Fibrinogenolytic Proteases Isolated from the Snake Venom
of Taiwan Habu: Serine Proteases with Kallikrein-like
and Angiotensin-Degrading Activities
1Chin-Chun Hung* and Shyh-Horng Chiou*
,†
,2*Institute of Biochemical Sciences, College of Science, National Taiwan University, Taipei, Taiwan; and †Institute of Biological Chemistry, P.O. Box 23-106, Academia, Taipei, Taiwan
Received January 16, 2001
Two venom proteases with fibrinogenolytic activity were isolated from the venom of Taiwan habu
(Trim-eresurus mucrosquamatus), one major crotalid snake
species in Taiwan. The purified enzymes showed a strong-fibrinogenolytic activity, cleaving -chain of fibrinogen molecules specifically. They also showed strong kallikrein-like activity in vitro, releasing bra-dykinin from kininogen. The purified enzymes did not coagulate human plasma, yet decreasing fibrinogen levels in plasma and prolonging bleeding without formation of fibrin clots, indicating that both pro-teases have specificities different from thrombin and thrombin-like proteases of snake venom reported pre-viously. They also exhibit amidase activity against
N-benzoyl-Pro-Phe-Arg-p-nitroanilide, which is a
spe-cific synthetic substrate for kallikrein-like proteases. Their stability at high temperatures was examined and found to be more stable when compared with an-crod and thrombin. Intravenous injection of either protease was shown to lower blood pressure in exper-imental rats. Most noteworthy is the observation that the proteases can cleave angiotensin I and release bra-dykinin from plasma kininogen in vitro, which is a strong vasodilator and probably responsible for the in
vivo hypotensive effect of these venom proteases.
© 2001 Academic Press
Key Words: -fibrinogenolytic activity;
kallikrein-like proteases; bradykinin; angiotensin I; hyperten-sion therapy.
Venoms from various snake species alter the
haemo-static and blood coagulation systems of human victims
or experimental animals in a complex manner.
Differ-ent venoms contain multiple componDiffer-ents which behave
as pro- or anticoagulants that directly (or indirectly)
induce or inhibit fibrinogen and/or platelet aggregation
and related complex biochemical processes, resulting
in common clinical complications of blood clotting or
uncontrolled hemorrhage by envenomation of
snake-bites (1–3). These apparently contrasting activities
have been attributed to the presence of fibrinogenolytic
or fibrinogen clotting enzymes in snake venoms (4 – 6).
On the other hand, platelet-aggregating enzymes in
venom generally lack fibrinogenolytic activity, but
can directly aggregate platelets in platelet-rich plasma
(7). Current interest is directed to some fibrinolytic
proteinases including metalloproteinases and
throm-bin-like enzymes because of their potential clinical
ap-plication in the treatment of vascular thrombotic
dis-eases (8).
It is well known that snake venoms contain complex
mixtures of pharmacologically active peptides and
pro-teins. Reptilian venoms particularly those obtained
from the snake families of Crotalidae and Viperidae,
are also shown to possess many different
fibrinogeno-lytic proteases which may initiate or affect blood
coag-ulation process associated with snakebites (6).
Differ-ent groups reported disparate proteases from venoms
of various crotalid snakes. They included crotalase,
a thrombin-like enzyme isolated from the
Ameri-can-Eastern diamondback rattlesnake (Crotalus
ada-manteus) (9), hemorrhagic toxins, anticoagulant
pro-teases and kallikrein-like enzymes from the
American-Western diamondback rattlesnake Crotalus atrox (10 –
12). We have previously evaluated the venom
compo-nents from Crotalus atrox and found that all fractions
isolated from the anion-exchange chromatography
showed varying extents of specific proteolytic activity
against
␣- and/or -chains of fibrinogen molecules (13,
14). Concurrently, studies on the toxin components
from Taiwan habu (Trimeresurus mucrosquamatus)
1The cDNA sequences encoding similar venom serine proteases to
Tm-VIG and Tm-IIG isolated from Taiwan habu (Trimeresurus
mu-crosquamatus) have been deposited in the EMBO database under the
Accession Nos. X83221 and X83225.
2To whom correspondence should be addressed. Fax:
(886)-2-26530014. E-mail: shchiou@gate.sinica.edu.tw.
doi:10.1006/bbrc.2001.4452, available online at http://www.idealibrary.com on
1012 0006-291X/01 $35.00
(15–17), a major and abundant crotalid species in
Tai-wan, indicated several kinds of fibrinogenases present
in this phylogenetically related species to those
Amer-ican rattlesnakes.
Concerning the pharmacological action of Formosan
snake venoms on blood coagulation, it was reported
early in 1921–1925 that the crude venoms of two
cro-talid snake species, Agkistrodon acutus and
Trimeresu-rus gramineus, had a coagulant action on whole blood
and plasma, while the venom of another species
Tri-meresurus mucrosquamatus of the same family showed
an inhibitory action (1). The inhibitory action on blood
coagulation was believed to be caused mostly by
de-struction of fibrinogen in the case of the venom of
Trimeresurus mucrosquamatus. Therefore it is deemed
imperative to isolate venom enzymes which are
respon-sible for these fibrinogen-degrading activities that
de-stroy the precursor fibrinogen molecules with the
re-sult of excluding the formation of fibrin clot in the blood
plasma. In this study we have made an effort in the
search and characterization of these fibrinogenases
from Taiwan habu, which show an unexpectedly strong
kallikrein-like hypotensive activity upon experimental
rats and may find their clinical applications in
hyper-tension therapy.
MATERIALS AND METHODS
Crude venom and chemicals for protein isolation and purification.
The lyophilized venom powder was obtained from the local snake farm, and the venom gland of Taiwan habu was donated from the National Institute of Preventive Medicine, Taipei, Taiwan. The syn-thetic substrates, human fibrinogen, angiotensin I, bradykinin, kal-likrein, snake venoms of other species and various protease inhibi-tors were from Sigma Chemical Co. (St. Louis, MO). High molecular weight kininogen was obtained from Enzyme Research Laboratories Inc. (South Bend, IN).
Thermostability study of purified proteases. The thermostability for purified kallikrein-like fibrinogenases together with ancrod and human thrombin was determined by pre-incubating enzymes in 0.1 M Tris, pH 8.0 buffer, at 25, 35, 45, 55, 65, 75, 85, and 95°C for 30 min. After preincubation, the enzyme was mixed with synthetic substrates and proteolytic activities then determined on a spectrophotometer by measuring absorbances at A405nm. The amidolytic activity towards
var-ious chromogenic substrates was measured with a Ultrospec 4000 spec-trophotometer (Amersham/Pharmacia) in a plastic cuvette with 1-cm path length. Assays were performed in 50 mM Tris-HCl, pH 8.0 in a total volume of 750l at 37°C. The final concentrations of enzymes were 1.0 nM for Tm-VIG and Tm-IIG, 0.02 U/ml for human thrombin (930 NIH U/mg protein based on Biuret assays) and 0.04 U/ml for Ancrod (500 NIH U/mg protein based on Biuret assays). The final concentration for chromogenic substrates was 0.1 mM. The formation of p-nitroaniline was monitored at 405 nm as a function of time.
Amino acid composition and sequence analyses. The amino-acid compositions were determined with a Beckman 6300 amino acid analyzer using a single-column system based on conventional ion-exchange chromatography system. The special rapid procedure for the preparation of protein hydrolysates using heat-resistant reus-able Pyrex tubes and high temperature (150°C, 1.5 h) for amino acid analysis was essentially according to the previous report (20).
N-Terminal sequence analysis was carried out by automated Ed-man degradation with a microsequencing sequencer (Model 477A,
Applied Biosystems). The lyophilized column fractions each contain-ing about 1–5 nmoles of protein were dissolved in 200l of 0.1% trifluoroacetic acid (TFA) or 0.1% SDS/0.1% TFA (1:1 v/v) and 10l each for sequence determinations.
Determination of cleavage sites using biological peptides as sub-strates. Cleavage of angiotensin I and bradykinin was measured by incubating 50l of angiotensin I (1 mg/ml) or bradykinin (1 mg/ml) in 0.05 M Tris-HCl, pH 7.5, with 5g of proteases at 37°C for 3 h, the mixture was filtered with a microcentrifugation filter (molecular mass cutoff⫽ 10,000). The filtrate was analyzed by HPLC (Bio-Rad Bio-Sil ODS-5S C18column, 4⫻ 250 mm). The HPLC was run for 35
min in a linear gradient of 0 –75% solvent B (95% acetonitrile con-taining 0.1% trifluoroacetic acid (TFA)) with 5% acetonitrile/0.1% TFA (solvent A) as the starting and equilibration eluent. The flow rate of column eluates was set at 1 ml/min and monitored at UV 214 nm. Peak fractions were collected and amino acid compositions were performed as described previously.
Kallikrein-like activity. The kallikrein-like activity was assayed on an SDS-polyacrylamide slab gel (5% stacking/8% resolving gel). Small vials containing about 5g high molecular weight kininogen in 50 mM Tris-HCl, pH 8.0 buffer were incubated at 37°C with 0.2g of human plasma kallikrein, 0.2g of TM-VIG or TM-IIG, 0.2 units of Ancrod in a total volume of 10l for various time intervals. After the incubation digestions were stopped by adding 0.1% SDS/1% -mercaptoethanol and heated at 100°C for 5 min. The proteolytic activity was monitored by observing the cleavage patterns of kinino-gen on Coomassie blue-stained gels after electrophoresis.
Kinin-releasing assay. High molecular-weight kininogen (20g) was incubated with Tm-VIG or Tm-IIG (60g/ml) in 50 mM Tris-HCl, 1 mM EDTA, pH 8.0 at 37°C for 4 h. The released kinin was identified by HPLC (Bio-Rad Bio-Sil ODS-5S C18column, 4⫻ 250
mm) as described previously (21), and its molecular mass analyzed in an LCQ mass spectrometer (Finnigan, San Jose, CA).
Total clottable fibrinogen assay. Fibrinogen assay was calibrated using Fibrinogen Standard (Dade Behring, Marburg, Germany). The Control Plasma N of normal concentration range (Behring) was used for fibrinogen assay. Various concentrations of venom proteases were incubated with Control Plasma N for 2 min at 37°C, and clotting time measurement was performed with Multifibren U reagent (Behring) on the Humaclot coagulometer (Human Gesellschaft fu¨ r Biochemica und Diagnostica mbH, Taunusstein, Germany).
Bleeding-time measurements. Bleeding time of mice was mea-sured by a modification of the method described by Kung et al. (22). Three-month-old ICR mice were used to determine bleeding times. Saline or solutions containing proteases at various concentrations were injected intravenously through a lateral vein of each mouse. After 5 min, the tail was completely transected 2–3 mm from the tip with a sharp surgical blade. To evaluate bleeding from the incision, a Whatman filter paper was applied to the cutting edge near the clot forming place every 30 s for 10 min, taking care not to dislodge the clot. Blood that continued to flow from the cut was allowed to fall on the filter paper during 30-s intervals. Bleeding times were deter-mined by measuring the time point with blood stains first disappear-ing on the filter paper.
In vivo hypotension assay. Blood pressure was assayed by the method as described previously (23). Rats (Sprague–Dawley, body weights of 250 –300 g) of either sex were anesthetized with sodium pentobarbital (50 – 60 mg/kg, intraperitoneally). The trachea was cannulated with a glass cannula for recording respiratory move-ments via a volumetric pressure transducer Grass PT 5A (Astro-Med. Inc., West Warwick, RI) and also for artificial ventilation with a rodent respirator (Model 680; Harvard Apparatus, Inc., Holliston, MA). The right common carotid artery and femoral vein were can-nulated with polyethylene tubings filled with heparinized saline. The carotid tubing was attached to a pressure transducer (Model P23-ID, Statham, Murray Hill, NJ) connected to a Grass Model 7 polygraph
(Astro-Med. Inc., West Warwick, RI). The femoral vein was used as an application route for injection of kallikrein-like fibrinogenases or saline.
Statistical analysis. All data are expressed as the mean⫾ SEM (n). Student’s t test was used to assess the statistical differences.
RESULTS AND DISCUSSION
Most venoms from snake species induced either
bleeding or blood clotting. These activities have been
attributed to fibrinogenolytic or fibrinogen clotting
en-zymes (4 – 6). In the previous study (17) we have
ap-plied multiple-step chromatographies for the isolation
and purification of a novel family of kallikrein-like
fibrinogenolytic enzymes from the venom of Taiwan
habu (Trimeresurus mucrosquamatus, denoted as Tm),
named Tm-VIG (with Val-Ile-Gly as the first three
N-terminal residues) and Tm-IIG (with Ile-Ile-Gly as
the first three N-terminal residues), which possess
rel-atively specific and strong activities on
-chain of
hu-man fibrinogen and kallikrein substrate. In this study
we have further extended the characterization of these
strong proteases with hypotensive effect. The purified
native enzymes Tm-VIG and Tm-IIG are acidic
pro-teins with isoelectric points lying between 5.5 to 6.9
and comprise less than 3% of total crude venom. They
can be distinguished from crotalase, thrombin, and
kallikrein-like enzymes reported previously from the
closely-related crotalid species based on the effects of
various protease inhibitors, amino acid compositions
and sequence comparison (17).
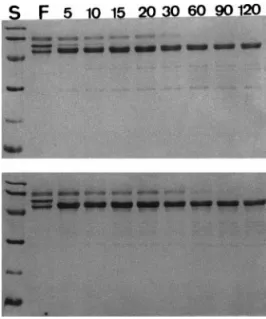
Fibrinogenolytic activity and substrate specificity of
Tm-VIG and Tm-IIG.
Both groups of fibrinogenolytic
enzymes, i.e., Tm-VIG and Tm-IIG hydrolyzed B

chain of fibrinogen within 5 min with relatively lower
activity on A
␣ chain while ␥ chains remained intact
even at the end of 120 min (Fig. 1). VIG and
Tm-IIG can also cleave p-nitroaniline from several
syn-thetic colored peptide substrates.
N-benzoyl-Pro-Phe-Arg p-nitroanilide, a specific synthetic substrate for
kallikrein-like proteases was most susceptible to
hy-drolysis by Tm-VIG and Tm-IIG. They also showed
relatively high activities towards N-p-tosyl-arginine
methyl ester (TAME), indicating that both groups are
members of serine proteases family. However
D-Val-Leu-Lys p-nitroanilide which is a specific substrate for
plasmin was demonstrated to be a very poor substrate
for these two types of fibrinogenases (unpublished
re-sults), attesting to some distinct features of these
fibrinogen-digesting proteases as compared with
con-ventional serine proteases involved in the process of
blood coagulation.
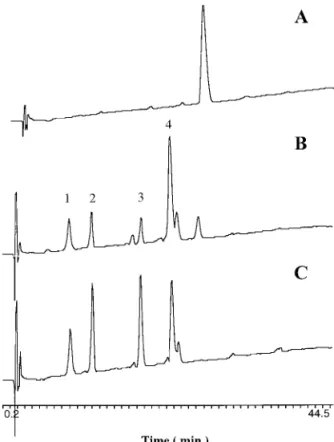
Incubation of angiotensin I with these two venom
proteases resulted in similar degradation patterns.
The four major peptide fragments released by specific
cleavage on angiotensin I with Tm-VIG and Tm-IIG as
determined from amino acid compositions of these
pep-tide fragments are as follows: His-Leu,
Asp-Arg-Val-Tyr, Ile-His-Pro-Phe, and
Asp-Arg-Val-Tyr-Ile-His-Pro-Phe (corresponding to four major peptide peaks in
Fig. 2). This would indicate that these two proteases
act on the same sites in angiotensin I. In addition,
when kininogen was incubated with purified Tm-VIG
or Tm-IIG, the disappearance of kininogen coupled
with the formation of the major degradation protein
fragment of 58 kDa chain is very similar to that
ob-served for human kallikrein (Fig. 3). The kinin
re-leased by Tm-VIG and Tm-IIG from kininogen was
further identified by reverse-phase HPLC. Comparison
of the fragmentation profiles by mass spectroscopy
us-ing synthetic bradykinin as a marker standard
identi-fied one of the released peptides as bradykinin (data
not shown), pointing to the fact that these two venom
proteases may possess genuine hypotensive effect in
vivo through bradykinin.
Stability of purified venom proteases with
-fibrino-genolytic activity.
We have carried out thermal
sta-bility analysis of Tm-VIG and Tm-IIG by incubating
these proteases at different temperatures for 30 min
and examined the fibrinogenolytic activity after
heat-ing. It is of surprise to find that these proteases similar
to another snake venom protease (ancrod) (24, 25)
iso-lated from Malayan pit viper Calloselasma rhodostoma
were heat-stable to about 95°C. They still maintained
FIG. 1. Time-course study of fibrinogenolytic activity of purified proteases (Tm-VIG and Tm-IIG) on SDS–PAGE. Lane F, purified fi-brinogen in the absence of proteases, the three subunit chains are A␣, B and ␥ chains of fibrinogen respectively from the top end downwards; Lane S, standard molecular mass markers (in kDa): phosphorylase b (94), bovine serum albumin (66), ovalbumin (45), carbonic anhydrase (30), soybean trypsin inhibitor (20), and␣-lactalbumin (14). Lanes with indicated numbers 5–120 denote time-course digestion of fibrinogen with purified proteases Tm-VIG (top) and Tm-IIG (bottom) at 37°C for 5, 10, 15, 20, 30, 60, 90, and 120 min, respectively. Note that the proteases show specific cleavages first on B and then A␣ chains with ␥ chain relatively resistant to digestion.their activity at a level of 50 – 65% activity even after
30 min heating at this high temperature whereas
hu-man thrombin lost activity completely at about 65°C
(Fig. 4). Both proteases are also more stable than
var-ious fibrinogenases prevvar-iously identified from
Ameri-can rattlesnake venoms (13, 14), which are also stable
only to about 60 – 65°C.
Effects of proteases on total clottable fibrinogen and
bleeding time.
The conversion of fibrinogen into fibrin
plays an important role in coagulation and hemostasis.
The final and most defined function of blood
coagula-tion is its effect on plasma clottability. Thus the
mea-surement of clottable fibrinogen in plasma has become
a standard protocol for comparison of effects of various
biological factors or pharmaceutical agents on the
blood clotting process (26). We have used the
clotting-time measurement to determine clottable fibrinogen in
plasma after treating with Tm proteases. Both venom
proteases could prolong clotting times by degrading
plasma fibrinogens directly. Total clottable fibrinogen
levels were decreased after incubation with purified
Tm-VIG/Tm-IIG for 2 min (Fig. 5). However, the blood
clotting cannot be induced and clotting-times
length-ened indefinitely when the concentrations of proteases
were higher than 1
g, corroborating the strong
fibrin-FIG. 2. HPLC chromatograms of angiotensin I cleavage inducedby Tm-VIG and Tm-IIG. Chromatography was analyzed by HPLC (Bio-Rad Bio-Sil ODS-5S C18column, 4⫻ 250 mm). The HPLC was
run for 35 min in a linear gradient of 0 –75% solvent B (95% nitrile containing 0.1% trifluoroacetic acid (TFA)) with 5% aceto-nitrile/0.1% TFA (solvent A) as the starting and equilibration eluent. The flow rate of column eluates was set at 1 ml/min. Each chromato-gram represents angiotensin I alone (A) and angiotensin I digested with Tm-VIG (B) or Tm-IIG (C). The labeled peaks indicate 4 major proteolytic fragments by digestion.
FIG. 3. Time-course study of kallikrein-like activity of venom pro-teases on SDS–PAGE. High-molecular-weight kininogen (114 kDa) was incubated with plasma kallikrein or Tm-VIG (top, left to right), and Tm-IIG or Ancrod (bottom, left to right) at 37°C for 15, 30, and 60 min, respectively. Arrows a and b indicate a 58-kDa light-chain fragment and a 45-kDa modified light-chain fragment formed after cleavage with plasma kallikrein. Lane S, standard high-molecular-mass markers (in kDa): myosin (212),-galactosidase (116), phosphorylase b (94), bovine serum albumin (66), catalase (57), and aldolase (40).
FIG. 4. Effect of temperature on the activity of purified fibrino-genolytic proteases, ancrod and human thrombin. Activity was mea-sured by using 0.1 mM N-benzoyl-Pro-Phe-Arg p-nitroanilide as sub-strate for Tm-VIG and Tm-IIG, and 0.1 mM N-p-tosyl-Gly-Pro-Arg
p-nitroanilide for ancrod and thrombin due to different substrate
spec-ificities among these proteases. Percent activity at different tempera-tures with reference to that at the ambient room temperature (100%) was compared for these four proteases. The proteolytic activities using synthetic chromogenic substrates were measured on a spectrophotom-eter at 405 nm. Data are presented as mean⫾ SEM (n ⫽ 3).
ogenolytic activity associated with these two novel
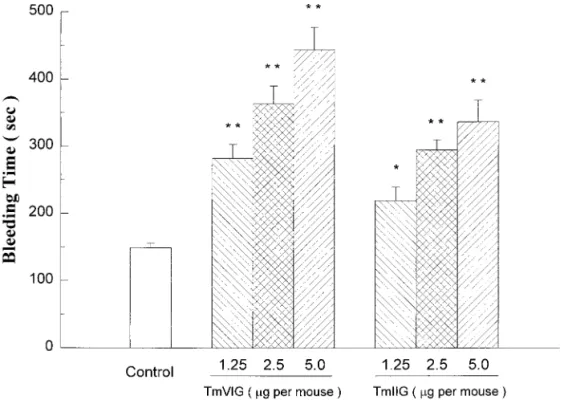
venom proteases. The bleeding times measured after
surgical transections on tails upon intravenous
admin-istration of proteases to mice were significantly
pro-longed in a dose-dependent manner (Fig. 6). It is
note-worthy that the anti-clotting or bleeding effect of
Tm-VIG was stronger than that of Tm-IIG significantly,
which deserves a further study on detailed structural
determination of these two proteases in the future.
Similar to clottable fibrinogen assays, the bleeding
times were found to lengthen to more than 10 min and
hemorrhagic side-effects appeared when amounts of
proteases injection were higher than 5
g per mouse.
Therefore from the in vitro clottable fibrinogen assays
and in vivo bleeding time measurements, it is
conceiv-able that Tm-VIG and Tm-IIG may be directly involved
in decreasing the levels of fibrinogen in the plasma
through defibrinogenation. In contrast, ancrod and
ba-troxobin, which are members of thrombin-like enzymes,
can cleave specifically fibrinopeptides A or B from
fibrin-ogen resulting in formation of fibrin clots (27).
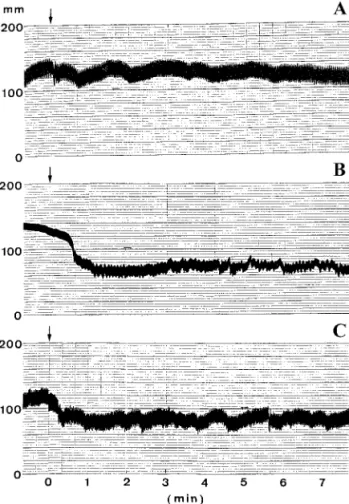
Hypotensive effects of Tm proteases on experimental
rats.
The hypotensive effects of proteases on rat blood
pressure were investigated by injecting these proteins
into cannulated rats. A significant blood pressure drop
was observed with Tm-VIG injection (Fig. 7). Injection
of Tm-IIG showed a milder effect. The hypotensive
effect exhibited by these venom proteases are likely
due to their inherent kallikrein-like activity. There is a
possibility that Tm-VIG and Tm-IIG may directly
af-fect the blood coagulation pathway by specifically
cleaving B
 chains of animal fibrinogens and act like
plasma kallikreins to cleave kininogen. This
kalli-krein-like activity is especially intriguing since the
reported
␣-fibrinogenases like ancrod did not show
such a high specificity against kininogen (data not
shown). Moreover both Tm-VIG and Tm-IIG
demon-strated no obvious ability to induce or inhibit platelet
aggregation, in great contrast with some venom
anti-thrombotic factors reported in the literature.
FIG. 5. Effect of kallikrein-like Tm proteases on apparent fibrino-gen concentration in plasma measured with a coagulometer. The sam-ples from human Control Plasma N were mixed with various concen-trations of venom proteases. Values are given as percent of original fibrinogen concentration measured in the absence or presence of venom proteases using the Control Plasma N solution without protease as reference. Data are assayed in triplicate measurements.
FIG. 6. Effect of kallikrein-like Tm proteases on tail bleeding time in mice by a filter paper method. Bleeding time was measured 5 min after the intravenous administration of saline or various doses of proteases. Data are presented as mean⫾ SEM (n ⫽ 6), with significance levels at *P⬍ 0.05 or **P ⬍ 0.01 as compared with the control.
In 1949 Rocha e Silva et al. (28) first reported that
trypsin and certain snake venoms acted on plasma
globulin to produce a substance that lowered blood
pressure. They termed this substance bradykinin. It is
likely that kallikrein-like
-fibrinogenases reported
here may be the snake venom enzymes responsible for
the generation of bradykinin from endogenous HMW
kininogen to lower blood pressure. The in vivo
hypo-tensive effect on rats by these enzymes (Fig. 7) attested
to the effectiveness of using these fibrinogenases as
antihypertensive agents. An additional activity of
these Tm fibrinogenases, the degradation of the
hyper-tensive peptide angiotensin I (Fig. 2), may also
poten-tiate hypotensive effect exhibited by these Tm venom
proteases. Further functional and structural
charac-terization regarding the structure/biological activity
correlation should shed some insight into the
mecha-nism underlying their kallikrein-like hypotensive
ef-fect and fibrinogenolytic action.
In conclusion, enzymes which interfere with
haemo-stasis in vertebrates are common components in snake
venoms. Two reptilian venom proteases ancrod (24, 25)
and batroxobin (29, 30) from viperid snakes have been
found clinically useful for the treatment of thrombotic
diseases (31, 32) during the past three decades. Venom
proteolytic enzymes with kallikrein-like activity
char-acterized in this study are strong and very heat-stable
fibrinogenolytic proteases first reported from Taiwan
habu, which is an evolutionarily more remote crotalid
species when compared with two viperid snake species
that contain batroxobin and ancrod. The comparison of
activity and thermal stability of habu proteases with
ancrod and batroxobin indeed shows great potentials
in exploiting these novel
-fibrinogenases with
kalli-krein-like activity as effective antithrombotic and
anti-hypertensive agents.
ACKNOWLEDGMENTS
This work was supported in part by Academia and the National Science Council (NSC Grants 87-2311-B-002-068, 88-2311-B-002-061 and 89-2311-B-001-190 to S.-H. Chiou), Taipei, Taiwan. This report will be submitted as part of a dissertation by C.-C. Hung to National Taiwan University in partial fulfillment of the degree of Doctor of Philosophy. We thank Professor Wan-Wan Lin at the Department of Pharmacology, College of Medicine, National Taiwan University for assisting hypotensive assays for the isolated fibrino-genases.
REFERENCES
1. Ouyang, C. (1957) J. Formosan Med. Assoc. 56, 435– 448. 2. Meaume, J. (1966) Toxicon 4, 25–58.
3. Kini, R. M., and Evans, H. J. (1990) Toxicon 28, 1387–1422. 4. Brinkhous, K. M., and Smith, S. V. (1988) in Hematology,
Hae-mostasis and Animal Venoms (Pirkle, H., and Markland, F. S., Jr., Eds.), Vol. 7, pp. 363–375, Marcel Dekker, New York. 5. Stocker, K. F. (1990) in Medical Use of Snake Venom Proteins
(Stocker, K. F., Ed.), pp. 97–160, CRC Press, Boston, MA. 6. Tu, A. T. (1982) in Rattlesnake Venoms: Their Actions and
Treatment (Tu, A. T., Ed.), pp. 247–312, Marcel Dekker, New York.
7. Serrano, S. M. T., Mentele, R., Sampaio, C. A. M., and Fink, E. (1995) Biochemistry 34, 7186 –7193.
8. Markland, F. S., Jr. (1998) Thromb. Haemostasis 79, 668 – 674.
9. Markland, F. S., and Damus, P. S. (1971) J. Biol. Chem. 246, 6460 – 6473.
10. Bjarnason, J. B., and Tu, A. T. (1978) Biochemistry 17, 3395– 3404.
11. Pandya, B. V., and Budzynski, A. Z. (1984) Biochemistry 23, 460 – 470.
12. Bjarnason, J. B., Barish, A., Direnzo, G. S., Campbell, R., and Fox, J. W. (1983) J. Biol. Chem. 258, 12566 –12573.
13. Chiou, S.-H., Hung, C.-C., and Lin, C.-W. (1992) Biochem. Int. 26, 105–112.
14. Chiou, S.-H., Hung, C.-C., and Huang, K.-F. (1992) Biochem.
Biophys. Res. Commun. 187, 389 –396.
15. Ouyang, C., and Teng, C. M. (1976) Biochim. Biophys. Acta 420, 298 –308.
FIG. 7. Hypotensive effect on rat blood pressure of kallikrein-like Tm fibrinogenases from Taiwan habu. (A) Blood pressure change after intravenous (i.v.) injection with normal saline; (B) blood pres-sure change after i.v. injection of Tm-VIG (0.5g/g weight); (C) blood pressure change after i.v. injection of Tm-IIG (0.5 g/g weight). Arrows indicate starting points for sample injections. Note that Tm-IIG seemed to show a lower hypotensive activity than Tm-VIG under similar experimental conditions.
16. Huang, K.-F., Hung, C.-C., and Chiou, S.-H. (1993) Biochem.
Mol. Biol. Int. 31, 1041–1050.
17. Hung, C.-C., Huang, K.-F., and Chiou, S.-H. (1994) Biochem.
Biophys. Res. Commun. 205, 1707–1715.
18. Laemmli, U. K. (1970) Nature 227, 680 – 685. 19. Roe, J. H. (1955) J. Biol. Chem. 212, 335–343. 20. Chiou, S.-H. (1988) Biochem. Int. 17, 981–987.
21. Fiedler, F., and Geiger, R. (1988) Methods Enzymol. 163, 257– 262.
22. Kung, S. H., Hagstrom, J. N., Cass, D., Tai, S. J., Lin, H. F., Stafford, D. W., and High, K. A. (1998) Blood 91, 784 –790. 23. Lin, W. W., Chen, Y. M., Lee, S. Y., and Lee, C. Y. (1991)
Asia-Pacific J. Pharmacol. 6, 277–286.
24. Burkhart, W., Smith, G. F. H., Su, J. L., Parikh, I., and LeVine, H. III. (1992) FEBS Lett. 297, 297–301.
25. Au, L. C., Lin, S. B., Chou, J. S., Teh, G. W., Chang, K. J., and Shih, C. M. (1993) Biochem. J. 294, 387–390.
26. Gaffney, P. J., and Wong, M. Y. (1992) Thromb. Haemostasis 68, 428 – 432.
27. Markland, (1998) Toxicon 36, 1749 –1780.
28. Rocha e Silva, M., Beraldo, W. T., and Rosenfeld, G. (1949)
Am. J. Physiol. 156, 261–273.
29. Holleman, W. H., and Weiss, L. J. (1976) J. Biol. Chem. 251, 1663–1669.
30. Itoh, N., Tanaka, N., Mihashi, S., and Yamashina, I. (1987)
J. Biol. Chem. 262, 3132–3135.
31. Stocker, K. (1978) in Handbook of Experimental Pharmacology (Markwardt, F., Ed.), Vol. 46, pp. 451– 484, Springer-Verlag, Berlin.