to spin coating and this makes it less suitable for large-scale production.
The preparation of PI films by VDP was introduced independently by Iijima et al. in Japan [22] and by Salem et al. [23] in the United States in 1985 and 1986, respectively. In 1987 Grunze and Lamb demonstrated its feasibility for in situ PI/metal interface studies [24,25]. In most of subsequent investigations of PI films prepared by vapor depositing oxydiamine (ODA) and PMDA were used as diamine and dianhydride components, respectively. Only in a few cases other diamine and/or dianhydride precursors, such as decamethylene diamine (DMDA) [26,27], m- and p-phenylene diamine (m- and p- PDA) [28,29], 4,4’-diaminobiphenyl disulfide (DAPS) [30,31], benzidine (BZD), 2,2’-bis(trifluoromethyl)-4,4’-diaminobiphenyl (TFDB), and 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) [32] have been used.
Deposition system
A typical VDP system consists of a vacuum chamber equipped with two separately heatable sources for the diamine and the dianhydride components, a system to control the fluxes from the sources, a shutter system, and a sample holder (Figure 1.4) [33]. The source cell which are commonly made out of metals or metal alloys are heated resistively or radiatively to temperature up to 200℃ in an ultra-high-vacuum. A fast and uniform heat transfer to the cells is necessary to obtain a good control over evaporation rate and ratio of the precursors. Quartz crystal oscillators are frequently used to monitor the evaporation rates from the source cells. Once calibrated, the source cells are heated to predetermined temperatures to obtain the desired deposition rate and ratio. A shutter is placed between evaporator and sample to avoid contamination of the sample prior to deposition. Any solid sample can be used as a substrate. The deposition is conformal rather than planar and the coverage of the substrate is determined by the distance and orientation of the substrate relative to the evaporator.
Mechanism of the VDP polyimides
The reaction scheme for the formation of PMDA/ODA PI by VDP is shown in Figure 1.5. After co-deposition on to a substrate kept at room temperature, a mixture between reacted and unreacted material is obtained [22,23]. The FTIR spectrum shows bands at 1850 and 1780 cm-1 due to anhydride carbonyl groups. They have been interpreted as unreacted trapped PMDA molecules. As an alternative, these bands could indicate anhydride end groups of short oligomer chains. The line-shape of the anhydride bands of a room temperature deposit does not agree well with line-shape observed for unreacted PMDA in PMDA/ODA mixture. It rather resembles the line-shape for terminal anhydride groups in short oligomer chains [34].
XPS and FTIR measurements reveal that the room temperature deposit consists mostly of the intermediate C or an ammonium carboxylate salt of PAA (D) formed through the reaction of the carboxylic acid with free amino groups [35]. The carbonyl band for the carboxylic acid group of form E expected at 1720 cm-1 is almost entirely absent. The intermediate C is formed through the attack of the amine at an anhydride carbonyl group and opening of anhydride ring. High-resolution electron energy loss spectroscopy measurements on PMDA/p-PDA and PMDA/aniline further confirmed that intermediate C can be formed and that it is stable at room temperature.
growth. This step is accompanied by forming carboxylic acid groups of PAA. Complete imidization is achieved after heating at 250℃ for 30 min. Subsequent heating at 400℃ for 30 min does not result in significant additional changes in FTIR spectrum. Similar to spin-coated films the VDP films remains stable at this temperature. O O O
+
H2N C C O O N O H H C C O O H N OH C C O O H N O H3N + Base + Base - Base N O O N O N A B C D E F G -H2O -H2O -Base +Base -H2OFigure 1.5 Reaction mechanism for the formation of VDP polyimides. A, anhydride endgroup; B, amino endgroup; C, intermediate carboxylate form; D, ammonium carboxylate salt of poly(amic acid); E, poly(amic acid); F, polyimide with imine branching; G, polyimide.
1.2 Characterization
Wholly aromatic PIs, like Kapton, have such incredible mechanical and thermal properties that they are used in replace of metals and glass in many high performance applications in the electronics, automotive, and even the aerospace industries. These properties come from strong intermolecular forces between the polymer chains [1].
As mentioned above, the PAA polymerization rates are dominated by dianhydrides as an electron acceptor and diamines as an electron donor. This suggests that the PAA polymerization proceeds via a charge transfer (CT) complex intermediate [36]. Indeed, one often observes the formation of a yellow-green solution due to the CT complex at the initial polymerization stage, followed by yield of a viscous light-yellowish PAA solution.
Dine-art and Wright originally proposed that CT may occur in PIs and imides, based on color, melting point, and solubility data of model compounds [37]. Since that study, many workers have described aromatic PIs as chains of alternating segments of electron-donating and electron-accepting character in which either intramolecular or intermolecular CT occurs. Furthermore, they have used CT theory to explain a number of properties of imides and PIs, such as color, photodegradation, fluorescence, photoconductivity, electroconductivity, glass transition and melting temperatures [38].
This sector reviews the accumulated fundamental research results for the CT theory, CT in PIs and the effect of CT in PI properties.
1.2.1 General charge transfer theory
Intermolecular CT in polyimides
Mulliken developed a general theory for charge transfer that many have found useful for describing intermolecular charge transfer complex [39]. The CT complex is comprised of two species, one acting as an electron donor, the other acting as an electron acceptor. For most complexes, the ground state CT interactions are small; upon excitation by UV/Vis light, an intermolecular CT transition occurs such that electron density transfers from the donor to the acceptor. The resulting excited state is mostly comprised of the CT state. The lowest energy transition is often a result of interactions between the highest occupied molecular orbital (HOMO) of the donor
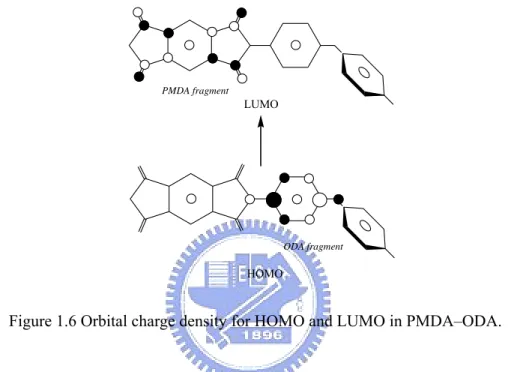
and the lowest unoccupied molecular orbital (LUMO) of the acceptor, which correspond closely to the ionization potential of the donor and the electron affinity of the acceptor, respectively. The schematic orbital charge density for HOMO and LUMO in the PMDA-ODA is shown in Figure 1.6. It should be noted that the charge at HOMO and LUMO is localized on the ODA and PMDA residues, respectively. This means that CT can take place via the one-electron HOMO to LUMO transition.
LUMO
HOMO
ODA fragment PMDA fragment
Figure 1.6 Orbital charge density for HOMO and LUMO in PMDA–ODA.
It is known that the UV/Vis absorption of the PMDA–ODA film consists of three peaks centered at 194 nm (6.4 eV), 210 nm (5.9 eV), 280 nm (4.4 eV), and a very weak absorption tail around 376 nm (3.3 eV) [40]. LaFemina et al. [40] on the basis of the experimentally measured adsorption spectrum, computed the electronic transition energies in PMDA-ODA using the spectroscopically paramerized CNDO/S3 model and compared with the experimental results.
The results of LaFemina et al. led to 413 nm (3.0 eV) as the HOMO-LUMO transition with a considerably low oscillator strength, corresponding the very weak lowest energy absorption tail in the PMDA-ODA film. The superjacent (second) LUMO also displays similar charge localization on the PMDA fragment, indicating that the HOMO-superjacent LUMO transition also causes CT. This transition at 280 nm (eV), which corresponds well to the actual absorption peak, occurs with a much higher transition probability (oscillator strength) than the HOMO-LUMO transition at 3.0 eV. In addition to these calculated transitions, the 320 nm band due to the pyromellitimide fragment, which is actually observed as a shoulder in the PMDA-ODA spectrum, should also be involved. Nonetheless, no 320 nm transition
was shown in the calculated transitions. Figure 1.7a shows an energy diagram draw by the present authors according to the descriptions mentioned above. LaFemina et al. also observed the very weak CT fluorescence upon excitation at 290 nm and proposed a simple emission mechanism in PMDA-ODA as shown in Figure 1.7b.
Figure 1.7 Schematic diagrams for: (a) HOMO−LUMO and HOMO−second LUMO transitions; and (b) CT emission in PMDA-ODA.
Intramolecular CT in polyimides
Analogous to the intermolecular charge transfer, intramolecular charge transfer can occur between contiguous donor and acceptor fragments [1]. One can describe the
electronic transitions of such systems in terms of two types: local transitions that correspond to the transitions of the unperturbed fragments, and intramolecular CT transitions that correspond to the transfer of electron density from the bonding molecular orbital of one fragment to the antibonding molecular orbital of adjacent fragment. An essential feature of this charge transfer is its dependence on the torsional angle, ω, defined as the angle between the planes of the donor and acceptor fragments (Figure 1.8). The probability for the intramolecular CT absorption transition should be highest in the limit of maximal π overlap, when the fragments are orthogonal. Furthermore, the intensity of the corresponding CT band has been shown experimentally to be proportional to cos2ω. Although deviations may occur for various reasons, Suzuki has explained this relationship with approximations leading to the cos2ω dependence of the dipole strength, oscillator strength, and molar extinction coefficient of the intramolecular CT complex.
N N O O O O n
ω
donor acceptorFigure 1.8 Torsional angle ω and the intramolecular donor/acceptor nature of the polyimide.
1.2.2 Polyimide properties influenced by CT interactions
Fluorescence
Hasegawa et al. [41] first demonstrated experimentally that wholly aromatic PIs films show CT fluorescence. The first approach was to measure the fluorescence spectra of several commercial PI films (50 μm thick). All the commercial samples used exhibited broad and structureless fluorescence in the long-wavelength range as shown in Figure 1.9. Another spectral feature is that the fluorescence intensity tends to reduce as the peak positions shift toward lower energy. This phenomenon is
characteristic of low molecular weight CT complex systems.
Figure 1.9 Fluorescence spectra of several commercial PI films (50 μm thick) upon excitation at 300 nm [41].
Wachsman and Frank [42] observed a fluorescence intensity enhancement with increasing final cure temperature for the PMDA-ODA system and proposed that this behavior is caused either by coplanarization between the benzimide and N-phenyl molecular conformational rearrangement, or by intermolecular CT complex formation promoted by dense molecular aggregation.
It seems not easy to separate the intermolecular and intramolecular CT fluorescence as for the CT absorption. However, it is most likely that the intermolecular CT mainly contributes to the fluorescence in the solid state of wholly aromatic PIs. This is according to the fact that the fluorescence intensity of the PI film prepared at 400 ℃ for 1h (rapid cure) was approximately 10 times higher than that cured upon the mildest condition (250 ℃/5h) [43]. In other words, the intramolecular CT fluorescence is much weaker than the intermolecular one. This may be rationalized in terms of a model compound result in which the benzimide-phenyl coplanarization leads to an essentially non-fluorescent character.
Photoconductivity
High-temperature PIs are used as an excellent electrical insulator in microelectronic devices. This is due to the fact that the CT complexes formed in PI films are classified into a category of weak CT complex, and therefore, have practically no contribution to the charge-separated structure at the ground state. Fainshtein et al. [44] showed that the increases in pressured and temperature cause a decrease in the electrical resistance in the dark for PIs derived from a fixed ODA with PMDA, BTDA, and 3,3’,4,4’-diphenylsulphonetetracarboxylic dianhydride. These results were explained in terms of an electron conductance mechanism based on interchain CT complex formation.
Freilich [45] reported that electron donor-loaded Kapton film results in a striking photocurrent enhancement by five orders of magnitude as compared to the donor-free film. Figure 1.10 displays the photocurrent of the 7.6 μm thick Kapton films containing 7.6 wt.-% DMA as a function of applied electric field (105–106 V cm-1) upon illumination at 480 nm. The difference absorption spectrum between DMA-loaded and DMA-free Kapton films showed a new broad band peaking at 460 nm, which coincides with the photocurrent action spectrum in position. Also, a linear relationship was observed between the transition energy and the ionization potential of the dopants. From these, he concluded that this photocurrent enhancement results from CT complex formation between the pyromellitimide fragment in the PI backbone and DMA.
Figure 1.10 Photocurrent responses as a function of applied electric field for 7.5 μm thick Kapton film and DMA-loaded Kapton film upon excitation at 480 nm [45].
Lee et al. [46] measured the photoconductivity of a semi-aromatic PI derived from PMDA and an alicyclic diamine, 4,4’-methylene bis(cyclohexylamine) (MBCHA). Unexpectedly, this CT-inhibiting semi-aromatic PI showed somewhat higher photoconductivity than CT-allowing PMDA-ODA over the whole electric field examined. For PMDA-MBCHA film cured at 200℃/1h, prolonged annealing at 200 ℃ increased the photocurrent by one order of magnitude. In contrast, dimethyl-substituted PI remains loosely packed owing to the steric hindrance even if annealed. On the basis of these results, a mechanism of charge carrier photogeneration was proposed for the PMDA-MBCHA: the pyromellitimide fragments weakly interact with the alicyclic portions of different chains under an assumption that PI chains take the mixed layer packing arrangement. Then, upon photoirration, the electron transfer occurs from the ground-state alicyclic portions to the excited state pyromelltimide fragment, finally charge carriers are produced by field-assisted thermal dissociation of the photogenerated exciplex to form separated ion pairs.
Thermal, mechanical and melt viscosity properties
Weak CT complexes look like exciplex in the viewpoint that both undergo charge separation only at the excited state. However, the latter has no attractive interaction between the donor-acceptor molecules at the ground state, whereas the former possesses a few kcal mol-1 of bonding energies even in solution at the ground state, comparable or lower than the hydrogen bonding energies [47]. The CT complexes in PIs may also be the case. An electron donor-acceptor miscible blend between
poly[(N-alkylcarbazol-3-yl)methyl methacrylate] and poly{2-[(3,5-dinitrobenzoyl)oxy]ethyl methacrylate} is a typical example. The
interchain donor-acceptor attractive interactions results in a pronounced positive deviation from the additive property in the Tg–composition curve [48]. DSC
thermograms confirmed that the blend includes the CT complexes formed exothermically.
Interchain CT interaction also may improve the mechanical properties of PI films. Sulzberg et al. [49] reported that 1:1 blending of anisylimino group-containing polycarbonate and nitro group-containing polycarbonate resulted in a higher tensile modulus (360,000 psi) than those of the component polymers (3335,000 psi for the donor polymer and 260,000 psi for the acceptor polymer). Similar mechanical property improvement was also observed in the 1:1 blend composed of N,N-dimethylamino-group and dinitro group pendant poly(propylene terephthalate)
polymers.
The interchain CT interactions in PIs tend to influence the softening behavior above Tg rather than the Tg’s themselves. St. Clair et al. [50] found that the addition of a
small amount of a low molecular weight diimide compound into high molecular weight thermoplastic PIs has resulted in considerably decreased melt viscosities without significant Tg decrease. Two possible mechanisms were proposed for the
pronounced results: the first is that the additives possessing a higher molecular mobility can have stronger interaction with the PI chains than the chains have with each other. Namely, the PI–additives CT interaction can break up the interchain CT interactions. In other words, this mechanism means that the CT interaction survive even in the molten state above the Tg. The second hypothesis is an Mw decrease of the
PI caused by transimidation during melt mixing above the Tg, although measurements
of the Mw were not conducted.
Voltage holding ratio in alignment layers for liquid crystal displays
One of the most important necessary conditions in recent LCD such as thin film transistor and super twisted nematic modes is to have higher voltage holding ratio (Rv
>95%) even in a high-temperature range (60℃), which is required for the display stability of LCD. Kikuchi et al. [38] synthesized a large numbers of PIs and measured the Rv values to clarify a relation with the PI structures using a cyano-containing LC.
Wholly aromatic PIs have a trend to show low values (40−70%). On other hand, in addition to wholly aliphatic PIs, semi-aromatic PIs exhibited very high values exceeding 90% except for the PIs using BAPP and BDAF as diamine components. These results lead to a speculation that the Rv is related to CT interactions in PIs,
although the detailed mechanism is still poorly understood. Rv depends on not only
the PI structure but also on the LC used. Much work has been carried out to improve this parameter through the chemical modification of LCs themselves, rather than through the survey of low Rv PIs.
References
[8] M. K. Ghosh and K. L. Mittal, Polyimides: Fundamentals and Applications, Marcel Dekker: New York, 1996.
[9] D. Wilson and P. Hergenrothe (Ed:H. D. Stenzenberger), Polyimides, Chapmam & Hall, London, 1990.
[10] C. E. Sroog. Prog. Polym. Sci. 1991, 16, 561.
[11] M. Ree, K. J. R. Chen, G. Czornyi, Polym. Eng. Sci. 1992, 32, 924.
[12] S. W. Lee, S. I. kim, B. W. Choi, B. Chae, S. B. Kim, M. Ree, Macromolecules 2003, 36, 6527.
[13] T. Takekoshi, J. E. Kochanowski, J. S. Manello, M. J. Webber, J. Polym. Sci. 1985, 23, 1759.
[14] A. Y. Ardashnikov, I. Y. Kardash, A. N. Pravednikov, Polym. Sci. 1971, 13, 2092.
[15] Y. S. Vygodskii, T. N. Spirina, P. P. Nechayev, Polym. Sci. 1977, 19, 1738. [16] G. M. Bower, L. Frost, J. Polym. Sci. 1963, A1, 3135.
[17] C. E. Sroog, A. L. Endrey, S. V. Abramo, C. E. Berr, J. Polym. Sci. 1965, A3, 1373.
[18] R. A. Dine-Hart, W. W. Wright, J. Polym. Sci. 1967, 11, 609.
[19] R. A. Orwoll, T. L. St. Clair, K. D. Dobbs, J. Polym. Sci., Polym. Phys. Ed., 1981, 19, 1385.
[20] C. C. Walker, J. Polym. Sci. Polym. Chem. 1988, 26 ,1649.
[21] W. Volksen, P. M. Cotts, in Polyimides: Synthesis, Characterisation and Properties, Plenum, New York, 1984, pp. 163-170.
[22] J. B. Huang, B. M. Gong, J. Vac. Sci. Technol. 1985, B3, 253. [23] A. I. Baise, J. Appl. Polym. Sci. 1986, 32, 4043.
[24] J. A. Kreuz, A. L. Endrey, F. P. Gary, C. E. Sroog, J. Polym. Sci. 1966, A1-4, 2607.
[25] M. M. koton, T. K. Meleshko, V. V. Kudryavtsev, Polym. Sci. USSR, 1982, A24, 791.
[26] M. M. koton, T. K. Meleshko, V. V. Kudryavtsev, Polym. Sci. USSR, 1982, 26, 2839.
[27] F. W. Harris, S. L. C. Hsu, High Perf. Polym. 1989, 1, 3.
[28] F. W. Harris, S. O. Norris, L. H. Lanier, n Polyimides: Synthesis, Characterisation and Properties, Plenum, New York, 1984, pp. 3-14.
[29] M. Iijima, Y. Takahashi, Ki Inagawa, A. Itoh, J. Vac. Soc. Jpn. 1985, 28, 437. [30] J. R. Salem, F. O. Sequeda, J. Duran, W. Y. Lee, R. M. Yang, J. Vac. Sci.
Technol. 1986, A4, 369.
[31] M. Grunze, R. N. Lamb, Chem. Phys. Lett. 1987, 133, 283. [32] M. Grunze, R. N. Lamb, J. Vac. Sci. Technol. 1987, A5, 1685.
[33] K. Iida, T. Nohara, K. Totani, S. Nakamura, G. Sawa, Jpn. J. Appl. Phys. 1989,
28, 2552.
[34] A. Kubono, H. Higuchi, S. Umemoto, N. Okui, Thin Solid Films 1993, 232, 256.
[35] B. G. Frederick, M. R. Ashton, N. V. Richardson, T. S. Jones, Surface Sci. 1993, 292, 33.
[36] N. V. Richardson, B. G. Frederick, W. N. Unertl, A. El Farrash, Surface Sci. 1994, 307, 124.
[37] A. Gölzhäuser, S. Panov, Ch. Wöll, Surface Sci. 1994, 314, 849. [38] A. Gölzhäuser, S. Panov, Ch. Wöll, Surface Sci. 1995, 334, 235.
[39] Y. Y. Maruo, Y. Andoh, S. Sasaki, J. Vac. Sci. Technol. 1993, A11, 2590.
[40] S. C. Chen, W. T. Whang, Thesis, A study on vapor deposition polymerization of polyimides and their electroluminescent characteristics in light emitting diodes (in Chinese), 2001, National Chiao Tung university, Taiwan.
[41] A. K. Saini, C. M. Carlin, H. H. Patterson, J. Polym. Sci. Polym. Chem. Ed. 1992, 30, 419.
[42] C. Hahn, T. Strunskus, D. Frankel, M. Grunze, J. Electron spectrosc. Relat. Phenom. 1990, 54/55, 1123.
[43] M. I. Bessonov, M. M. Koton, V. V. Kudryavtsev, L. A. Laius, Polyimides: thermally stable polymers, Plenum, New York, 1987.
[44] R. S. Dine-Hart, W. W. Wright, Makromol. Chem. 1971, 143, 189. [45] M. Hasegawa, K. Horie, Prog. Polym. Sci. 2001, 26, 259.
[46] R. S. Mulliken, Molecular Complexes, John Wiley & Sons, New York, 1969. [47] J. P. LaFemina, G. Arjavalingam, G. Hougham, J. Chem. Phys. 1989, 90, 5154. [48] M. Hasegawa, M. Kochi, I. Mita, R. Yokota, Polym. Prepr. Jpn. 1987, 36,
1158.
[49] E. D. Wachsman, C. W. Frank, Polymer 1988, 29, 1191.
[50] M. Hasegawa, M. Kochi, I. Mita, R. Yokota, Eur. Polym. J. 1989, 25, 349. [51] Y. Fainshtein, L. A. Igonin, G. A. Lushcheikin, Polym. Sci. USSR, 1976, 18,
661.
[52] S. C. Freilich, Macromolecules 1987, 20, 973.
[53] S. A. Lee, T. Yamashita, K. Horie, J. Polym. Sci. B 1998, 36, 1433.
[54] N. Mataga, T. Kubota, Molecular interactions and electronic spectra, Marcel Dekker, New York, 1970.
[56] T. Sulzberg, R. J. Gotter, J. Polym. Sci. A1 1970, 8, 2747.
[57] T. L. S. Clair, J. R. Pratt, D. M. Stoakley, H. D. Burks, Polyimides: materials, chemistry, and characterization, Elsevier, Amsterdam, 1989.

![Table 1.2 Basicity pK a of diamines and their reactivityes toward PMDA [1]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8690831.198271/26.892.239.683.886.1143/table-basicity-pk-diamines-reactivityes-pmda.webp)