行政院國家科學委員會專題研究計畫 成果報告
利用原子力顯微鏡觀測自然軟骨細胞與人工分化軟骨細胞
之超微構造之差異
研究成果報告(精簡版)
計 畫 類 別 : 個別型 計 畫 編 號 : NSC 95-2314-B-002-147- 執 行 期 間 : 95 年 08 月 01 日至 96 年 07 月 31 日 執 行 單 位 : 國立臺灣大學醫學院骨科 計 畫 主 持 人 : 江清泉 共 同 主 持 人 : 李宣書、陳敏慧、江鴻生、林世明、蔡芷季 計畫參與人員: 博士班研究生-兼任助理:謝昌訓 處 理 方 式 : 本計畫可公開查詢中 華 民 國 96 年 10 月 05 日
Receptor mapping for human chondrocytes
Introduction:
The mechanical properties of chondrocytes influence the maintenance of the articular cartilage extracellular matrix. The chondrocytes in the cartilage detect different loading conditions, respond to mechanical signals, and thereby maintain the homeostasis of the cartilaginous tissues. They regulate the metabolic activity by means of complex biologic and biophysical interactions with the extracellular matrix (ECM) that affects the development of cartilage and the progression of joint diseases and aging. In addition, the inducing chondrocytes from mesenchymal stem cells (MSCs) were used to be materials for tissue engineering and research. The surface ultrastructures and mechanical properties of chondrocytes from differential sources were unclear. Atomic force microscope (AFM) permits the characterization of biologic samples ranging from a single molecule to whole cell (nanometers to micrometers), it is a powerful tool to explore the shape of a single cell, the properties of the cellular membrane, or the interaction of intermolecular forces. To differentiate the mechanical properties of chondrocytes among a young (normal modulus), an old (osteoarthritic, OA modulus), artificiality (MSCs inducing chondrocytes) and native (from the same patient as artificiality group), we used an AFM to probe the surface ultrastructure and to measure the adhesion force and stiffness.
Material and Methods:
Human bone marrow and articular chondrocytes were prepared from surgical specimens of patients with sports-related injury (patient age, 21–45 years) or patients with OA (patient age, older than 65 years) who needed artificial knee replacement at the ages between 21–81 years in the National University of Taiwan Hospital. First, MSCs were isolated from bone marrow in the primary culture system. For chondrocytes isolation, the cartilage was cut to pieces (1 – 3 mm3), washed by PBS, and treated with 2 mg type II collagenase per ml in a 37 water bath for 4 hours. The cells were ℃ washed three times by PBS and cultured in T75 flasks with culture medium. For chondrogenic differentiation, the hMSCs were plated with a density of 5x107 cells/ml in pallet culture system. Briefly, the hMSCs were treated with 10ng/ml TGF – 1 in chondrogenic medium. In the control group, the cells were cultured in chondrogenic medium without TGF- 1. The chondrogenic differentiation was demonstrated by immunohistochemical staining of anti-collagen type II antibodies. To monitor the surface unltrastructure of differential chondrocytes, the AFM is an important tool for studying biologic samples. With this technique, a minute tip is used as a sensor, and a cantilever serves as a transducer to measure surface and force interactions between the tip of the AFM and the sample by means of cantilever-deflection signals. This optical signal can be converted to an electric signal by using a photodiode detector with quadrants phases and then recorded on a computer. The experiments were performing by using a scanning probe microscope controlled by a probe station unit (SPA-300HV and SPI 3800N, respectively; SII Nano Technology
Inc. Tokyo, Japan). Commercial silicone cantilevers (Pointprobe CONT; Nanoworld, Matterhorn, Switzerland) with a spring constant of 0.21 N/m were used to measure force strength and to obtain surface images with the contact modes of the AFM in air. Figure 1 shows a typical cycle for force measurement. Arrowheads indicate the relative position of the surface of the chondrocyte with respect to the tip of the AFM. For the approach, as the piezo scanner is moved toward the tip of the AFM at constant velocity from point a to point b, the attractive force pulls down the cantilever, which jumps to contact the surface at point b. As the cantilever approaches the sample, it bends upward until it reaches point c. For retraction, when the tip reaches point c, the piezo scanner is moved away from the tip, and the cantilever begins to retract. As it does so, it bends downward until it reaches point d. The tip-sample complex starts to break from point d. A sharp, adhesive pull-off of approximately 150 pN, a specific tip-sample interaction, is observed in the retract trace. Finally, the cantilever returned at point e, same as the original equilibrium state at point a. All force measurements were obtained at a position on the cell surface in the 10 × 10-μm2 area. The time necessary to acquire one set of approach and retraction force curves was 5 seconds.
Results:
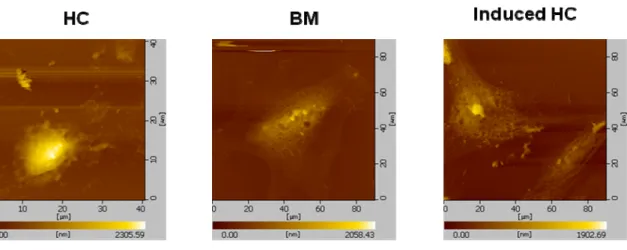
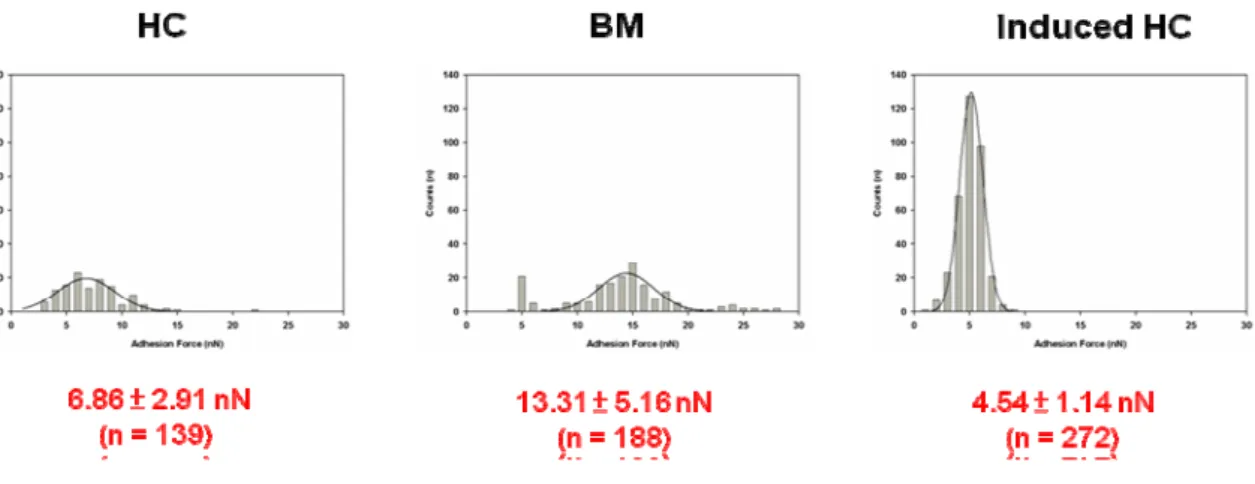
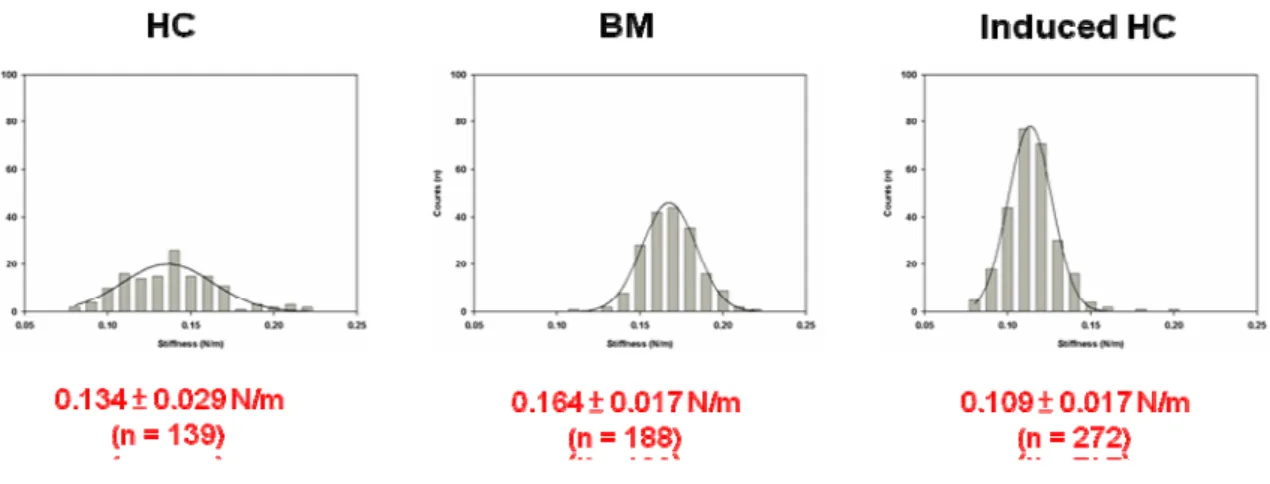
A single-cell AFM image (scanning area 50 × 50 μm2) provided topographic information about OA, native, inducing chondrocytes and mesenchymal stem cells. MSCs not only have the remarkable potential of different cell types but also can develop into various cellular lineages. In our results, MSCs could be differentiated to chondrocytes by TGF- 1. The figure 2 was shown the inducing chondrocytes that expressed type II collagen, which is the marker protein of chondrocytes. The various source of chondrocytes including OA, native, inducing chondrocytes and MSCs were measured the mechanical properties by AFM. In our previous result, the OA chondrocytes had lower adhesion force and stiffness than normal chondrocytes. The figure 3 was shown the morphology of human chondrocyte (HC), bone marrow (BM) and induced human chondrocyte (Induced HC). The adhesion force of BM was higher than HC and Induced HC (figure 4). In addition, the induced human chondrocyte had lower adhesion force than native human chondrocyte. The figure 5 was shown the BM had higher stiffness than HC and induced HC. The stiffness of HC was higher than induced HC.
Discussion:
The MSCs were isolated from adult bone marrow from the surgical specimens of femur of patients. The MSCs could be proliferation and expansion through continuous division. After differential inducing factors stimulating, the MSCs could differentiate to adipogenesis, myogenesis, osteogenesis and chondrogenesis. In the chondrogenesis, the large quantity of MSCs was compressed to form high density pellet and been induced by TGF- 1. After 21 days, the inducing chondrocytes still formed a pellet, but the non-inducing chondrocytes, the pellet was destroyed and fell apart. In our previous results, the mechanical property might represent the health of
chondrocytes. In these data, the MSCs had stronger in the physical mechanics than native chondrocytes. The differential properties might be based on differential cell population. The mechanical properties would be changed through MSCs differentiate to chondrocytes. In addition, the induced chondrocytes had lower stiffness and adhesion force than native chondrocyte. The stiffness and adhesion force of induced chondrocyte lower than native chondrocyte might duce less mechanical stress stimulation in differentiation process. We want to establish the AFM system to monitor the mechanical properties of chondrocytes from differential source.
Fig 2:
Chondrogenic differentiation of human MSCs. The collagen type II special antibody staining of the cells was shown in the chondrogenetic-induced group after chondrogenetic induction on day 28.