Various forms of mouse lactoferrins: purification and
characterization
*
Sheng-Hsiang Li, Yee-Hsiung Chen
Institute of Biochemical Sciences, College of Science, National Taiwan University and Institute of Biological Chemistry,
Academic Sinica, P.O. Box 23-106, Taipei, Taiwan
Received 22 June 1998; received in revised form 21 December 1998; accepted 20 January 1999
Abstract
This work was conducted to study the microheterogeneity of mouse lactoferrin (LF). Two forms, LF and LF , could be1 2 purified from uterine luminal fluid by ion-exchange HPLC on a Protein PAK SP 5PW column. Another form, LF , was3
purified from the epididymis homogenate by affinity chromatography on a column of Protein A-Sepharose coupled with the purified LF antibody that was prepared to give no crossreaction with serum albumin. Both LF and LF showed a M2 1 2 r
74 000 band while LF gave a M 70 000 band on reducing SDS–PAGE. All of them were reduced to a M 68 000 band after3 r r they had been digested with N-glycosidase F. The data from automated Edman degradation confirmed the completely identical 19 amino acid sequences in the N-terminal regions of these three LFs, except the lack of N-terminal Lys–Ala of LF / LF in LF . LF in tissue homogenates was immunodetected by Western blot procedure using the purified LF antibody.2 3 1 2
Different amounts of LF with a molecular mass of the 70 000 or 74 000 were distributed in the non-sexual organs such as kidney, spleen, lung, heart and liver and the sexual glands including epididymis, vagina, uterus, ovary and prostate. No LF was detected in stomach, intestine, testis and seminal vesicle. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Lactoferrins
1. Introduction and Furmanski suggests it to be a potential transcrip-tion activator [11].
Lactoferrin (LF) is present mainly in milk [1], LF has been described in the human reproductive other external secretions [2] and the secondary tract during the menstrual cycle [12] and in human granules of neutrophils [3]. It has been associated seminal plasma as one of the major sperm-coating with iron adsorption in newborn infants [4], bac- antigens [13,14]. However, its role in reproductive tericidal activity [5,6], antiviral activity [7], growth- biology has not received attention until it was stimulatory activity [8], and immune modulation (for identified to be a major protein secreted from the reviews, see Refs. [9,10]). The recent study of He uterine epithelial cells of both adult and immature female mice after estrogen treatment [15,16]. De-tailed analyses of LF and its RNA message during the natural estrus cycle [17,18] and the preimplanta-tion period [19] reveal the regulapreimplanta-tion of its
expres-*Corresponding author. Fax: 1886-2-2363-5038.
E-mail address: bc304@gate.sinica.edu.tw (Y. Chen) sion by ovarian steroids. LF is present in the
0378-4347 / 99 / $ – see front matter 1999 Elsevier Science B.V. All rights reserved. P I I : S 0 3 7 8 - 4 3 4 7 ( 9 9 ) 0 0 0 4 6 - 8
epididymis of mouse and porcine [20,21]. Its expres- oil with a daily dosage of 100 ng / g of body mass sion in the prepubertal epididymis can be stimulated was injected subcutaneously to immature females for by estrogen [20]. three consecutive days. We collected uterine luminal LF is glycosylated. The heterogeneity due to fluid (ULF) of either the DES-treated animals on day different glycosylation occurs in porcine and human 24 of age or the mature females in the proestrus LF [22,23]. Various forms of mouse LF have been stage, which was verified by the vaginal test. Twenty suspected also [16,17]. This work was conducted to ml of 500 mM EDTA and 100 ml of 100 mM PMSF
clarify the microheterogeneity of LF in the mouse in isopropanol were added to 10 ml of ULF and the reproductive tracts. We purified and identified two preparation was stored at 2708C before use. forms of M 74 000 LF in uterine luminal fluid, andr
one form of M 70 000 LF in epididymis homoge-r 2.3. Purification of LF from mouse ULF nate. Moreover, our results confirmed no LF other
than M 70 000 or 74 000 in the sexual and non-r LF was partially purified from ULF by a modified sexual organs. method [16]. Briefly, the ULF preparation was directly subjected to gel filtration on a Sephadex G-100 column (8032.6 cm), and the LF fraction was
2. Experimental rechromatographied on a CM-Affi-Gel Blue column (1031.5 cm). About 500 mg of the partially purified 2.1. Materials protein sample in 200 ml of 20 mM phosphate buffer at pH 7.4 or 150 ml of the ULF preparation from the Sephadex G-100, protein molecular mass markers mature females or DES-treated immature females and Protein A-Sepharose CL-4B were purchased was further resolved by high-performance liquid from Pharmacia (Uppsala, Sweden). CM-Affi-Gel chromatography (HPLC) on a Protein PAK SP 5PW Blue and Econo columns were obtained from Bio- column (7.5 cm37.5 mm) preequilibrated with the Rad Labs. (Richmond, CA, USA). A Protein PAK SP same buffer. The column was washed with a linear 5PW column was obtained from Waters (Milford, gradient of 0–0.6 M sodium acetate in the buffer at MA, USA). N-Glycosidase F, protease inhibitor room temperature. Fractions (500 ml) were collected cocktail tablets and phenylmethylsulfonyl fluoride and were monitored by the optical density at 280 nm. (PMSF) were products of Boehringer-Mannheim The chromatography was performed with a Waters (Mannheim, Germany). Aminolink gel, BCA protein 650E advanced protein purification system attached assay kit and enhanced chemiluminescent substrate to a Waters 486 tunable absorbance detector. were obtained from Pierce (Rockford, IL, USA).
Mouse serum albumin, horseradish peroxidase- 2.4. Purification of LF antibody and LF2 3
conjugated goat anti-rabbit antibody and
diethyl-stilbestrol (DES) were from Sigma (St. Louis, MO, According to the procedures recommended by the USA). All chemicals were reagent grade. commercial source, Aminolink gel covalently linked with mouse serum albumin or LF2 was prepared. 2.2. Animals Around 1.0 mg of mouse serum albumin or 0.5 mg
of LF was coupled to 1.0 ml of the affinity gel.2
ICR mice (Charles River Labs., Wilmington, MA, Antiserum against LF was raised in New Zealand2
USA) were maintained and bred in the animal center White rabbits. LF in normal saline (2 mg / ml) was2
at the College of Medicine, National Taiwan Uni- mixed with Freund’s complete adjuvant (1:2, v / v). versity. Animals were treated following the institu- Rabbit received intrasplenic injection of 300 ml of tional guidelines for the care and use of experimental the mixture. The antiserum was collected eight animals. The mature males (6–8 weeks old), mature weeks later. Around 1.5 ml of the antiserum was females (6–8 weeks old) and immature females (21 applied to a column (2.531.0 cm) of Aminolink gel days old) were used throughout the study. Animals coupled with mouse serum albumin. The column was were sacrificed by cervical dislocation. DES in corn washed with phosphate-buffered saline (PBS) (1.5
mM KH PO , 8.1 mM Na HPO , 137 mM NaCl,2 4 2 4 mM EDTA, 0.5% Nonidet P-40, and 10 mM sodium 2.7 mM KCl, pH 7.4) and the non-retarded portion azide for 16 h at 378C.
was further subjected to affinity chromatography on Around 5.0 g of tissue from both the mature male a column (2.531.0 cm) of Aminolink gel linked with mice and the mature female mice was homogenized LF2 preequilibrated with PBS at 48C. The column in 1.5 ml of PBS containing the protease inhibitor was washed with PBS to remove the non-retarded cocktail and 1.0 mM PMSF. The homogenates were portion and antibody to LF was then eluted from the2 centrifuged at 120 000 g for 30 min. The protein column with 0.1 M glycine (pH 3.0) at a flow-rate of concentration in the supernatant was determined with 18 ml / h that was controlled by a peristaltic pump. a BCA protein assay kit. The supernatant containing The eluent was neutralized with 0.1 volume of 1.0 M 100 mg of the protein extract was mixed with equal Tris–HCl at pH 8.2, dialyzed against deionized water volume of the sample buffer (0.125 M Tris–HCl, at 48C and lyophilized. About 0.8 mg of the LF2 4.0% SDS, 20% glycerol, 10% 2-mercaptoethanol, antibody could be prepared from 1.5 ml of an- pH 6.8) and heated at 1008C for 3 min prior to tiserum. The lyophilized sample was redissolved in SDS–polyacrylamide gel electrophoresis (PAGE) PBS containing 10% glycerol and 0.05% NaN at a3 which was performed on an 8.0% gel slab (8.23 final concentration of 10 mg / ml and stored at 7.330.075 cm) according to the method of Laemmli
2208C. The specificity of the antibody was verified [25]. The protein components were transferred from by assessment of its immunoaffinity to purified LF2 the gel to a polyvinylidene difluoride (PVDF) mem-and mouse serum albumin using the Western blot brane by a diffusion method. Briefly, the SDS– procedure. PAGE gel was sandwiched by two pieces of PVDF The affinity gel of the LF antibody–Protein A-2 membrane and immersed in PBS containing 1.0 mM Sepharose was prepared by following the method of EDTA in a TE22 transphor tank (Hoefer, Mighty Schneider et al. [26]. Around 3 mg of the antibody Small Transphor Tank). The solution was gently was coupled to 1.5 ml of Protein A-Sepharose CL- stirred at 48C for 60 h. Proteins were almost com-4B. The affinity gel was packed into a small Econo pletely transferred to the membranes as checked by column (1.531.0 cm). Around 20 mg of protein in staining the gel with 0.1% Coomassie Brilliant Blue. the soluble portion of mouse epididymal homoge- The membrane was blocked in PBS containing 0.1% nates was applied to the column preequilibrated with Tween 20 and 5.0% skim milk. LF was immuno-PBS at 48C. The column was washed to remove the detected by Western blot procedure, using the non-retarded portion with the same buffer and LF3 purified LF antibody (0.4 mg / ml) and horseradish2
was washed out from the column with 0.1 M glycine peroxidase-conjugated goat anti-rabbit antibody di-(pH 3.0) at a flow-rate of 12 ml / h. Recovery of the luted to 1:8000 in the blocking solution. Finally, protein sample was followed the procedures men- enhanced chemiluminescent substrate was employed
tioned above. to visualize the signal.
2.5. Protein analysis and Western blot procedure 3. Results
The amino acid sequences of a protein were 3.1. Purification of LF and its antibody determined by automated Edman degradation with a
gas-phase sequenator (Applied Biosystems, Foster Previously, one M 74 000 LF in ULF of CD-1r
City, CA, USA). mice was purified by a series of isolation procedures Removal of the N-glycoconjugate from a including chromatography and rechromatography on glycoprotein was followed the method of Tarentino a Sephadex G-100 column and affinity chromatog-and Plummer Jr. [24]. Protein was boiled in 1.0% raphy on a CM-Affi-Gel blue column [16]. The last sodium dodecyl sulfate (SDS) and incubated with step was attempted to remove the contaminant of
N-glycosidase F (40 U / mg of protein) in 20 mM serum albumin. We followed these procedures to sodium phosphate at pH 7.2 in the presence of 50 isolate LF from ULF of the DES-stimulated
imma-ture ICR mice. However, the protein sample could be by the appearance of a shoulder in the front side of further resolved by ion-exchange HPLC on a SP the peak.
column to four components, two minor ones denoted Both peaks III and peak IV of Fig. 1A gave only I and II and two major ones denoted III and IV in one M 74 000 band on reducing SDS–PAGE gelr
Fig. 1A. We applied the HPLC method to resolve (lanes 1 and 2 of Fig. 2). The M 74 000 protein wasr
directly the components of ULF. Two peaks corre- not found in peaks I and II of Fig. 1A. Apparently, sponding to III and IV of Fig. 1A appeared in the peaks III and IV are two forms of lactoferrin. chromatograms of ULF freshly collected from the Tentatively, we designated peaks III and IV of Fig. DES-stimulated immature mice or the adult mice in 1A to be LF and LF , respectively. Only one M1 2 r
the proestrus stage (Fig. 1B and C). However, peak 74 000 band and trace of minor components other IV in Fig. 1B and C was heterogeneous as evidenced than LF / LF1 2 appeared on the electrophoretic pat-terns of peak III in either Fig. 1B or C (lanes 3 and 5 of Fig. 2). The same situation happened in both peak IV and its shoulder in either Fig. 1B and C (lanes 4, 6 and 7 of Fig. 2). These data supported the presence of both LF1 and LF2 in ULF and suggested an heterogeneous population of different LF form.2
In spite of our efforts to purify LF / LF , trace1 2
amounts of serum albumin remained in the protein sample. As a result, the antiserum collected from rabbits immunized with LF retained weak immuno-2
affinity to mouse serum albumin in addition to its strong immunoaffinity to LF / LF . To eliminate the1 2
immunoactivity due to serum albumin, we passed the antiserum through an affinity column of Aminolink gel coupled with mouse serum albumin. The LF2
antibody was further purified by affinity chromatog-raphy on a column of Aminolink gel coupled with LF . As shown in Fig. 3, we could detect LF to a2
Fig. 1. Purification of LF in the mouse ULF. The protein samples were subjected to HPLC on a Waters Protein Pak SP 5PW column
preequilibrated with 20 mM phosphate buffer at pH 7.4. A linear Fig. 2. Determination of the homogeneity of LFs by SDS–PAGE. gradient of 0–0.6 M sodium acetate indicated by a dashed line The protein samples were resolved by reducing SDS–PAGE on an was applied to the column at a flow-rate of 1 ml / min for 40 min. 8.0% gel slab (8.237.330.075 cm): lane 1, peak III of Fig. 1A (1 (A) The LF sample partially purified from ULF of the DES- mg); lane 2, peak IV of Fig. 1A (1 mg); lane 3, peak III of Fig. 1B
stimulated immature mice through CM-Affi-Gel Blue affinity (2.5 mg); lane 4, peak IV of Fig. 1B (2.5 mg); lane 5, peak III of chromatography according to the previous method [16]. (B) ULF Fig. 1C (2 mg); lane 6, peak IV of Fig. 1C (2 mg); lane 7, the collected from the DES-treated immature mice. (C) ULF collected shoulder of peak IV of Fig. 1C (2 mg). The gel was stained with from mature mice in proestrus stage. 0.1% Coomassie Brilliant Blue to reveal the protein bands.
with N-glycosidase F reduced their molecular mass to a similar one about M 68 000 (cf. lanes 2, 4 and 6r
of Fig. 4A). Automated Edman degradation of LF ,1
LF or LF up to 19 cycles gave reliable data for2 3
their N-terminal sequences, which can be completely aligned with the protein sequences of LF deduced from the cDNA of CD-1 mice [15] (Fig. 4B). The N-terminal sequences of LF are identical to those of1
LF except the loss of N-terminal Lys–Ala of LF in2 2
LF . This manifests LF to be a truncated form of1 1
LF . The N-terminal sequence of LF is completely2 3
identical to those of LF , suggesting that they share2
with the same protein core. Taken together, the smaller molecular size of LF as compared with LF3 2
is attributed to their difference in the N-glycoconju-gates.
3.3. Tissue distribution of LF
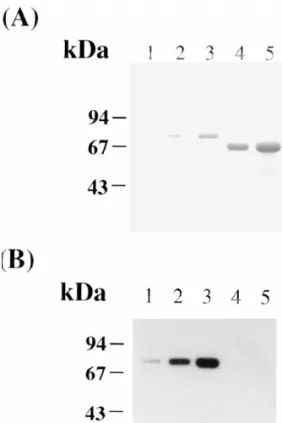
Based on the molecular mass of immunoreactive LF band in Western analysis shown in Fig. 3, either2
a M 70 000 protein or a M 74 000 one, but notr r Fig. 3. Specificity of the purified LF antibody. The proteins were2
both, was immunoreacted with the purified LF
resolved by SDS–PAGE on an 8.0% polyacrylamide gel slab 2
(8.237.330.075 cm). (A) The proteins were stained with antibody in the blot pattern of western analysis for
Coomassie Brilliant Blue dye; lanes 1–3, 50 ng, 200 ng and 500 100 mg of proteins in each tissue homogenate of ng of LF ; lanes 4 and 5, 2 and 5 mg of mouse serum albumin.2 mature mice (Fig. 5). In the non-sexual organs the (B) The proteins in the gel (A) were transferred to a PVDF
M 74 000 LF was detected in spleen, lung and heartr membrane by a diffusion method and immunodetected by Western
but no LF was detected in stomach and intestine. The
blot with the purified LF antibody in the blocking solution (0.42
mg / ml) (see Sections 2.4 and 2.5 for details). latter was not attributed to the technical problem of
Western blot procedures, because the transfer of LF low limit of 50 ng by Western blot procedure, using from the polyacrylamide slab gel to PVDF mem-the purified LF antibody which showed no immuno-2 brane was almost complete and the detection of LF activity to mouse serum albumin even when 5 mg of on the membrane could reach a low limit of 50 ng. the protein was tested. Relatively, the M 74 000 LF seemed to exist in liverr
LF is present in epididymis [20,21]. We purified and kidney. In the reproductive tracts, the M 74 000r
the protein from the soluble fraction of epididymis LF was detected in vagina and uterus, but the Mr
homogenates by affinity chromatography on a col- 70 000 LF was found in the Vas Deferens and umn of Protein A–Sepharose coupled with the epididymis. We detected traces of LF in ovary and purified LF2 antibody. The protein was tentatively prostate and no LF in testis and seminal vesicle. designated as LF (see Experimental).3 Based on the immunostaining intensity, LF is abun-dant in epididymis and vagina as compared the 3.2. Characterization of LF amount of LF in other sexual organs. This is congruent with the previous result detected by We characterized LF and LF of Fig. 1A and LF1 2 3 enzyme-linked immunosorbent assay (ELISA) [20]. by comparing their molecular sizes and N-terminal Because of the absence of LF mRNA in vas de-sequences. LF is smaller than LF / LF . It gave a3 1 2 ferens, the appearance of LF in this organ is likely
M 70 000 band on reducing SDS–PAGE gel (cf.r from epididymal secretion [20]. The vagina is among lane 5 of Fig. 4A). Digestion of LF , LF1 2 or LF3 the female sexual organs and the epididymis is
Fig. 4. Characterization of LFs. (A) Determination of molecular size by SDS–PAGE. LF and LF of Fig. 1A and LF were digested with1 2 3 N-glycosidase F. The protein sample (1 mg) was subjected to SDS–PAGE on an 8.0% gel slab: lane 1, LF ; lane 2, the digested LF ; lane 3,1 1 LF ; lane 4, the digested LF ; lane 5, LF ; lane 6, the digested LF . The gel was stained with 0.1% Coomassie Brilliant Blue. (B) Alignment2 2 3 3 of the signal peptide sequences of mammal LFs and the N-terminal sequences of LF , LF and LF . Signal peptide sequences of several LFs1 2 3 and the junction with mature protein are aligned. Vertical lines denote the conserved residues. The glutamate residue in mouse LF is indicated by a dot. The N-terminal sequences of LF , LF and LF were obtained from automated Edman degradation. The cDNA-deduced1 2 3 amino acid sequences of LF in ULF of CD-1 mice are listed for comparison. ‘‘X’’ represents an unidentified amino acid, which is most likely to be Cys, in the amino acid sequence determination by automated Edman degradation.
among the male sexual organs to contain a higher LF have a blocked N-terminus [16], but the N-terminal concentration. Lys of LF isolated from mouse milk is not blocked [27]. The N-termini of LFs purified from several species of mammal are not blocked also. This work
4. Discussion reveals the microheterogeneity of LF in the re-productive tracts of ICR mice. Two forms, LF and1
Among the reproductive tracts of adult mice, LF is LF , are present in ULF and one form, LF , exists in2 3
mainly distributed in epididymis, uterus and vagina. epididymis. Our results indicate that: (a) they are LF isolated from ULF of CD-1 mice was claimed to glycoproteins with the N-linked glycoconjugate in
Fig. 5. Reexamination of the tissue distribution of LF in various mouse tissues. One hundred mg of protein from each tissue homogenate was resolved by SDS–PAGE on an 8.0% gel slab, transferred to a PVDF membrane and immunodetected by Western blot procedures with the purified LF antibody (see Section 2.5). Tissues examined are Vas Deferens (lane 1), kidney (lane 2), spleen (lane 3), lung (lane 4), heart2 (lane 5), liver (lane 6), stomach (lane 7), intestine (lane 8), ovary (lane 9), uterus (lane 10), vagina (lane 11), testis (lane 12), epididymis (lane 13), prostate (lane 14), and seminal vesicle (lane 15).
nature; (b) their N-terminal residues are not blocked type site has been found in the precursor of bovine growth hormone [30] and artificial mutant [31]. and can be determined directly by Edman
degra-The association of LF with serum albumin is well dation; (c) they may share the same protein core
known [32,33]. We made a great effort to prepare the except the deletion of the N-terminal Lys–Ala of
LF antibody with no crossactivity to mouse serum LF / LF in LF ; (d) LF and LF may have similar2 3 1 1 2 2
albumin. The antibody immunoreacted only with the glycan content if not identical glycan chain
com-M 70 000 or 74 000 LF, which is distinct from com-M
position, and LF contains less amount of carbohy-3 r r
65 000 serum albumin, in the Western analysis of drate than LF / LF . Apparently, the microhetero-1 2
tissue homogenates (Fig. 5). This excludes the geneity of LF arises from the different
post-transla-existence of a LF with a molecular mass similar to tional cleavage of a precursor protein or the different
serum albumin as suspected in the previous reports extent of glycosylation on a putative protein.
[16,17]. The signal peptide of an eukaryotic protein usually
contains a hydrophilic N-terminal domain with net positive charge, a hydrophobic core domain of at
Acknowledgements
least seven residues and a polar C-terminal domain of 4–6 residues which comprise Pro or Gly that
This work was partially supported by a grant interrupts the ordered secondary structure in order to
(NSC 88-2311-B-001-012) from National Science facilitate the post-translational cleavage. The typical
Council, Taiwan. We thank the technical assistance tripartite domain occurs in the 19 amino acid
res-from Mr. Shui-Tsung Chen of our institute. idues of signal peptide in each mammalian LF listed
in Fig. 4B. There appears a highly conserved 10
210 21
residues, namely FLGALGLCLA , in the
C-28 References
terminal region in which Gly is mutated to Glu in mouse LF. As mentioned in the previous works [28],
[1] P.L. Masson, J.F. Heremans, Comp. Biochem. Physiol. 39B
the presence of a charged residue in the hydrophobic
(1971) 119.
region of a signal peptide may affect the cleavage
[2] P.L. Masson, J.F. Heremans, C. Dive, Clin. Chim. Acta 14
efficiency or / and the cleavage site by signal peptid- (1966) 735.
ase. This seems to happen in the generation of LF1 [3] T. Rado, J. Bollekens, G. St. Laurent, L. Parker, E.J. Benz
and LF . The post-translational cleavage sites at2 Jr., Blood 64 (1984) 1103.
21 12 [4] J.H. Brock, Arch. Dis. Child. 55 (1980) 417.
Ala and Ala in the yield of LF and LF are not1 2
[5] R.R. Arnold, M.F. Cole, J.R. McGhee, Science 197 (1976)
contradictory to (–3, –1) rule, which states a small
263.
and neutral residue at positions –1 and –3 relative to [6] W. Bellamy, M. Takase, K. Yamauchi, H. Wakabayashi, K. the cleavage site of signal peptide [29]. In fact, the Kawase, M. Tomita, Biochim. Biophys. Acta 1121 (1992)
[7] L. Lu, G. Hangoc, A. Oliff, L.T. Chen, R.N. Shen, H.E. [20] L.C. Yu, Y.H. Chen, Biochem. J. 296 (1993) 107. Broxmeyer, Cancer Res. 47 (1987) 4184. [21] Y.Z. Jin, S. Bannai, F. Dacheux, J. Dacheux, N. Okamura, [8] P. Gentile, H.E. Broxmeyer, Blood 61 (1983) 982. Mol. Reprod. Dev. 47 (1997) 490.
[9] L. Sanchez, M. Calvo, J.H. Brock, Arch. Dis. Child. 67 [22] J.J. Rogerts, J.C. Boursnell, J. Reprod. Fertil. 42 (1975) 579. (1992) 657. [23] W.J. Hurley, R.C. Grieve, C.E. Magura, H.M. Hegarty, S. [10] B. Lonnerdal, S. Iyer, Annu. Rev. Nutr. 15 (1995) 93. Zou, J. Dairy Sci. 76 (1993) 377.
[11] J. He, P. Furmanski, Nature 373 (1995) 721. [24] A.L. Tarentino, T.H. Plummer Jr., Methods Enzymol. 230 [12] M.S. Cohen, B.E. Britigan, M. French, K. Bean, Am. J. (1994) 44.
Obstet. Gynecol. 157 (1987) 1122. [25] U.K. Laemmli, Nature (Lond.) 227 (1970) 680. [13] P.F. Tauber, L.J.D. Zaneveld, D. Propping, G.F.B.
[26] C. Schneider, R.A. Newman, D.R. Sutherland, U. Asser, Schumacher, J. Reprod. Fertil. 43 (1975) 249.
M.F. Greaves, J. Biol. Chem. 257 (1982) 10766. [14] S.A. Goodman, L.G. Young, J. Reprod. Immunol. 3 (1981)
[27] J.M. Kinkade, W.W.K. Miller III, F.M. Segars, Biochim. 99.
Biophys. Acta 446 (1976) 407. [15] B.T. Pentecost, C.T. Teng, J. Biol. Chem. 262 (1987) 10134.
[28] M.P. Caulfield, L.T. Duong, R.K. Baker, M. Rosenblatt, [16] C.T. Teng, B.T. Penticost, Y.H. Chen, R.R. Newbold, E.M.
M.O. Lively, J. Biol. Chem. 264 (1989) 15813. Eddy, J.A. McLachlan, Endocrinology (Baltimore) 124
[29] G. Von Heijne, Eur. J. Biochem. 133 (1983) 17. (1989) 992.
[30] V.R. Lingappa, A. Thiery-Devillers, G. Blobel, Proc. Natl. [17] D.K. Walmer, M.A. Wrona, C.L. Hughes, K.G. Nelson,
Acad. Sci. USA 74 (1977) 2432. Endocrinology 131 (1992) 1458.
[31] R.J. Folz, S.F. Nothwehr, J.I. Gordon, J. Biol. Chem. 263 [18] R.R. Newblod, C.T. Teng, W.C. Beckman, W.N. Jefferson Jr.,
(1988) 2070. R.B. Hanson, J.V. Miller, J.A. McLachlan, Biol. Reprod. 47
[32] A. Hekman, Biochim. Biophys. Acta 251 (1971) 380. (1992) 903.
[19] M.T. McMaster, C.T. Teng, S.K. Dey, G.K. Andrews, Mol. [33] F. Lampreave, A. Pineiro, J.H. Brock, L. Sanchez, M. Calvo, Endocrinol. 5 (1992) 101. Intern. J. Biol. Macrom. 12 (1990) 2.