行政院國家科學委員會補助專題研究計畫成果報告
※※※※※※※※※※※※※※※※※※※※※※※※※
自由基在有機合成上的運用
※※※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:v 個別型計畫 □整合型計畫
計畫編號:NSC89-2113-M-002-013
執行期間:88 年 8 月 1 日至 89 年 7 月 31 日
計畫主持人:蔡蘊明
共同主持人:
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
執行單位:國立台灣大學化學系
中 華 民 國八十九年十月十三日
行政院國家科學委員會專題研究計畫成果報告
國科會專題研究計畫成果報告撰寫格式說明
NSC Pr oject Repor ts
計畫編號:NSC 89-2113-M-002-013
執行期限:88 年 8 月 1 日至 89 年 7 月 31 日
主持人:蔡蘊明 台大化學系
一、中文摘要 矽基酮與 NPTS 在酸的催化下可於其α位植入一苯硫基 關鍵詞:矽基酮、α苯硫基 Abstr actThe reactions of acylsilanes with N-(phenylthio)succinimide in the presence of p-toluenesulfonic
acid in acetonitrile give α-sulfenylated acylsilanes in good yields. Aldehydes with α-alkyl substituent afford moderate yields of α-sulfenylated products under the same conditions.
Keywor ds: imides; silicon and compounds; sulfides.
二、緣由與目的
α-Sulfenylated carbonyl compounds are useful intermediates in organic synthesis.1 Preparation of these
compounds often involves SN2 displacement of α-halogenated carbonyl compounds.1a Direct
sulfenylation methods are also available;1a,c however, these methods usually require basic conditions. Recently, we needed to prepare α-sulfenylated acylsilane 2 from bromoacylsilane 1.2,3 Due to the
presence of a bromo functional group, we tried to avoid using basic conditions.4 We found that
N-(phenylthio)succinimide (3)5 could be used to convert acylsilanes to α-sulfenylated acylsilanes under acidic conditions.6,7 MePh2Si O Br MePh 2Si O Br SPh N O O SPh 1 2 3 三、研究報告應含的內容
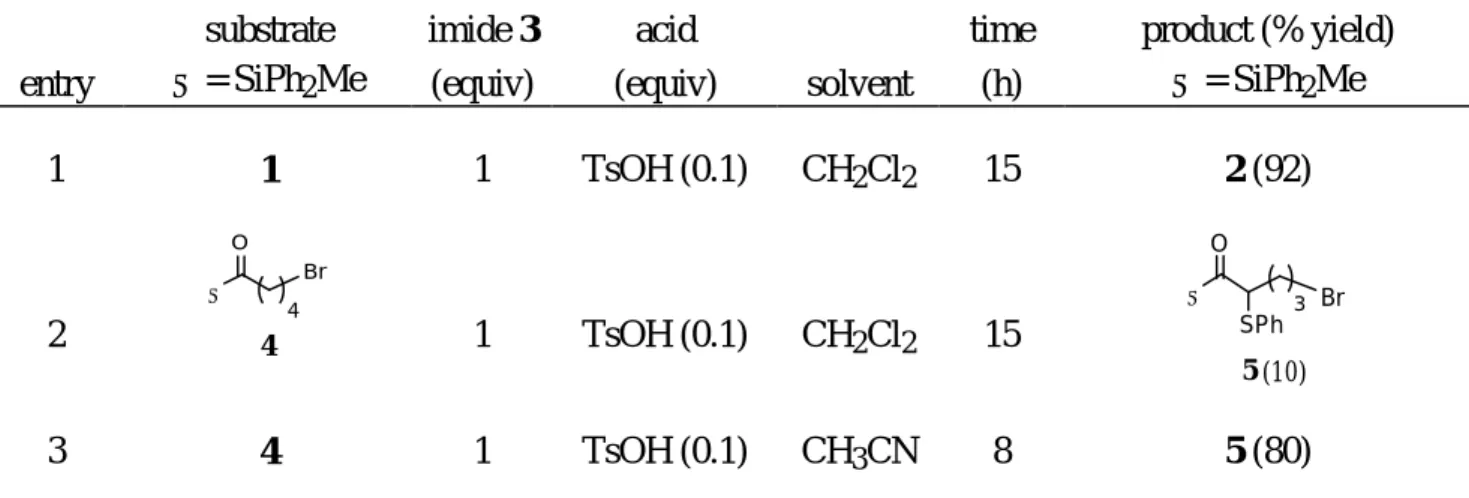
Our results were shown in Table 1. The reaction of acylsilane 1 (entry 1) with one equivalent of imide 3 and 0.1 equivalent of p-toluenesulfonic acid in dichloromethane at room temperature overnight gave
sulfide 2 in excellent yield (92%). Surprisingly, under the same reaction condition (entry 2), acylsilane
42 afforded 10% of sulfide 5. The difference between acylsilanes 4 and 1 is a single methylene unit.
We found that this type of reaction is faster in a more polar solvent such as acetonitrile. When acylsilane 4 was treated with imide 3 (1 equiv) in the presence of catalytic amount of p-toluenesulfonic
toluenesulfonic acid. In the presence of one equivalent of p-toluenesulfonic acid, propanoylsilane 10
reacted with imide 3 in acetonitrile for 5 h to afford 90% yield of sulfide 11.
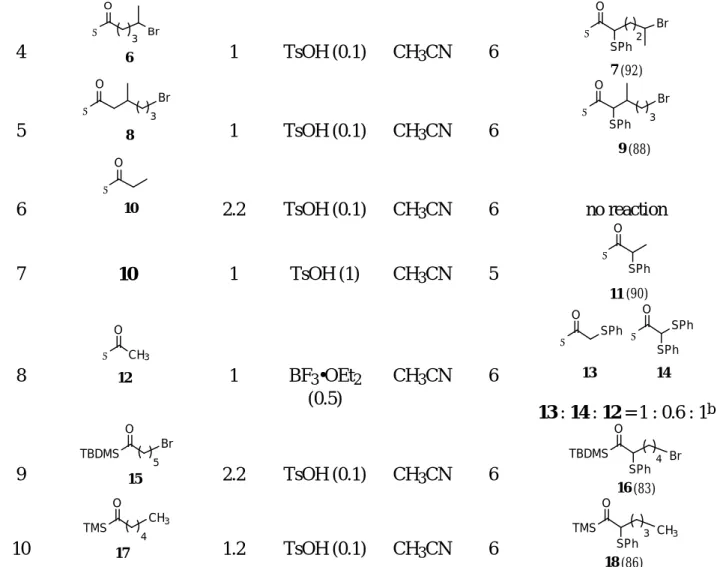
Ethanoylsilane 12 (entry 8) was the least reactive one that we have studied. Under the condition described above for the preparation of sulfide 11 (entry 7), we observed no reaction for ethanoylsilane 12. Reaction occurred when we used borontrifluoride etherate; however, bis-sulfenylation became competitive. As shown in entry 8, the reaction of ethanoylsilane 12 with imide 3 in the presence of borontrifluoride etherate (0.5 equiv) in acetonitrile for 6 h proceeded in about 60% conversion. The monosulfide 13 and bis-sulfide 14 were present in a 3/2 ratio, respectively.
The α-sulfenylation reactions are not limited to acylmethyldiphenylsilanes. As shown in entry 9 (Table 1), bromoacylsilane 152 with a bulky t-butyldimethylsilyl group (TBDMS) underwent α-sulfenylation
with imide 3 (2.2 equiv) in the presence of p-toluenesulfonic acid (0.1 equiv) in acetonitrile (6 h) to give
sulfide 16 in 83% yield. Under similar reaction condition, acyltrimethylsilane 178 (entry 10) afforded
sulfide 184a in good yield (86%).
When we applied this method to aldehydes and ketones, only α-substituted aldehydes were sulfenylated in mild yields. As shown in Table 2, a dismal 19% yield of sulfide 209 (entry 1) was obtained by the
reaction of straight chain hexanal (19) with imide 3 in the presence of borontrifluoride etherate (1 equiv) at room temperature in acetonitrile for 21 h. With the presence of an α-methyl substituent, aldehyde 21 (entry 2) led to 68% of sulfide 22 in 3 h under similar condition. All the ketones that we have studied reacted poorly as shown in entries 3 and 4. Sulfenylation occurred preferentially at the more substituted side of the unsymmetric ketones with low yields.
The reactivity pattern observed in these sulfenylation reactions correlates well with the enol-content of the carbonyl compounds.10 It was reported recently that the enol-content of acylsilanes is higher than
aldehydes and ketones.11 In this study, the acylsilanes show the highest reactivity towards imide 3
under acidic conditions. Among the acylsilanes, ethanoylsilane 12 is the least reactive. Presumably, acylsilane 12 is the least enolizable acylsilane that we have studied. Ketones are known to have lower enol content than aldehyde.10 Here, we found that the ketones are poor substrates in this reaction.
Table 1 α-Sulfenylation of acylsilanes with N-phenylthiosuccinimide (3)a
entry substrate Σ = SiPh2Me imide 3 (equiv) acid (equiv) solvent time (h) product (% yield) Σ = SiPh2Me 1 1 1 TsOH (0.1) CH2Cl2 15 2 (92) 2 Σ Br O 4 4 1 TsOH (0.1) CH2Cl2 15 Σ O Br SPh 3 5 (10) 3 4 1 TsOH (0.1) CH3CN 8 5 (80)
4 Σ O Br 3 6 1 TsOH (0.1) CH3CN 6 Σ O SPh 2 7 (92) Br 5 Br 3 8 Σ O 1 TsOH (0.1) CH3CN 6 Br 3 9 (88) Σ O SPh 6 Σ O 10 2.2 TsOH (0.1) CH3CN 6 no reaction 7 10 1 TsOH (1) CH3CN 5 Σ O 11 (90) SPh 8 Σ CH3 O 12 1 BF3•OEt2 (0.5) CH3CN 6 Σ O 13 SPh Σ O SPh SPh 14 13 : 14 : 12 = 1 : 0.6 : 1b 9 TBDMS Br O 15 5 2.2 TsOH (0.1) CH3CN 6 TBDMS O Br SPh 4 16 (83) 10 TMS CH3 O 17 4 1.2 TsOH (0.1) CH3CN 6 TMS O CH3 SPh 3 18 (86)
aAll reactions were performed at room temperature.
bProducts were not isolated, and the ratio was determined by 1H NMR analysis.
Table 2 α-Sulfenylation of aldehydes and ketones with N-phenylthiosuccinimide (3)a
entry substrate imide 3 (equiv) acid (equiv) time (h) product (%yield) 1 H O 19 1.0 BF3•OEt 2 (1.0) 21 H O SPh 20 (19) 2 H O 21 1.0 BF3•OEt 2 (1.0) 3 H O SPh 22 (68)
3 O 23 2.0 TsOH (1.0) 7 O SPh 24 (24) 4 25 O 1.0 BF3•OEt 2 (1.0) 14 O SPh 26 (22)
aThese reactions were performed in acetonitrile at room temperature.
五、參考文獻
1. (a) Trost, B. M. Chem. Rev. 1978, 78, 363–382. (b) Trost, B. M. Acc. Chem. Res. 1978, 11, 453–
461. (c) Foray, G.; Pe齌隳ry, A. B.; Rossi, R. A. Tetrahedron Lett. 1997, 38, 2035–2038.
2. Chang, S.-Y.; Jiaang, W.-T.; Cherng, C.-D.; Tang, K.-H.; Huang, C.-H.; Tsai, Y.-M. J. Org. Chem.
1997, 62, 9089–9098.
3. For recent reviews about acylsilanes, see: (a) Ricci, A.; Degl'Innocenti, A. Synthesis 1989, 647–
660. (b) Page, P. C. B.; Klair, S. S.; Rosenthal, S. Chem. Soc. Rev. 1990, 19, 147–195. (c)
Cirillo, P. F.; Panek, J. S. Org. Prep. Proc. Int. 1992, 24, 553–582. (d) Page, P. C. B.; McKenzie,
M. J.; Klair, S. S.; Rosenthal, S. In Acyl silanes; Rappoport, Z; Apeloig, Y., Eds. The chemistry
of organic silicon compounds. John Wiley: New York, 1998; Vol. 2, Chap. 27, pp. 1599–1665. 4. For the preparation of α-sulfenylated acylsilanes through enol silyl ethers, see: (a) Minami, N.;
Abe, T.; Kuwajima, I. J. Organometal. Chem. 1978, 145, C1–C3. (b) Reich, H. J.; Holtan, R. C.;
Bolm, C. J. Am. Chem. Soc., 1990, 112, 5609–5617. (c) Thomas, S. E.; Tustin, G. J. Tetrahedron
1992, 48, 7629–7640.
5. Groebel, W. Chem. Ber. 1960, 93, 284–285.
6. For α-selenylation of ketones and aldehydes with N-(phenylseleno)phthalimide under acidic
conditions, see: Cossy, J.; Furet, N. Tetrahedron Lett. 1993, 34, 7755–7756.
7. For the use of sulfenamide derivatives to convert ketones to their β-ketosulfides, see: (a) Mukaiyama, T.; Kobayashi, S.; Kumamoto, T. Tetrahedron Lett. 1970, 5115–5118. (b)
Kumamoto, T.; Kobayashi, S.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 1972, 45, 866–870.
8. Miller, J. A.; Zweifel, G. Synthesis 1981, 288–289.
9. Seebach, D.; Teschner, M. Chem. Ber. 1976, 109, 1601–1616.
10. Guthrie, J. P.; Cullimore, P. A. Can. J. Chem. 1979, 57, 240–248.