國 立 交 通 大 學
生 物 科 技 系 暨 研 究 所
碩士論文
胃幽門螺旋桿菌感染病人血清中之抗體可增強病原熱休克蛋白 60
誘發前發炎性細胞激素產生的活性
Antibodies in the sera of patients with Helicobacter pylori infection
promote the activities of pathogenic heat shock protein 60 to
induce the expressions of proinflammatory cytokines
研 究 生:楊筑婷
指導教授:廖光文 教授
胃幽門螺旋桿菌感染病人血清中之抗體可增強病原熱休克蛋白 60 誘 發前發炎性細胞激素產生的活性
Antibodies in the sera of patients with Helicobacter pylori infection promote the activities of pathogenic heat shock protein 60 to induce the
expressions of proinflammatory cytokines
研 究 生:楊筑婷 Student:Chu-Ting Yang 指導教授:廖光文 Advisor:Kuang-Wen Liao 國 立 交 通 大 學 生 物 科 技 系 碩 士 論 文 A Thesis
Submitted to Department of Biological Science and Technology College of Biological Science and Technology
National Chiao Tung University in partial Fulfillment of the Requirements
for the Degree of Master
in
Biological Science and Technology
July 2009
Hsinchu, Taiwan, Republic of China
胃幽門螺旋桿菌感染病人血清中之抗體可增強病原熱休克蛋白 60 誘發前發 炎性細胞激素產生的活性 學生: 楊筑婷 指導教授: 廖光文 教授 國立交通大學 生物科技學系 碩士班 摘要 胃 幽 門 螺 旋 桿 菌 的 熱 休 克 蛋 白 (HSP60s) 誘 導 前 發 炎 性 細 胞 激 素 (proinflammaroty cytokine) 的表現跟胃部發炎有很大的關連性。在臨床的 研究中,胃幽門螺旋桿菌感染病人的血清裡可以偵測到抗胃幽門螺旋桿菌 熱休克蛋白 60 抗體。令人感到驚訝的,16 位患有胃癌、胃炎、十二指腸 潰瘍或胃潰瘍病人的血清並無法中和重組胃幽門螺旋桿菌熱休克蛋白 60 誘導腫瘤壞死因子-甲型 (TNF-) 和介白素-8 (IL-8) 的釋放,反而增加前 發炎性細胞激素的表現。研究結果顯示,不論來自何種物種的抗胃幽門螺 旋桿菌熱休克蛋白 60 多株抗體 (anti-HpHSP60 polysera) 都可以影響胃幽 門螺旋桿菌熱休克蛋白 60 增加腫瘤壞死因子-甲型和介白素-8 的表現。更 近一步研究發現此增強效應為血清中專一抗胃幽門螺旋桿菌熱休克蛋白 60 抗體所導致,而非血清中其他因子或非專一性抗體。最後,實驗結果推 測 Fc 接受器 (Fc receptor) 在抗胃幽門螺旋桿菌熱休克蛋白 60 抗體增加發 炎的影響中扮演一個角色。綜合這些研究結果,我們揭露當胃幽門螺旋桿 菌熱休克蛋白 60 存在時,病人血清中的抗胃幽門螺旋桿菌熱休克蛋白 60 抗體可能造成更嚴重的發炎。這樣的發現可能可以提供未來胃幽門螺旋桿 菌感染病人在臨床上治療的應用。
Antibodies in the sera of patients with Helicobacter pylori infection promote the activities of pathogenic heat shock protein 60 to induce the expressions
of proinflammatory cytokines
Student: Chu-Ting Yang Advisor: Dr. Kuang-Wen Liao Department of Biological Science and Technology
National Chiao Tung University
Abstract
H. pylori-derived heat shock proteins (HSP60s) are closely associated with gastric inflammation to induce the expressions of proinflammatory cytokines. In clinical examination, anti-HpHSP60 antibodies could be detected in H.pylori-postive patients’ sera. Surprisingly, the sera from 16 patients with gastric cancer, gastritis, duodenal ulcer or peptic ulcer could not neutralize the activities of recombinant HpHSP60 to induce releases of TNF- and IL-8 but they raised the expressions of proinflammatory cytokines. The results showed the no matter what species’ anti-HpHSP60 polysera all could cause an enhancive effect on HpHSP60 to induce TNF- or IL-8 expression. Furthermore, the specific antibodies in sera but not other factors or non-specific antibodies contributed to the enhancive effect. Finally, the results suggested Fc receptor plays a role in the enhancive effect of anti-HpHSP60. Together these results, we explored that the patients’ anti-HpHSP60 antibodies in sera may result in more serious inflammation in the presence of HpHSP60. This finding may provide further application in clinical treatments for the patients with H. pylori infection.
誌 謝
回想兩年的碩士研究時光轉眼就過了,過程中的心情五味雜陳,當初毅 然決然的從醫藥化學跨到生物科技這個領域,一切都是新的嘗試跟新的挑 戰,起初對於實驗相當不熟悉,在指導教授廖光文教授教導之下也逐漸的 步上軌道,不論在學業或生活上都給予細心指導與愛護,並且提供一個良 好的研究環境,讓我能無慮的完成學問,在此致上由衷的感謝!此外,特 別感謝吳彰哲教授、鄭添祿教授及蔡女滿教授,對於論文的賜教及指正, 使學生論文更完善。學生修業期間藉由許多人的幫忙才得以完成論文,其 中特別感謝毛仁淡講座教授,在學生學習單株抗體技術上給予相當大的資 源及技術上的支持,在此致上最誠摯的謝意。 在實驗指導上,感謝陳文亮博士給予教導與協助,而且毫無保留提供 我寶貴的單株抗體技術與經驗。感謝賴以祥博士在免疫分析實驗技術的指 導與協助,讓我的研究得以順利完成。感謝第一個家中的于鈴學姐(在我沮 喪時開導我)、彥谷學長、小莉(同窗五年可愛室友)、小溫、何姵、阿伯, 維瞳(陪伴我純化蛋白、討論實驗、認識上帝的優秀學妹)、小薇(實驗上軌 道的真實獅子座搞笑咖,有妳坐旁邊很開心)、靜敏(抗體接班人)、馬馬、 雙雙、小美、晨洋。以及第二個家的繼鋒學長(實驗開導大師)、詩璇、俐 穎(跑步談心好姐妹)、宛伶(搞笑小可愛)、柏如(漂亮美女)、沁紜、善綺(善 解人意大美人)及在我心中很有份量的高醫好友禕庭、玲宇,在你們大家的陪伴之下,我順利渡過碩士生涯,在此一併致上我由衷的謝意,謝謝你們!! 最後感謝我最親愛的家人,父親楊茂源先生、母親蔡淑鈴女士、哥哥楊 程凱,因為你們的支持與鼓勵,讓我能無後顧之憂的順利完成碩士學位。 ---僅以此畢業論文獻給所有關心及幫助我的人
目錄
中文摘要---I Abstract---II Acknowledgements---III 目錄---V Chapter 1: Introduction---11. The morphology of Helicobacter pylori---1
2. The epidemiology of Helicobacter pylori---1
3. Helicobacter pylori associated diseases---2
4. The background of Heat shock protein 60 (HSP60)---3
5. The proinflammatory roles of HSP60s---4
6. The antibody response to HSP60---5
7. Fc receptor---6
Chapter 2: Materials and Methods---8
Materials---8
1. Reagent---8
2. Kit---9
4. Instrument---11
5. Cell line---11
6. Animal---11
7. Other---12
Methods---13
1. Cell culture condition---13
2. Expression and purification of HpHSP60---13
3. Detection of cytokines production in the THP-1 cells---17
4. Measurement of patients’ sera antibody to HpHSP60---17
5. Determination of TNF- and IL-8 levels in sera condition by ELISA---18
6. Production of monoclonal antibody---18
7. Determination of TNF- and IL-8 levels in mAb condition by ELISA---22
Chapter 3: Results---24
1. The preparation of recombinant H. pylori HSP60---24
2. The patient sera with anti-HpHSP60 antibodies could enhance the expressions of TNF- and IL-8---25 3. The effects of mouse and rabbit anti-HpHSP60 polyclonal antibodies on the
a b i l i t i e s o f H p H S P 6 0 t o i n d u c e e x p r e s s i o n s o f T N F - a n d IL-8---26 4. The effects of monoclonal antibodies on the abilities of HpHSP60 to induce expressions of TNF- and IL-8---27
Chapter 4: Discussion---29 Figures and Legends---33
Figure 1. SDS-PAGE and Western blot analyses of recombinant HpHSP60---33 Figure 2. Production of proinflammatory cytokines in THP-1 cells---34 Figure 3. The relative ratio of serum antibodies to HpHSP60 in H. pylori-positive patients---35 Figure 4. The patients’ sera with anti-HpHSP60 antibodies could enhance the expressions of TNF- and IL-8---37 Figure 5. The effect of mouse polyclonal antibodies on the abilities of HpHSP60 to induce expressions of TNF- and IL-8---39 Figure 6. The effect of rabbit polyclonal antibodies on the abilities of HpHSP60 to induce expressions of TNF- and IL-8---41 Table1. The characterizations of monoclonal Abs---42 Figure 7. The effect of monoclonal antibodies on the abilities of HpHSP60 to
induce expressions of TNF- and IL-8---44 Figure 8. The effect of non-related mAb on the abilities of HpHSP60 to induce expressions of TNF- and IL-8---46 Figure 9. The non-related mAbs interfere with anti-HpHSP60 monoclonal antibodies to enhance the abilities of HpHSP60 to induce expressions of TNF- Figure 10. The non-related mAbs interfere with anti-HpHSP60 monoclonal antibodies to enhance the abilities of HpHSP60 to induce expressions of IL-8---50
Chapter 1: Introduction
1. The morphology of Helicobacter pylori
Helicobacter pylori (Hp) is a well-known gastric-parasitical pathogen. In 1982, Marshall and Warren were the first group for isolation of Helicobacter pylori organisms are spiral-shape, gram-negative bacteria approximately 2.5 to 5.0 micrometers long and 0.5 to 1 micrometer wide. In gastric biopsy specimens, H. pylori (Hp) organisms have four to six unipolar sheathed flagella, which are approximately 30 µm long and are essential for motility (Goodwin, McCulloch et al. 1985; Goodwin and Armstrong 1990). When staining with tannic acid, it can be observed that the outer membrane of H. pylori is coated with a glycocalyx-like structure (Goodwin, McCulloch et al. 1985). The surface of viable H. pylori cells grown on agar plates is coated with 12 to 15 nm ring-shaped aggregates of urease and HSP60 (Austin, Doig et al. 1992). In gastric biopsy specimens, it has been shown that both urease and HSP60 are located in the cytoplasm and surface of all bacterium (Dunn, Vakil et al. 1997).
2. The epidemiology of Helicobacter pylori
Overall H. pylori prevalence among the many studies varied from 11% to 69% (Thjodleifsson, Asbjornsdottir et al. 2007). It has been suggested that the low socioeconomic status and high densities of living are associated with high rates of infection (Bruce and Maaroos 2008). In addition, it is well accepted that H. pylori infection is acquired in childhood (Malaty, Logan et al. 2001). There have been several studies assessed the risk of gastric caner in the presence of H. pylori. By the several meta-analysises, it has been indicated that H. pylori infection is associated with approximately a two-fold increased risk of developing gastric cancer (Eslick, Lim et al. 1999; Xue 2000; Huang and Hunt 2003). In addition, some s studies have shown virulence factors of H. pylori may increase the risk of gastric cancer e.g. CagA, VacA, and dupA (Wen 2008).
3. Helicobacter pylori associated diseases
It has become clear that H. pylori infection was strongly associated with inflammation in the gastric mucosa, and cause polymophonuclear cell infiltration (Blaser 1990). The persistent infection of H. pylori could lead to gastritis, gastric ulcer and duodenal ulcer. In addition, chronic gastritis that H. pylori-induced has been indicated to linked to the development of gastric adenocarcinoma (Correa 1992). In 1994, the International Agency for Cancer
Research, an arm of the World Health Organization, declared that H. pylori was a carcinogen of humans (1994). H. pylori infection also associated with the development of gastric non-Hodgkin’s lymphomas and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (Eidt, Stolte et al. 1994; Parsonnet, Hansen et al. 1994). It has been indicated that eradicate H. pylori often contributes to regression of MALT lymphoma (Bayerdorffer, Neubauer et al. 1995). Together, H. pylori has been clearly associated with many of the most important gastroduodenal disease.
4. The background of Heat shock protein 60 (HSP60)
Heat shock proteins (HSPs) are ubiquitous and evolutionary conserved proteins. The first report the fundamental role of HSPs in cellular homeostasis and cell viability was F. Ritossa in 1962. He recognized HSPs by exposing Drosophila to 37 ℃ and proteins of 70 and 26 kDa were highly expressed, suggesting they are indispensable to overcome heat stress (Ritossa 1962). Since then, sequential studies have shown heat shock response is ubiquitous in all organisms from bacteria to plants and animals, and it is an essential defense mechanism for protecting cells from a wide range of damage condition, such as heat shock, alcohol, oxidative stress, fever, or inflammation (Lindquist 1986;
Morimoto 1993). According to molecular weight, HSPs are functionally related proteins classified into several families: HSP100, HSP90, HSP70, HSP60, HSP40 and small HSPs. HSPs act as housekeeping function and molecular chaperones that are important for the survival of eukaryotic and prokaryotic cells (Jolly and Morimoto 2000). In addition, HSPs such as HSP60 from several microorganisms may elicit a strong immune response in mammalian hosts (Zugel and Kaufmann 1999).
5. The proinflammatory roles of HSP60s
HSP60 from several microorganisms such as bacteria, protozoa, fungi and helminthes have been shown to induce host immune response after infection with these organisms (Zugel and Kaufmann 1999). Antibodies specific to Mycobacterial tuberculosis HSP60 have been detected in patients with tuberculosis and leprosy and also in mice (Young, O'Neill et al. 1988; Barrios, Tougne et al. 1994). In addition, the HSP60 of M. tuberculosis was demonstrated to activate secretion of pro-inflammation cytokines, IL1-β and TNF-α from human monocytes (Friedland, Shattock et al. 1993). HSP60 of Chlamydia pneumoniae have been demonstrated to cause acute pulmonary inflammation via TLR4 in mice and increase the level of IL-6 in the bronchoalveolar lavage fluid
(Bulut, Shimada et al. 2009). Bulut and colleagues have identified the Chlamydia trachomatis HSP60 could activate macrophages and endothelial cells trough TLR4 to activate NF-κB and promote inflammation (Bulut, Faure et al. 2002). Similarly, HSP60 of Helicobacter pylori has been shown to induce series of pro-inflammation cytokine expression such as IL-1β, IL-8, and IL-6 through TLR2 and 4 from human monocytes and gastric epithelium cells (Gobert, Bambou et al. 2004; Lin, Ayada et al. 2005; Zhao, Yokota et al. 2007).
6. The antibody response to HSP60
Helicobacter pylori is associated with gastritis and peptic ulcer disease in humans. H. pylori infection induces the host’s constitutional immune response against various antigens of this bacterium (Yunoki, Yokota et al. 2000). The detection of immunoglobulin G (IgG) antibodies to H. pylori is useful for the diagnosis of infection. Some investigators reported that the titers of these antibodies declined during therapy for H. pylori eradication (Veenendaal, Pena et al. 1991; Kosunen, Seppala et al. 1992; Perez-Perez, Brown et al. 1994; Wang, Chen et al. 1994; Cutler and Prasad 1996; Perez-Perez, Cutler et al. 1997). Yunoki et al. measured the titers of IgG antibodies to the HSP60, urease, and whole-cell lysates of H. pylori in sera from patients with peptic ulcer during
antimicrobial treatment of H. pylori and then assessed its usefulness for the monitoring of eradication therapy. In recent years, it has also been accepted that host immune reactions play an important role in the pathogenesis of H. pylori infection (Ishii, Yokota et al. 2001). Although H. pylori is a noninvasive bacterium and is restricted to gastric epithelial cells, infection with H. pylori induces humoral and cellular immune responses in the gastric mucosa (Mattsson, Quiding-Jarbrink et al. 1998; Sommer, Faller et al. 1998). In the research of Ishii et al., rHpHSP60 was expressed, and the levels of antibodies to HSP60 in sera were measured in patients with gastric ulcer, duodenal ulcer, gastritis, and gastric MALT lymphoma to demonstrate the immunological role of HSP60 in H.
pylori-infected patients(Ishii, Yokota et al. 2001). Besides, to investigate
whether the antibodies against HpHSP60 involved in inflammatory reactions to further cause gastric disease is very important.
7. Fc receptor
Fc receptors are found on some cells of the immune system. These include phagocytes like macrophages and monocytes, granulocytes like neutrophils and eosinophils, and lymphocytes of the innate immune system or adaptive immune system (Sarfati, Fournier et al. 1992; Sulica, Chambers et al. 1995; Selvaraj,
Fifadara et al. 2004). They allow these cells to bind to antibodies that are attached to the surface of microbes or microbe infected cells, helping these cells to identify and eliminate microbial pathogens. The Fc receptors bind the antibodies at their Fc region, an interaction that activates the cell that possesses the Fc receptor (Raghavan and Bjorkman 1996). Activation of phagocytes is the most common function attributed to Fc receptors. Also Fcγ receptors (FcγR) trigger inflammatory reactions in response to immunoglobulin-opsonized pathogens and antigen-antibody complexes. Thus, in our study we will investigate whether the inflammatory reactions are associated with specific antibodies.
Chapter 2: Materials and Methods
Material 1. Reagent
The following reagents obtained were described as following: RPMI1640 and Dulbecco’s modified Eagle’s medium (DMEM) were from Invitrogen Inc. (Gaithersburg, MD, USA). Fetal Bovine Serum (FBS) were from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Penicillin/ streptomycin/ amphotericin (PSA) were from Biological industries (Beithaemek, Israel). Kanoamycin and Tris were from MDBio Inc. (Rockville, MD, USA). Isopropyl-beta-D-thiogalactopyranoside, NaCl, yeast extract, agar, Tis-HCl, Triton X-100, TEMED, imidazole, glycin, and 2-mercantoethanol were from Amresco Inc. (Solon, OH, USA). Sephadex G-25 Medium was from Amersham Bioscciences (Uppsala, Sweeden). Heparin , sodium dodecyl sulfate (SDS), 3,3´,5,5´-tetramethylbenzidine (TMB), dimethyl sulfoxide (DMSO), 3,3’-di-aminobenzidine (DAB) and ammonium persulfate (APS) were from Sigma-Aldrich (Steinheim, Germany). Goat anti-human IgG, IgA, IgM antibody was from Millipore Co. (Billerica, MA, USA). Goat anti-mouse IgG (H + L chain-specific)-HRP was from Jackson Immunoresearch Laboratories (West Grove, PA, USA). Nitrocellulose membrane (Hybond-ECL extra; Amersham,
Buckingham, UK). HAT and HT medium (Hybri-Max; Sigma Chemical Co., St. Louis, MO).
2. Kit
Human IL-8, and TNF-α ELISA kit was obtained from R&D systems (Minneapolis, MN, USA). Coomasie PlusTM Protein Assay Reagent kit were from Pierce (Rockford, IL, USA).
3. Buffer
z 1╳ PBS
NaCl 8.18 g, KCl 0.2 g, Na2HPO4 1.41 g, KH2PO4 0.245 g in 1 L of DDW
z LB solution
Tryptone 10 g, Yeast extract 5 g, NaCl 10 g in 1 L of DDW z LB agar
Tryptone 10 g, Yeast extract 5 g, NaCl 10 g, Agar 15 g in 1 L of DDW z Alkaline lysis solution I
50 mM of Tris-HCl, 10 mM of EDTA, 100 µg/ml of RNase A in 100 ml of DDW
0.02M of NaOH, 1% SDS in 100 ml of DDW z Alkaline lysis solution III
2.8 M of KOAc in 100 ml of DDW, pH = 5.1 z Buffer N2
KCl 34g, Tris-base 6.057g, 75 ml of Ethanol, 0.75 ml of TritoX100, in 500 ml of DDW, pH= 6.3 z Buffer N3 Tris-HCl 6.057 g, KCl 42.86 g, 79 ml of Ethanol in 500 ml of DDW, pH= 6.3 z Buffer N5 Tris-HCl 6.057 g, KCl 37.3 g, 79 ml of Ethanol in 500 ml of DDW, pH= 8.5 z Binding buffer for protein purification
20 mM of Na2HPO4, 0.5 M of NaCl, 40 mM of Imidazole in 500 ml of
DDW, pH= 7.4
z Elution buffer for protein purification
20 mM of Na2HPO4, 0.5 M of NaCl, 500mM of Imidazole in 500 ml of
DDW, pH= 7.4
z SDS-PAGE running buffer
z SDS-PAGE loading buffer
12mM Tris-HCl, pH 6.8, 0.4% SDS, 5% glycerol, 0.02% bromphenol blue z Transfer buffer
25mM Tris, 192mM glycine, 20% methanol, 0.0375% SDS (pH8.3)
4. Instrument
HisTrapTM HP column was from GE healthcare (Uppsala, Sweeden). Sunrise remote control (TECAN). Fluorescence microscopy was from Olympus (Hicksville, NY, USA). FACScan flow cytometry (Becton Dickinson, Moutain View, CA). Semi-dry transfer cell obtained from Bio-Rad Laboratories (Hercules, CA, USA). ELISA plate (Nunc, Roskilde, Denmark).
5. Cell line
The human monocytic cell line (THP-1) and mouse myeloma cell line (F0) were obtained from the Bioresourece Collection and Research Center (Hsinchu, Taiwan).
6. Animal
Rabbit obtained from Council of Agriculture, Executive Yuan, R.O.C.
7. Other
Escherichia coli (BL21 and DH5α) were obtained from Yeastern Biotech
Co. H. pylori genome was from Department of Internal Medicine, College of Medicine, National Taiwan University.
Method
1. Cell culture condition
THP-1 were cultured in RPMI 1640 medium supplemented with 0.05 mM 2-mercantoethanol, 2g/L sodium bicarbonate, 50µg/ml of PSA, and 10% heat-inactivated FBS. FO were cultured in DMEM medium supplemented with 1.5g/L sodium bicarbonate, 10% heat-inactivated fetal bovine serum (FBS), 50ug/ml of PSA. Hybridoma were cultured in medium DMEM supplemented with 1.5g/L sodium bicarbonate, 20% heat-inactivated FBS and 50µg/ml of PS.
2. Expression and purification of HpHSP60
Transformation of E. coli
Transformation was assayed by using competent cells of E. coli. DH5α was used for plasmid amplification, and BL21 was used for protein expression. First, the competent cells were mixed gently with 1 ng DNA, and then incubated on ice for 30 min. After incubation, cells were heat shock at 42 ℃ for 90 seconds, and chilled on ice for 2 min. Next, place the competent cells to 250 µl LB and incubate at 37 ℃ with shaking 225 rpm for 1 hour. Cells were plated onto LB agar plate containing 30 µg/ml of kanamycin and incubate at 37 ℃ for
Midi plasmid DNA preparation
Pick up a single colony of transformed bacteria and transfer into 100 ml LB medium containing 30 µg/ml of kanamycin. Incubate the culture at 37 ℃ with vigorous shaking for 16 hours. The bacteria were recovered by centrifugation at 8000 rpm for 15 minutes. Remove the supernatant as dry as possible, and then resuspend the bacterial pellet with 8 ml of alkaline lysis solution I. Add 8 ml of Alkaline lysis solution II for lysis cells and mix the contents by inverting five times, then incubate for 3 min. Add 8 ml of ice-cold alkaline lysis solution III for neutralizing and gently invert the tube several times, then incubate on ice for 3 minutes. Centrifuge the bacterial lysates at 12000 rpm for 30 minutes. After washing NeucleoBond ion-exchange resin with 5 ml of buffer N2, the supernatant of bacteria lysates was added to the column; following by 20 ml of buffer N3. The plasmid DNA was eluted by adding 5 ml of buffer N5 and then separating the eluted mixture into microfuge tubes. Next, add 700 µl of isopropanol to each tube for precipitating DNA and place the tube on ice for 10 minutes. Collect the precipitated DNA by centrifugation at 13000 rpm for 45 min and then remove the supernatant. The precipitated DNA was washed by adding 1 ml of 70 % ethanol, and then dissolved into DDW. Measure the absorbance at 260 and 280 nm and assay by using restriction enzyme
digestion for checking the DNA quantity and quality.
rHpHSP60 protein induction
E. coli (BL21) were transformed with 1ng of pET-HpHSP60 plasmid, and then growth on LB plates containing 30 µg/ml of kanamycin at 37 ℃ for 16 hours. After incubation, pick up five colonies from the LB plates then inoculated into 100 ml of LB medium containing 30 mg/ml of kanamycin at 37 ℃ for 16 hours with vigorous shaking. Next, transfer the 100 ml of culture broth into 900 ml of LB medium containing 30 µg/ml of kanamycin and incubate at 37 ℃ with vigorous shaking until the value of OD600 reach the range from 0.6 to 0.8. Add 1.25 ml of IPTG (800mM) and continually incubate for 4 hours.
rHpHSP60 protein purification
After HpHSP60 induction, collect the bacterial by centrifugation at 8000 rpm for 20 min and then remove the supernatant. Resuspend the bacterial pellet with 30 ml of binding buffer, then disrupted by sonicate the whole cells mixture on ice for 15 min. After centrifugation, the supernatants were harvested. The rHpHSP60 were purified by HisTrapTMHP column according the manufacturer’s instructions. The purity of rHpHSP60 was examined by SDS-PAGE and confirmed with mass spectrometry analysis.
Gel Electrophoresis
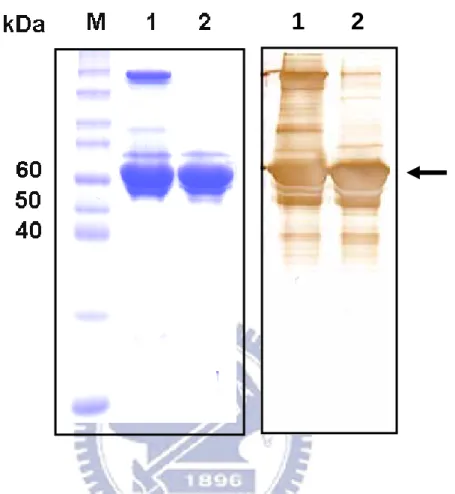
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out on 1.5-mm-thick slab gel, using discontinuous system. The gel containing 10% (for non-reducing and reducing samples) polyacrylamide was used with a top stacking gel of 5% polyacrylamide. Approximately 10µg of the protein was loaded onto the gels and each tested sample was preheated at 100 ℃ for 10 min in a loading buffer without or with 140mM 2-mercaptoethanol. The samples were then run for about 1 hr at 100V and stained by Coomassie brilliant blue (Fig 1).
Western Blot Analysis
Following the SDS-PAGE, the gel was soaked briefly and instantly in the transfer buffer for 30 s. The gel was then immediately electrotransferred to a nitrocellulose membrane at 90 mA for 45 min in a semi-dry transfer cell. The membrane was immersed in 2% skim milk for 1 h with gentle shaking. Following 3 x washes with PBS for 5 min, the membrane was then treated with polyclonal antibodies or mAb and developed with 3-3’-diaminobenzidine (3,3’,4,4’-tetra-amino-biphenyl) according to the method previously described (Yang and Mao 1999).
3. Detection of cytokines production in the THP-1 cells
THP-1 cells (5×105) were seeded in 24- well culture plates and incubated at 37℃, 5% CO2 atmosphere for 1hours. And 10µg HpHSP60 were treated into
seeded cells for 24 hr. The supernatants with or without HpHSP60 stimulation were collected. TNF-α, IL-1β, IL-6, and IL-8 levels in the supernatants was measured by ELISA, according to the manufacturer’s specifications.
4. Measurement of patients’ sera antibody to HpHSP60
The serum samples were obtained from National Taiwan University Hospital. The serum samples derived from the patients which were diagnosis as Helicobacter pylori infection. According to the titer, samples were separated to 2 groups. And there were four symptoms including gastric cancer (HC), gastritis (HS), duodenal ulcer (HD) or peptic ulcer (HU). Serum antibodies to HpHSP60 were measured by enzyme-linked immunosorbent assay (ELISA). First, 96-well plates were coated with 100µL of HpHSP60 (1 mg/mL) and in phosphate-buffer saline overnight at 4 ℃ . After the wells were blocked with 300 µl of phosphate-buffer saline tween-20 containing 5% skim milk for 1 hour, plates were incubated with sera at a dilution of 1: 10,000 for anti-HpHSP60 antibody for 1 hour at room temperature and washed three times with phosphate-buffered
saline tween-20. Peroxidase-labeled goat anti-human IgG, IgA, IgM antibody was added (1:10,000), and the plate was incubated for 1 hour at room temperature. After plate was washed, each well was reacted with 3,3´,5,5´-tetramethylbenzidine solution for 20 minutes. After plate was reacted, the wells were added HCl to stop reaction. The optical density was measured at 450 nm on an ELISA plate reader.
5. Determination of TNF-α and IL-8 levels in sera condition by ELISA
THP-1 cells were seeded in 24- well culture plates with 0.2 ml of cell suspension (5×105/well) and incubated at 37℃, 5% CO2 atmosphere for 1hours.
And 5µg HpHSP60 were preincubated with sera (patients’ sera 1:250, anti-HpHsp60 mouse polyclonal antibodies 1:1000 and anti-HpHsp60 rabbit polyclonal antibodies 1:1000/200/100/50) in volume of 0.8ml for 30 min then treated into seeded cells for 24 hr. All sera were diluted then filtered by 0.22 nm filter. TNF-α, and IL-8 levels in the supernatants was measured by ELISA, according to the manufacturer’s specifications.
6. Production of monoclonal antibody Animal care and use
The monoclonal antibody productions were utilized Balb/c mice with 5-7 weeks of age. The mice were fed in animal room from Chiao Tung University during the period of immunization. Feed and water were available daily. CO2.
was used as a method of sacrificing and the other management was conducted according to guidelines established by NSC of Taiwan.
Preparation of mouse polyclonal antibodies against HpHSP60
Female Balb/c mice, aged 5-7 weeks, were used for immunization. HpHSP60 in sterilized phosphate buffered saline (PBS), containing 0.12 M NaCl, 0.02 M phosphate, pH 7.4, was mixed and homogenized with an equal volume of complete Freund’s adjuvant by a three-way stopcock. Each mouse was initially given a total emulsion of 0.3 ml containing 100 µg of protein with 6 subcutaneous injections onto the back and an intraperitoneal injection. At day 7, an identical dose with incomplete adjuvant was given intraperitoneally followed by two intramuscular injections without adjuvant at day 14. Seven days following a final booster, blood was collected in 0.1% (wt/vol) EDTA and plasma was obtained. This plasma was used as a source for conventional polyclonal antibody against HpHSP60. Additional booster injections were given when necessary.
Female rabbit were used for immunization. HpHSP60 in sterilized phosphate buffered saline (PBS), containing 0.12 M NaCl, 0.02 M phosphate, pH 7.4, was mixed and homogenized with an equal volume of incomplete Freund’s adjuvant by a three-way stopcock. The rabbit was initially given a total emulsion of 2 ml containing 1 mg of protein with 6 subcutaneous injections onto the back. At day 30, an identical dose with incomplete adjuvant was given onto back followed by intramuscular injections at day 60. Then blood was collected in 0.1% (wt/vol) EDTA and plasma was obtained. This plasma was used as a source for conventional polyclonal antibody against HpHSP60. Additional booster injections were given when necessary. The titers of this antibody were over 1:20,000 as judged by an ELISA (described below).
Production of monoclonal antibody
After immunization, the titers of this antibody were over 1:6,400 as judged by an ELISA (described below). The spleen obtained was used for preparing hybridoma fusion. Monoclonal antibodies were produced according to the standard procedures (Mao, Rechtin et al. 1988; Mao, Rechtin et al. 1990). In brief, myeloma cell line (FO) was fused with spleen cells from immunized Balb/c mice at a ratio of 1:5. Fusion was carried out within 2 min at 37 ℃ using 1 ml of 50% (wt/vol) polyethylene glycol containing 10% (vol/vol) DMSO. Cell
mixture was then washed and resuspended in HAT medium containing approximately 1 x 105 FO cells per ml. The suspended cells were distributed as 100 µl per well in 96-well microtiter plates and incubated at 37℃ in a 5% CO2-
incubator followed by an addition of 100 µl of fresh HAT medium after 7 days. Subsequently, culture medium was assayed for the production of specific antibodies, between 14 and 21 days following the fusion, using a solid-phase ELISA described below. After primary screening, desired hybridomas were selected, expanded, and subcloned.
Enzyme linked immunosorbent assay
Initially, approximate 1 µg of HpHSP50 in 100 µl of PBS was coated on each well of an ELISA plate for screening hybridoma antibodies. Unbound proteins were washed with PBS 3 x and subsequently blocked by an addition of 300 µl of 2% (wt/vol) skim mlik for 1 hr. Following washes with PBS, 100 µl of hybridoma culture medium (2-3 weeks following the fusion) were added and incubated at room temperature for 60-90 min. Each well was washed 3 x with PBS containing 0.5% skim milk and 0.05% Tween-20. Bound antibodies were detected using a goat anti-mouse IgG (H + L chain-specific) conjugated with horseradish peroxidase (1:10,000) for 1 hr in PBS containing 0.5% skim milk and 0.05% Tween-20. Finally, each well was washed and developed with 0.04%
(wt/vol) 2,2-Azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) containing 0.01% (vol/vol) H2O2 in PBS.
Purification of HpHSP60 mAbs
Twice volume of 0.06 M sodium acetate buffer was added to hybridoma culture medium then added caprylic acid stirring at room temperature for 30 min. Centrifuge at 4000 g for 30 min and retains the supernatant. By a 50% saturated ammonium, the precipitate was dissolved in 2 ml PBS followed by extensive dialyses against a 20 mM phosphate buffer at pH7.2. to remove the remaining ammonium sulfate.
7. Determination of TNF-α and IL-8 levels in mAbs condition by ELISA
THP-1 cells were seeded in 24- well culture plates with 0.9 ml of cell suspension (5×105/well) and incubated at 37℃, 5% CO2 atmosphere for 1hours.
And 5µg HpHSP60 were preincubated with mAb and non-related mAb (0.4, 2, 10, 50 µg) for 30 min in volume of 0.1 ml then treated into seeded cells for 24 hr. On the other hand, the protocol of non-related mAb pretreating experiments, THP-1 cells were seeded in 24- well culture plates with 0.9 ml of cell suspension (5×105/well) contained with non-related mAb (50µg) and incubated at 37℃, 5% CO2 atmosphere for 1 h. And 5µg HpHSP60 were preincubated
with 5A8, 5A12 and 5B11 (10 µg) for 30 min in volume of 0.1 ml then added into seeded cells for 24 hr. TNF-α, and IL-8 levels in the supernatants was measured by ELISA, according to the manufacturer’s specifications.
Chapter 3: Results
1. The preparation of recombinant H. pylori HSP60
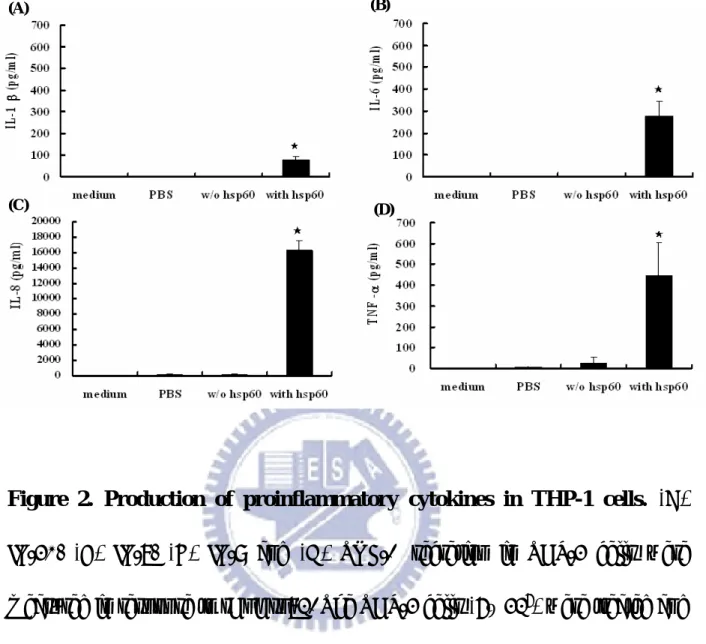
Several publishes have revealed that H. pylori HSP60 would stimulate IL-8 or IL-6 secretions in human monocytic cells or mouse macrophage respectively (Gobert, Bambou et al. 2004; Lin, Ayada et al. 2005). In this study, we cloned and expressed the recombinant HpHSP60 in E. coli expression system. The migration on SDS-PAGE revealed that the molecular weight of HpHsp60 is about 66 kDa (Fig. 1; left panel) and the rHpHSP60 could be recognized by mouse anti-HpHSP60 polyclonal antibodies. The results indicated that we successfully obtained rHpHSP60. Further, whether the rHpHSP60 proteins maintained their bioactivities to induce proinflammatory cytokines expressions on THP-1 cells as wild type of HpHSP60 was determined. The rHpHSP60 (10 µg/ml) were treated THP-1 cells for 24 h, and the supernatants were collected. Pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8 in the supernatant were analyzed by ELISA. The productions of TNF-α, IL-1β, IL-6, and IL-8 in response to 10 µg/ml rHpHSP60 were significantly increased comparing to the samples without rHpHSP60 treatment. The mean value of IL-1β, IL-6, IL-8 or TNF-α in the culture medium was 101 ± 38, 277 ± 66, 16301 ± 1305 or 449 ± 153 pg/ml, respectively (Fig. 2A-2D). The results
indicated that the rHpHSP60 still had the capability to induce the releases of proinflammatory cytokines.
2. The patient sera with anti-HpHSP60 antibodies could enhance the
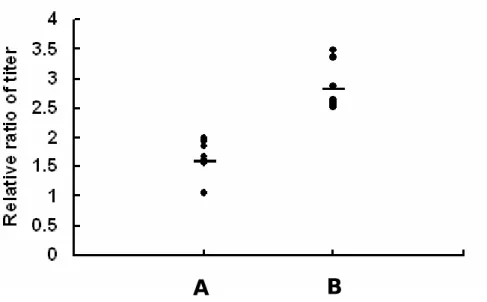
expressions of TNF-α and IL-8.
The anti-HpHSP60 antibodies in sera of H. pylori-infected patients were measured by ELISA (Fig 3). According to the results, samples were divided into two groups: low titer group and high titer group (A: relative titer ratio < 2.2; B: relative titer ratio > 2.2). The mean value ± SD of serum antibodies to H. pylori-positive patients determined by an ELISA in group A (n = 8) and B (n = 8) were 1.59 ± 0.36 and 2.82 ± 0.39.
To determine whether the anti-HpHSP60 antibodies in sera of H. pylori-infected patients could lower the ability of HpHSP60 to stimulate proinflammatory cytokine secretions, the patients’ sera from A and B groups were respectively incubated with rHpHSP60. The inductive amounts of TNF-α or IL-8 were determined and the results showed no matter which sera all could increase the expressions of TNF-α (Fig. 4A; A group: 770.57 ± 147.30 and B group: 740.75 ± 105.75 pg/ml) or IL-8 (Fig. 4B; A group : 10943.35 ± 4935.40 and B group: 10280.29 ± 2148.42 pg/ml) than the samples without sera
treatments (TNF-α: 554.99 ± 35.33 and IL-8:4355.42 ± 331.12 pg/ml). There were no significant differences between low titer group (A group) and high titer group (B group). In addition, the patients’ sera alone without rHpHSP60 treatments could not result in any increases in the expressions of TNF-α or IL-8.
3. The effects of mouse and rabbit anti-HpHSP60 polyclonal antibodies on
the abilities of HpHSP60 to induce expressions of TNF-α and IL-8.
To test the hypothesis that the anti-HpHSP60 polyclonal antibodies in H. pylori-infected patients’ sera could enhance the abilities of HpHSP60 to stimulate the expressions of TNF-α or IL-8 secretion, anti-HpHSP60 polyclonal antibodies derived from mice or rabbit immunized with rHpHSP60 were used to study the effects of polyclonal antibodies on the activities of HpHSP60 for inductions of proinflammatory cytokines. Combining with HpHSP60, all mouse anti-HpHSP60 sera significantly increased the expressions of TNF-α (1674.49 ± 188.12 pg/ml) or IL-8 (22402.75 ± 3341.75 pg/ml) comparing to the samples without sera treatment (TNF-α: 599.25 ± 19.93 and IL-8: 4587.82 ± 965.07 pg/ml) (Fig 5). While rHpHSP60 were treated with normal sera derived from naïve mice, TNF-α and IL-8 expressions were no significantly increase. Similarly, the sera only without rHpHSP60 treatments could not cause any
significant TNF-α and IL-8 expressions.
All treatments with different doses of rabbit anti-HpHSP60 polyclonal antibodies facilitated rHpHSP60 to induce the expressions of TNF-α and IL-8, which were in accordance with the mouse sera (Fig. 6 A and B).
4. The effects of monoclonal antibodies on the abilities of HpHSP60 to
induce expressions of TNF-α and IL-8.
So far, the previous results have revealed that sera against HpHSP60 no matter it were from patients, mice or rabbit can enhance the abilities of rHpHsp60 to stimulate TNF-α or IL-8 secretions. However, certain facts but not anti-HpHSP60 antibodies in serum may involve in or dominate this enhancive effect, which still could not be excluded. Thus, monoclonal antibodies against HpHSP60 were produced to verify the phenomenon. As shown in Table 1, from 390 hybridomas in a primary screening, 260 hybridomas could react with rHpHSP60 and 4 hybridomas were able to recognize the wild type HpHSP60. Finally, 3 hybridomas were established and used in this study (designated as 5A8, 5A12 and 5B11) (Table 1).
Different dosages (0.4, 2, 10, 50 µg/ml) of three monoclonal antibodies were incubated with rHpHSP60 to treat THP-1 cells. All mAbs (5A8, 5A12 and
5B11) significantly increased the abilities of HpHSP60 to trigger the expressions of TNF-α comparing to the samples without mAb treatment (Fig 7A). In IL-8, almost doses of tree mAbs could significantly enhance the secretions, except of 0.4 µg/ml of 5A8, 50 µg/ml of 5A12 and 50 µg/ml of 5B11 (Fig 7B). In addition, these mAbs could not enhance the secretions of proinflmmatory cytokines by themselves.
The previous results indicated that the additional mAbs could cause the enhancive effect but the antigen-binding activities were essential for the enhancive effect or not still was unclear. Thus, the non-related Abs (anti-bovine haptoglobin) were prior interacted with rHpHSP60 and the results showed that TNF-α or IL-8 expressions were higher decreased as additions of non-related Abs were more (Fig. 8A and B). Further, whether the non-related mAb could affect the enhancive effect of specific anti-HpHSP60 mAbs on the cytokines expressions induced by HpHSP60 was examined. Figure 9A-C showed the abilities of 5A8, 5A12 and 5B11 to enhance expressions of TNF-α was significantly suppressed by non-related mAbs. Similarly, the non-related mAbs also decreased the specific anti-HpHSP60 mAbs’ enhancive effects on the IL-8 expressions (Fig. 10A-C).
Chapter 4: Discussion
In this study, we explored that the anti-HpHSP60 antibodies in patients’ sera could significantly enhance the ability of HpHSP60 to induce TNF-α or IL-8. According the previous literatures, anti-HpHSP60 antibodies could be detected in H. pylori infected patients’ sera (Yunoki, Yokota et al. 2000; Ishii, Yokota et al. 2001; Tanaka, Kamada et al. 2009) and our results agreed well with these clinical examinations. Figure 3 pointed out that all H. pylori-infected patients had antibodies against HpHSP60 in their sera.
Furthermore, Dr. Perez-Perez et al. demonstrated that the titer of anti-HpHSP60 was correlated with the degree of gastric mucosal inflammation (Hussain, Shiratsuchi et al. 2000). As the knowledge from the literatures, extracellular HSPs are the most powerful ways of sending a ‘danger signal’ to the immune system in order to generate the responses that can help the organism manage an infection or disease and they are associating with the innate or adaptive immune systems activation (Ellis 1990; Young 1990). Furthermore, microbial HSP60s have been explored as an immunodominant antigen since it could induce powerful immune response after infection (Habich, Kempe et al. 2003). Bacterial HSPs such as HSP65 of Mycobacterial leprae have also been reported that they have the capability to induce releases of TNF-α, IL-6, and
IL-8 from human monocytic cells (Friedland, Shattock et al. 1993). Similarly, HpHSP60 has been reported to induce proinflammatory cytokines including IL-6, and IL-8 from human monocytic cells and/or gastric epithelium cells (Lin, Ayada et al. 2005; Zhao, Yokota et al. 2007), which is dominated by TLR-2 and TLR-4 pathways (Gobert, Bambou et al. 2004; Takenaka, Yokota et al. 2004).
Furthermore, some reports indicate that HSP60 is located on the surface of the bacteria (Yamaguchi, Osaki et al. 1996). These data indicate that hsp60 develops a tendency to be recognized by the host and may be closely related to H. pylori-induced inflammation. However, the role of anti-HpHSP60 antibodies in this inflammation is not clear. Ishii et al. have reported that the levels of IgG1 antibodies to HSP60 are elevated and it is closely associated with MALT lymphoma in H. pylori infected patients (Ishii, Yokota et al. 2001). Moreover, Hussain et al. also indicated the specific anti-PPD antibodies (IgG1) could up-regulate the activity of PPD to induce proinflammatory cytokines (Hussain, Shiratsuchi et al. 2000), which is similar as our finding on HpHSP60. In addition, different polyclonal anti-sera derived from different species confirm this effect again (Fig. 5 and 6), which indicated that the difference in species did not involved in the mechanism of enhancive effect on induction of proinflammatory cytokine release.
To sure the effect is because the anti-HpHSP60 antibodies but not the other factors in sera, we produced monoclonal antibodies against HpHSP60 to exclude the effects of non-specific anti-HpHSP60 antibodies or other factors in sera. Our data showed all purified mAbs (5A8, 5A12 and 5B11) could also cause higher levels of TNF-α or IL-8 expression in monocytes (Fig 7). Similar enhancive effect on pathogenesis by monoclonal antibodies is also explored for to H. capsulatum HSP60 (Guimaraes, Frases et al. 2009). So far, our results indicated the anti-HpHSP60 antibodies could result in this enhancive effect. Although these results revealed that different affinity of mAbs would cause different influences on cytokines expressions, seemingly the affinity is not the mechanism for the enhancive effect.
Our results indicated the specific anti-HpHSP60 antibodies could raise the capability of HpHSP60 to enhance the expressions of proinlammatory cytokines but the mechanisms were still unclear. Previous literatures have explored that HpHSP60 could induce expressions of proinflammatory cytokines through TLR-2 and TLR-4 pathways (Gobert, Bambou et al. 2004; Takenaka, Yokota et al. 2004). Although which factor(s) involved in the enhancive effects of specific antibodies did not completely identify, our results have indicated the non-related mAb could decrease the enhancive effect of specific mAbs (Fig 9 and 10).
Therefore, we proposed that Fc receptors may involve in the enhancive effects of specific antibodies. It has been reported that the Fc receptor can interact with the antibody’ Fc region (Raghavan and Bjorkman 1996) and the recent study has further found that Fcγ receptors can trigger inflammatory reactions in response to immunoglobulin-opsonized pathogens or antigen-antibody complexes (Liu, Masuda et al. 2005). Thus, prior combination of the anti-HpHSP60 antibody with HpHSP60 should posterior bind to Fc receptor, which could increase the probability of HpHSP60 to interact with TLR. Besides, the engagement of the Fc receptor could also trigger the signaling to contribute to the enhancive effect.
Finally, we indicated that the antibodies against HpHSP60 could enhance the ability of HpHSP60 to induce proinflammatory cytokines secretion in monocytes. In addition, we proposed that Fc receptor on monocyte involved in the enhancive effect of the anti-HpHSP60 antibodies. H. pylori infection causes the gastric diseases by induction of chronic inflammation. Although several virulent factors in this pathogenic microorganism have been identified, the pathogenic immunity induced by these factors are rare discussed. According our results, we proposed that patients’ the anti-HpHSP60 antibodies may bind to HpHSP60 to result in more serious inflammation and lead to more serious tissue damages in infectious sites.
Figures and Legends
Figure 1. SDS-PAGE and Western blot analyses of recombinant HpHSP60.
Left panel: Coomassie blue staining of rHpHSP60 run on a 10% SDS-PAGE under non-reducing and reducing conditions. Right panel: Western blot analysis of rHpHSP60 run on a 10% SDS-PAGE under non-reducing and reducing conditions. Lane M means the molecular marker and sample reacted without or with β-ME buffer was non-reducing conditions (lanes 1) and reducing conditions (lanes 2).
Figure 2. Production of proinflammatory cytokines in THP-1 cells. (A)
IL-1β, (B) IL-6, (C) IL-8 and (D) TNF-α secretion in THP-1 cells were measured in response to rHpHsp60. The THP-1 cells (5× 105) were treated and incubated with the rHpHsp60s (final concentration of 10 µg/ml) in 24-well plate for 24 h, and the cytokines in the medium were determined by ELISA. Results are representative three independent experiments. , ★ P<0.001 compared to samples without rHpHsp60 treatment. (n = 3). Source from (Lin 2008)
(A) (B)
Figure 3. The relative ratio of serum antibodies to HpHSP60 in H.
pylori-positive patients. The titer of anti-HpHSP60 antibodies from sera of H. pylori-positive patients were analyzed using ELISA. And there are four symptoms of samples including gastric cancer, gastritis, duodenal ulcer, and gastric ulcer. According to the titer, samples were divided into two groups including low titer group (A group, n = 8) and high titer group (B group, n = 8). Results are shown as mean ± SD.
(A.) (B.) -* ** HpHSP60 + + + Patients’ serum A B -*** * HpHSP60 + + Patients’ serum A B +
Figure 4. The patients sera with anti-HpHSP60 antibodies could enhance
the expressions of TNF-α and IL-8. The expressions of TNF-α (A.) and IL-8
(B.) were assessed by ELISA as described in material and method section. The patients’ sera of A and B groups were 1:250 diluted and incubated with HpHSP60 (5µg/ml) for 30 min then treated THP-1 cells (5×105 /well) for 24
hour. The horizontal lines of the data were expressed as the sample only with cells. Results are shown as mean ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001 as compare to samples without sera treatments.
(A.) (B.) HpHSP60 + + Polyclonal Abs - + *** HpHSP60 + + Polyclonal Abs - + ***
Figure 5. The effect of mouse polyclonal antibodies on the abilities of
HpHSP60 to induce expressions of TNF- α and IL-8. The expressions of
TNF-α (A.) and IL-8 (B.) were assessed by ELISA as described in material and method section. The mouse anti-HpHSP60 polyclonal antibodies were diluted (1:1000) and incubated with HpHSP60 (5µg/ml) for 30 min then treated THP-1 cells (5×105 /well) for 24 hour. The horizontal lines of the data were expressed as the sample only with cells. Results are shown as mean ± SD. ***p< 0.001 as compare to samples without sera treatments.
(A.) (B.) 0 200 400 600 800 1000 1200 1400 1600 TN F-a lpha c o nc . ( p g/ m l) HpHSP60 R polyclonal Ab - - 1:1000 1:200 1:100 1:50 + + + + + - + - ** ** ** ** 0 2000 4000 6000 8000 10000 12000 14000 16000 IL -8 c o n c . (p g /m l) HpHSP60 R polyclonal Ab - - 1:1000 1:200 1:100 1:50 + + + + + - + - *** *** *** ***
Figure 6. The effect of rabbit polyclonal antibodies on the abilities of
HpHSP60 to induce expressions of TNF- α and IL-8. The expressions of
TNF-α (A.) and IL-8 (B.) were assessed by ELISA as described in material and method section. The rabbit anti-HpHSP60 polyclonal antibodies were diluted (1:1000, 1:200, 1:100 and 1:50) and incubated with HpHSP60 (5µg/ml) for 30 min then treated THP-1 cells (5×105 /well) for 24 hour. Results are shown as mean ± SD. **p< 0.01 and ***p< 0.001 as compare to samples only with HpHSP60 treatments. (n = 3) R polyclonal Abs: Rabbit anti-HpHSP60 polyclonal antibodies.
(A.) (B.) (µg/ml) 0 200 400 600 800 1000 m A bs TN F-a lpha c onc . ( pg/ m l) 5A8 5A12 5B11 HpHSP60 0 5 5 5 5 5 0 0 0.4 2 10 50 mAbs ** ** ** ** ** ** * * *** *** *** ** 0 2000 4000 6000 8000 10000 m A b s IL -8 c o n c . (p g /m l) 5A8 5A12 5B11 HpHSP60 0 5 5 5 5 5 0 0 0.4 2 10 50 (µg/ml) mAbs ** * * ** ** * ** *** *
Figure 7. The effect of monoclonal antibodies on the abilities of HpHSP60
to induce expressions of TNF- α and IL-8. The expressions of TNF-α (A.) and
IL-8 (B.) were assessed by ELISA as described in material and method section. At first, HpHSP60 (5µg/ml) were incubated with various concentration of monoclonal antibodies (5A8, 5A12 and 5B11) including 0.4, 2, 10, 50 µg/ml for 30 min then treated THP-1 cells (5×105 /well) for 24 hour. Then the supernatants were harvested to be examined for the cytokines expression level. Results are shown as mean ± SD. *p< 0.05, **p< 0.01, ***p< 0.001 as compare to samples only with HpHSP60 treatments. (n = 3)
(A.) (B.) 0 200 400 600 800 1000 TN F-a lp h a co nc. ( pg /m l) non-related mAb HpHSP60 0 5 5 5 5 5 0 0 0.4 2 10 50 (µg/ml) Non-related mAbs 0 50 ** *** *** HpHSP60 0 5 5 5 5 5 0 0 0.4 2 10 50 (µg/ml) Non-related mAbs 0 50 0 2000 4000 6000 8000 10000 IL-8 c onc . ( pg/ m l) non-related mAb * ** **
Figure 8. The effect of non-related mAb on the abilities of HpHSP60
to induce expressions of TNF- α and IL-8. HpHSP60 (5µg/ml) were incubated
with various concentration of non-related mAb including 0.4, 2, 10, 50 µg/ml for 30 min then treated THP-1 cells (5×105 /well) for 24 hour. And the TNF-α (A.) and IL-8 (B.) levels in supernatant were measured by ELISA. Results are shown as mean ± SD. *p< 0.05, **p< 0.01, ***p< 0.001 as compare to samples only with HpHSP60 treatments. (n = 3) Non-related mAb: The monoclonal antibody against haptoglobin.
(A.) (B.) (C.) (µg/ml) 0 200 400 600 800 1000 TN F-al pha con c . (pg/ m l) 5B11 HpHSP60 5B11 Non-related mAb 0 0 5 0 0 0 0 10 5 50 10 5 ** ** 0 200 400 600 800 1000 T N F-al pha conc. (pg/ m l) 5A12 HpHSP60 5A12 Non-related mAb 0 0 5 0 0 0 0 10 5 50 10 5 (µg/ml) ** ** HpHSP60 5A8 Non-related mAb 0 0 5 0 0 0 0 10 5 50 10 5 0 200 400 600 800 1000 TNF -alpha con c. (p g/m l) 5A8 (µg/ml) ** *
Figure 9. The non-related mAbs interfere with anti-HpHSP60 monoclonal antibodies to enhance the abilities of HpHSP60 to induce expressions of TNF- α. (A.) 5A8, (B.) 5A12 and (C.) 5B11 (10 µg/ml) were incubated with
HpHSP60 (5µg/ml) for 30 min then treated to THP-1 cells (5×105 /well) which
were pretreated with the non-related Ab (50µg/ml) for 1 hr then incubated for 24 hr. And the TNF-α levels in supernatant were measured by ELISA. Results are shown as mean ± SD. *p< 0.05, **p< 0.01, as compare to samples only with HpHSP60 treatments or samples with non-related Ab treatments. (n = 3) Non-related mAb: The monoclonal antibody against haptoglobin.
(A.) (B.) (C.) (µg/ml) 0 2000 4000 6000 8000 10000 IL -8 c o n c . (p g /m l) 5A8 HpHSP60 5A8 Non-related mAb 0 0 5 0 0 0 0 10 5 50 10 5 ** ** 0 2000 4000 6000 8000 10000 IL -8 c o n c . (p g /m l) 5A12 (µg/ml) HpHSP60 5A12 Non-related mAb 0 0 5 0 0 0 0 10 5 50 10 5 ** *** 0 2000 4000 6000 8000 10000 IL -8 c o n c . (p g /m l) 5B11 HpHSP60 5B11 Non-related mAb 0 0 5 0 0 0 0 10 5 50 10 5 *** ***
Figure 10. The non-related mAbs interfere with anti-HpHSP60 monoclonal antibodies to enhance the abilities of HpHSP60 to induce expressions of
IL-8. (A.) 5A8, (B.) 5A12 and (C.) 5B11 (10 µg/ml) were incubated with
HpHSP60 (5µg/ml) for 30 min then treated to THP-1 cells (5×105 /well) which
were pretreated with the non-related Ab (50µg/ml) for 1 hr then incubated for 24 hr. And the IL-8 levels in supernatant were measured by ELISA. Results are shown as mean ± SD. **p< 0.01 and ***p< 0.001, as compare to samples only with HpHSP60 treatments or samples with non-related Ab treatments. (n = 3) Non-related Ab: anti-bovin haptoglobin antibody.
References
(1994). "Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994." IARC Monogr Eval Carcinog Risks Hum 61: 1-241.
Austin, J. W., P. Doig, et al. (1992). "Structural comparison of urease and a GroEL analog from Helicobacter pylori." J Bacteriol 174(22): 7470-3.
Barrios, C., C. Tougne, et al. (1994). "Specificity of antibodies induced after immunization of mice with the mycobacterial heat shock protein of 65 kD." Clin Exp Immunol 98(2): 224-8.
Bayerdorffer, E., A. Neubauer, et al. (1995). "Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group." Lancet 345(8965): 1591-4. Blaser, M. J. (1990). "Helicobacter pylori and the pathogenesis of
gastroduodenal inflammation." J Infect Dis 161(4): 626-33.
Bruce, M. G. and H. I. Maaroos (2008). "Epidemiology of Helicobacter pylori infection." Helicobacter 13 Suppl 1: 1-6.
Bulut, Y., E. Faure, et al. (2002). "Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway." J Immunol 168(3): 1435-40.
Bulut, Y., K. Shimada, et al. (2009). "Chlamydial heat shock protein 60 induces acute pulmonary inflammation in mice via the Toll-like receptor 4- and
MyD88-dependent pathway." Infect Immun 77(7): 2683-90.
Correa, P. (1992). "Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention." Cancer Res 52(24): 6735-40.
Cutler, A. F. and V. M. Prasad (1996). "Long-term follow-up of Helicobacter pylori serology after successful eradication." Am J Gastroenterol 91(1): 85-8.
Dunn, B. E., N. B. Vakil, et al. (1997). "Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies." Infect Immun 65(4): 1181-8.
Eidt, S., M. Stolte, et al. (1994). "Helicobacter pylori gastritis and primary gastric non-Hodgkin's lymphomas." J Clin Pathol 47(5): 436-9.
Ellis, R. J. (1990). "The molecular chaperone concept." Semin Cell Biol 1(1): 1-9.
Eslick, G. D., L. L. Lim, et al. (1999). "Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis." Am J Gastroenterol 94(9): 2373-9.
Friedland, J. S., R. Shattock, et al. (1993). "Mycobacterial 65-kD heat shock protein induces release of proinflammatory cytokines from human monocytic cells." Clin Exp Immunol 91(1): 58-62.
Gobert, A. P., J. C. Bambou, et al. (2004). "Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism." J Biol Chem 279(1): 245-50.
Goodwin, C. S. and J. A. Armstrong (1990). "Microbiological aspects of Helicobacter pylori (Campylobacter pylori)." Eur J Clin Microbiol Infect Dis
9(1): 1-13.
Goodwin, C. S., R. K. McCulloch, et al. (1985). "Unusual cellular fatty acids and distinctive ultrastructure in a new spiral bacterium (Campylobacter pyloridis) from the human gastric mucosa." J Med Microbiol 19(2): 257-67.
Guimaraes, A. J., S. Frases, et al. (2009). "Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of Histoplasma capsulatum." Infect Immun
77(4): 1357-67.
Habich, C., K. Kempe, et al. (2003). "Different heat shock protein 60 species share pro-inflammatory activity but not binding sites on macrophages." FEBS
Lett 533(1-3): 105-9.
Huang, J. Q. and R. H. Hunt (2003). "The evolving epidemiology of
Helicobacter pylori infection and gastric cancer." Can J Gastroenterol 17 Suppl
B: 18B-20B.
Hussain, R., H. Shiratsuchi, et al. (2000). "PPD-specific IgG1 antibody subclass upregulate tumour necrosis factor expression in PPD-stimulated monocytes: possible link with disease pathogenesis in tuberculosis." Clin Exp Immunol
119(3): 449-55.
Ishii, E., K. Yokota, et al. (2001). "Immunoglobulin G1 antibody response to Helicobacter pylori heat shock protein 60 is closely associated with low-grade gastric mucosa-associated lymphoid tissue lymphoma." Clin Diagn Lab
Immunol 8(6): 1056-9.
Jolly, C. and R. I. Morimoto (2000). "Role of the heat shock response and
molecular chaperones in oncogenesis and cell death." J Natl Cancer Inst 92(19): 1564-72.
Kosunen, T. U., K. Seppala, et al. (1992). "Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori." Lancet
339(8798): 893-5.
Lin, S. N., K. Ayada, et al. (2005). "Helicobacter pylori heat-shock protein 60 induces production of the pro-inflammatory cytokine IL8 in monocytic cells." J Med Microbiol 54(Pt 3): 225-33.
Lin, Y., Y (2008). "Heat shock protein 60 of helicobacter pylori induces proinflammatory cytokined secretion but diminishes monocyte activation." Lindquist, S. (1986). "The heat-shock response." Annu Rev Biochem 55: 1151-91.
Liu, Y., E. Masuda, et al. (2005). "Cytokine-mediated regulation of activating and inhibitory Fc gamma receptors in human monocytes." J Leukoc Biol 77(5): 767-76.
preschool and school-aged minority children: effect of socioeconomic indicators and breast-feeding practices." Clin Infect Dis 32(10): 1387-92.
Mao, S. J., A. E. Rechtin, et al. (1988). "Monoclonal antibodies that distinguish between active and inactive forms of human postheparin plasma hepatic
triglyceride lipase." J Lipid Res 29(8): 1023-9.
Mao, S. J., A. E. Rechtin, et al. (1990). "Characterization of a monoclonal antibody specific to the amino terminus of the alpha-chain of human fibrin." Thromb Haemost 63(3): 445-8.
Mattsson, A., M. Quiding-Jarbrink, et al. (1998). "Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects." Infect Immun 66(6): 2705-12.
Morimoto, R. I. (1993). "Cells in stress: transcriptional activation of heat shock genes." Science 259(5100): 1409-10.
Parsonnet, J., S. Hansen, et al. (1994). "Helicobacter pylori infection and gastric lymphoma." N Engl J Med 330(18): 1267-71.
Perez-Perez, G. I., W. R. Brown, et al. (1994). "Correlation between serological and mucosal inflammatory responses to Helicobacter pylori." Clin Diagn Lab Immunol 1(3): 325-9.
Perez-Perez, G. I., A. F. Cutler, et al. (1997). "Value of serology as a noninvasive method for evaluating the efficacy of treatment of Helicobacter pylori
infection." Clin Infect Dis 25(5): 1038-43.
Raghavan, M. and P. J. Bjorkman (1996). "Fc receptors and their interactions with immunoglobulins." Annu Rev Cell Dev Biol 12: 181-220.
Ritossa, F. (1962). "A new puffing pattern induced by temperature shock and DNP in Drosophila." Experientia 18: 571-573.
Sarfati, M., S. Fournier, et al. (1992). "Expression, regulation and function of human Fc epsilon RII (CD23) antigen." Immunol Res 11(3-4): 260-72.
Selvaraj, P., N. Fifadara, et al. (2004). "Functional regulation of human neutrophil Fc gamma receptors." Immunol Res 29(1-3): 219-30.
Sommer, F., G. Faller, et al. (1998). "Antrum- and corpus mucosa-infiltrating CD4(+) lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype." Infect Immun 66(11): 5543-6.
Sulica, A., W. H. Chambers, et al. (1995). "Divergent signal transduction
pathways and effects on natural killer cell functions induced by interaction of Fc receptors with physiologic ligands or antireceptor antibodies." Nat Immun 14(3): 123-33.
Takenaka, R., K. Yokota, et al. (2004). "Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells." Microbiology 150(Pt 12): 3913-22.
Tanaka, A., T. Kamada, et al. (2009). "Helicobacter pylori heat shock protein 60 antibodies are associated with gastric cancer." Pathol Res Pract.
Thjodleifsson, B., H. Asbjornsdottir, et al. (2007). "Seroprevalence of
Helicobacter pylori and cagA antibodies in Iceland, Estonia and Sweden." Scand J Infect Dis 39(8): 683-9.
Veenendaal, R. A., A. S. Pena, et al. (1991). "Long term serological surveillance after treatment of Helicobacter pylori infection." Gut 32(11): 1291-4.
Wang, W. M., C. Y. Chen, et al. (1994). "Long-term follow-up and serological study after triple therapy of Helicobacter pylori-associated duodenal ulcer." Am J Gastroenterol 89(10): 1793-6.
Wen, S. a. M., S. F. (2008). "Helicobacter pylori virulence facters in gastric carcinogenesis." Cancar Lett.
Xue, C. L. (2000). "[Diagnosis, treatment and prevention of Toxoplasma infection during pregnancy]." Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 18(1): 55-7.
Yamaguchi, H., T. Osaki, et al. (1996). "Flow cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori." J Med Microbiol 45(4): 270-7.
Yang, S. J. and S. J. Mao (1999). "Simple high-performance liquid
chromatographic purification procedure for porcine plasma haptoglobin." J Chromatogr B Biomed Sci Appl 731(2): 395-402.
Young, D., K. O'Neill, et al. (1988). "Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain." Proc Natl Acad Sci U S A 85(14): 5339-42.
Young, D. B. (1990). "Chaperonins and the immune response." Semin Cell Biol
1(1): 27-35.
Yunoki, N., K. Yokota, et al. (2000). "Antibody to heat shock protein can be used for early serological monitoring of Helicobacter pylori eradication treatment." Clin Diagn Lab Immunol 7(4): 574-7.
Zhao, Y., K. Yokota, et al. (2007). "Helicobacter pylori heat-shock protein 60 induces interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated protein (MAP) kinase pathway in human monocytes." J Med Microbiol 56(Pt 2): 154-64.
Zugel, U. and S. H. Kaufmann (1999). "Immune response against heat shock proteins in infectious diseases." Immunobiology 201(1): 22-35.