國
立
交
通
大
學
生物科技系
博
士
論
文
登革熱第二型病毒膜蛋白藉由胺基酸 K51 及 K241 與人
類 Ubc9 蛋白產生交互作用
The Dengue virus type II envelope protein interacts with human Ubc9, a
SUMO-conjugating E2 enzyme, via K51 and K241 amino acids
研 究 生:邱美惠
指導教授:楊昀良 博士
登革熱第二型病毒膜蛋白藉由胺基酸 K51 及 K241 與人類 Ubc9 蛋白產
生交互作用
The Dengue virus type II envelope protein interacts with human Ubc9, a
SUMO-conjugating E2 enzyme, via K51 and K241 amino acids
研 究 生:邱美惠 Student:Mei-Wui Chiu
指導教授:楊昀良 Advisor:Yun-Liang Yang
國 立 交 通 大 學
生 物 科 技 系
博 士 論 文
A ThesisSubmitted to Department of Biological Science and Technology National Chiao Tung University
in partial Fulfillment of the Requirements for the Degree of
phD in
Biological Science and Technology
July 2007
Hsinchu, Taiwan, Republic of China
登革熱第二型病毒膜蛋白藉由胺基酸
K51 及 K241 與人類
Ubc9 蛋白產生交互作用
學生:邱美惠
指導教授:楊昀良 博士
國立交通大學生物科技系博士班
摘要
登革熱為一經蚊蟲叮咬而感染人類之病毒,在全球暖化之氣候影響之下,其威脅性已不再侷限 於熱帶/亞熱帶而成為全球性之流行疾病。登革熱病毒之外套膜蛋白屬於結構性蛋白,在感染 宿主細胞時扮演很重要之角色,同時也是主要抗原之一。Chapter I 利用重組蛋白技術表現登革 熱第二型病毒strain PL046 之外套膜蛋白,該重組蛋白不包含 C 端之疏水性結構(transmembranedomain),而以一段 S peptide 取代作為純化之用。在 E. coli 表現之結果雖然形成 inclusion body,
但經過重新叠合後,此重組蛋白之結構未受影響。在病毒感染的同時加入重組膜蛋白能有效抑
制病毒斑(plaque)之形成,顯示該重組膜蛋白經由與病毒顆粒本身之膜蛋白競爭,減少病毒入
侵宿主細胞之機會。Chapter II 研究病毒膜蛋白與宿主細胞內蛋白之交互作用,藉由 Functional
Yeast Array 篩選 500 個人類蛋白是否與登革熱第二型病毒膜蛋白發生交互作用。篩選的結果
找出5 個人類蛋白,其中一個為 Ubc9。利用 co-precipitation assay 可再次驗證 Ubc9 與 DV2E
之蛋白質交互作用,點突變結果顯示Ubc9 可能以 K51 與 K241 作為與 DV2E 作用之位置。以
共軛焦顯微鏡觀察於BHK-21 細胞內表現 DV2E-GFP 及 Flag-Ubc9 兩種蛋白質之位置,發現當
共同表現DV2E-GFP 及 Flag-Ubc9 時,DV2E-EGFP 的位置由原本分布於細胞質範圍逐漸往細
胞核趨近。另外,若於BHK-21 細胞內大量表現 Ubc9 能減少病毒感染之病毒斑(plaque)之形成,
The Dengue virus type II envelope protein interacts with human Ubc9, a
SUMO-conjugating E2 enzyme, via K51 and K241 amino acids
student:
Mei-Wui ChiuAdvisors:Dr.
Yun-Liang YangDepartment of Biological Science and Technology
National Chiao Tung University
ABSTRACT
Dengue viruses (DVs) are mosquito-borne infectious pathogens. They have become an expanding public health problem in the tropics and subtropics. The dengue envelope (E) protein is one of the viral structure proteins responsible mainly for the virus attachment and entry onto host cells. It is also the major immunogen for virus neutralization. In chapter I, I have constructed a recombinant plasmid expressing a truncated E protein of DV-2 virus PL046 strain. The C-terminal hydrophobic domain of the E protein was removed and replaced with the sequence of S peptide to facilitate expression and purification. When expressed in Escherichia coli, the recombinant E proteins were found to be in the form of aggregated state. Through denaturation and dialysis processes, the receptor-interacting function of the purified recombinant E proteins was maintained, which was demonstrated by its ability to inhibit the DV-2 plaque-forming efficiency on mammalian BHK-21 host cells.
In chapter II, to identify the human cellular proteins interacting with the envelope protein of dengue virus serotype 2 inside host cells, I have performed a screening with the yeast
two-hybrid-based “Functional Yeast Array”. Interestingly, the Small Ubiquitin-like Modifier-1 Conjugating Enzyme 9 protein, modulating cellular processes such as those regulating signal transduction and cell growth, was one of the candidates interacting with the dengue virus envelope protein. With co-precipitation assay, it is demonstrated that the dengue envelope protein indeed could interact directly with the Ubc9 protein. Site-directed mutagenesis has demonstrated that Ubc9 might interact with the E protein via amino acid residues K51 and K241. Furthermore, immunofluorescence microscopy has shown that the DV2E-EGFP proteins tended to progress toward the nucleus membrane and co-localized with Flag-Ubc9 proteins around the nucleus membrane in the cytoplasm side, and DV2E-EGFP also shifted the distribution of Flag-Ubc9 from evenly in the nucleus toward concentrating around the nuclear membrane in the nucleic side. In addition, over-expression of Ubc9 could reduce the plaque formation of the dengue virus in mammalian cells. This is the first report that DV envelope proteins can interact with the protein of sumoylation system and Ubc9 may involve in the host defense system to prevent virus infection.
誌謝
首先誠摯的感謝指導恩師楊昀良博士,老師悉心的教導及指點,使我在這些年中獲益匪 淺,並得以一窺登革熱病毒領域的深奧。在多年的研究生涯中,承蒙老師照顧,無論是在研 究上或是在為人處事上,都以很大的包容力去指導我,在此深深感謝老師的付出。 感謝施修明博士在我的研究中給予無私的指導和許多研究資源的支持,老師的研究熱忱及 學術風範,使我十分敬佩。因緣際會得到施老師的教導,老師對於研究的熱愛以及縝密的思 考,也讓我留下深刻的印象,進而影響了我自己本身對於研究的態度。感謝楊裕雄博士,在 我還是碩士生時引領我進入研究殿堂,一路唸到博士以來都不斷的給予我許多協助與指引。 同時亦感謝岳嶽博士以及徐維莉博士在百忙之中願意抽空指導,兩位博士精闢的見解使我受 益良多。另外,感謝進階生物科技邱春龍總經理,承蒙總經理的支持,使得我得以利用在職 進修繼續完成學業。 博士班生涯裡,其實有許多起起伏伏,我曾灰心放棄過,於博三時休學。但師長、家人與 朋友們的支持,使我在兩年後復學繼續完成學業,對於這一路走來,我要感激的人真的很多 很多。因為同時有兩種身分(研究生與上班族),六年裡的日子,不但認識更多朋友,也很幸運 的得到兩倍的助力。實驗室裡、辦公室裡共同的生活點滴,學術上的討論、言不及義的閒扯、 讓人又愛又怕的大餐與宵夜、趕報告的革命情感、開會的狂風掃落葉、邊上班邊做實驗兩頭 忙的昏天暗地…。感謝眾位好友、同事們的共同砥礪(墮落?),你/妳們的陪伴讓我的研究生活 變得絢麗多彩,更值得回憶。 感謝好友晟洋的大力協助。因為有你的幫忙,使得我的研究能夠更完整而嚴謹。感謝靜雯 (Betty)、龍賢(Sam)、怡君(Jessica)、小慧、惠雯(Swenny)、徐坴暉副總(安東尼)、滿姐、國城、 咸靜(Genie)等同事們,一群搞笑的朋友以及三不五時的聚餐讓我有得以喘息放鬆的一刻,也 讓我的生活增添許多樂趣,更別提於公於私都十分義氣的鼎力相助。也感謝前任主管林杰良 博士的提拔,使我有機會可以延續自己的研究題目。感謝施修明老師實驗室多位先進,在我 後半段的實驗中給予許多協助,使我的實驗得以順利完成。自家實驗室的阿貴、柏吟、育穎、 宗瀚(小頭)、怡謹、欣彬、淑萍等學弟學妹們當然也不能忘記,你/妳們的幫忙我銘感在心。要 感謝的人實在太多,族繁不及備載,謹在此致上深深的謝意。 感謝建龍所付出的體諒與包容,不厭其煩的指出我研究中的缺失,且總能在我迷惘時為我 解惑、給我信心,支持我繼續前進,並且陪我堅持到最後。 最後,謹以此文獻給我摯愛的家人們,奶奶、父母親、哥哥嫂嫂、以及姑姑在背後的默默 支持,是我最大的精神支柱。Table of Contents
Abstract (Chinese)………...…… i Abstract (English)……… ii Acknowledgement………...…… iv Table of Contents……….…… v List of Figures………. viList of Tables……….. viii
Abbreviations and Symbols……… ix
General introduction………....………. 1
1.1 The history and resurgence as a global public health problem………...…… 1
1.2 Clinical spectrum of dengue infection………...…… 2
1.3 Transmission and infection with dengue virus………...…... 3
1.4 Epidemiology……… 4
1.5 Molecular biology of dengue viruses………... 5
1.6 Dengue virus replication………... 11
1.7 Viral pathogenesis……….…... 12
Thesis Objectives………... 14
Chapter I Blocking the dengue virus 2 infections on BHK-21 cells with purified recombinant dengue virus 2 E protein expressed in Escherichia coli Introduction……….….…... 15
Methods and Materials…..………...…... 17
Results………….………... 20
Discussion……… 23
Chapter II The Type 2 Dengue Virus Envelope Protein Interacts with Small Ubiquitin-like Modifier-1(SUMO-1) Conjugating Enzyme 9 (Ubc9) Introduction……….….…... 31
Methods and Materials…..………...…... 34
Results………….………... 43
Discussion……… 50
Reference…...……….……... 67 Appendix
The map of pET-32c
List of Figures
Figure 1.1 World distribution of dengue viruses and their mosquito vector, Aedes
aegypti, in 2005 ……….. 2
Figure 1.2 Transmission of dengue virus by Aedes aegypti .………... 4 Figure 1.3 The distribution of dengue confirmed cases in Taiwan (1987-2006)…………. 5 Figure 1.4 The genome organization of dengue virus …...……….. 6 Figure 1.5 Intracellular life cycle of dengue virus ……….. 12 Figure 2.1 Construction of pStag9 plasmid……….. 27 Figure 2.2 Detection of recombinant EStag9 protein expression. E. coli cells were
sonicated and followed by centrifugation before resolution by 12 %
SDS–PAGE ……….... 28
Figure 2.3 Competitive blocking assay ………... 29 Figure 2.4 Quantitative representation of dosage-dependent competitive blocking assay
of viral infection by the purified recombinant EStag9 proteins (●) and BSA
(○) ………... 30
Figure 3.1 Schematic presentation of DV2E-related constructs ………... 56 Figure 3.2 The scheme of Function Yeast Array ………. 57 Figure 3.3 Yeast two-hybrid analysis to assess the protein-protein interaction between
Ubc9 and DV2E……….. 58
Figure 3.4 Detection of DV2E protein with anti-EStag9 polyclonal antibody …………... 59 Figure 3.5 Ubc9 interacting with DV2E in vitro ………. 60 Figure 3.6 β-gal activity assay to quantify the interaction between DV2E and Ubc9 in
Figure 3.7 Double mutations on DV2E abolishing the interaction between the DV2E and
Ubc9 ………... 63
Figure 3.8 Subcellular localization of DV2E and Ubc9 by immunofluorescence ……….. 64 Figure 3.9 Determination of the Effect of Ubc9 over-expression on the propagation of
List of Tables
Table 2.1 Comparison of the DV-2 E sequence (1–402 amino acids) variations between Taiwan local strain PL046 and New Guinea-C strain (Accession
No. M29095)………. 26
Table 3.1 Primers used for mutagenesis ………... 53 Table 3.2 Comparison of traditional Yeast Two-Hybrid and Functional Yeast Array…... 54 Table 3.3 The candidates of interaction sites between Ubc9 and DV2E ………. 55
Abbreviations and Symbols
DF : Dengue fever
DHF : Dengue hemorrhagic fever (DF/DHF)
DSS : Dengue shock syndrome
DEN : Dengue virus
DV2E : dengue-2 virus envelope
CBA : Competitive Blocking Assay
SUMO : Small Ubiquitin-like Modifier
General Introduction
1.1 The history and resurgence as a global public health problem
Dengue fever and dengue hemorrhagic fever (DF/DHF) are caused by the dengue viruses, which belong to the genus Flavivirus, family Flaviviridae. There are four serotype (DEN-1, DEN-2, DEN-3 and DEN-4), not only all of which can cause DF/DHF but also have similar natural histories, including humans as the primary vertebrate host and Aedes mosquitoes of the subgenus Stegomyia as the primary mosquito vectors. Since the 1950s, dengue has been endemic in Southeast Asia, where DHF/DSS was first recognized. Patients of DHF have been reported sporadically since 1780 in the Philadelphia epidemic [Rush, 1789]. Significant numbers of cases of hemorrhagic disease were associated with several subsequent epidemics, including Charter Towers, Australia, in 1897, Beirut in 1910, Taiwan in 1916, Greece in 1928, and Taiwan in 1931 [Hare, 1898; Koizumi et al., 1916; Copanaris, 1928; Akashi, 1932; Halstead and Papaevangelou, 1980]. Today, DHF/DSS remains one of the 10 leading causes of hospitalization and is the leading cause of childhood mortality in several Asian countries (World Health Organization, 1997).
The reasons for global emergence of DF/DHF as a major public health problem in the waning years of the twentieth century are complex and not fully understood. However, the reasarch of Gubler have identified several important factors [Gubler and Trend, 1994]. First, the susceptible individuals living in urban areas provided a pool of reinfection by Ae. aegypti, and the subsequently occurred epidemics of dengue also provided increased opportunity for the viruses to move between countries, both within and out of the region. Second, in most dengue-endemic countries of the world the control of mosquito was ineffective [Gubler, 1989; Newton and Reiter, 1992]. Third, major global demographics have changed by the concurrent uncontrolled population growth and unplanned urbanization. The environment provides ideal habitats for the vector mosquito. A fourth factor for emergence of DF/DHF is the increased
traveling by airplanes. It is providing the opportunities for transporting dengue viruses between population centers of the tropics, and resulting in a constant exchange of dengue viruses and other pathogens.
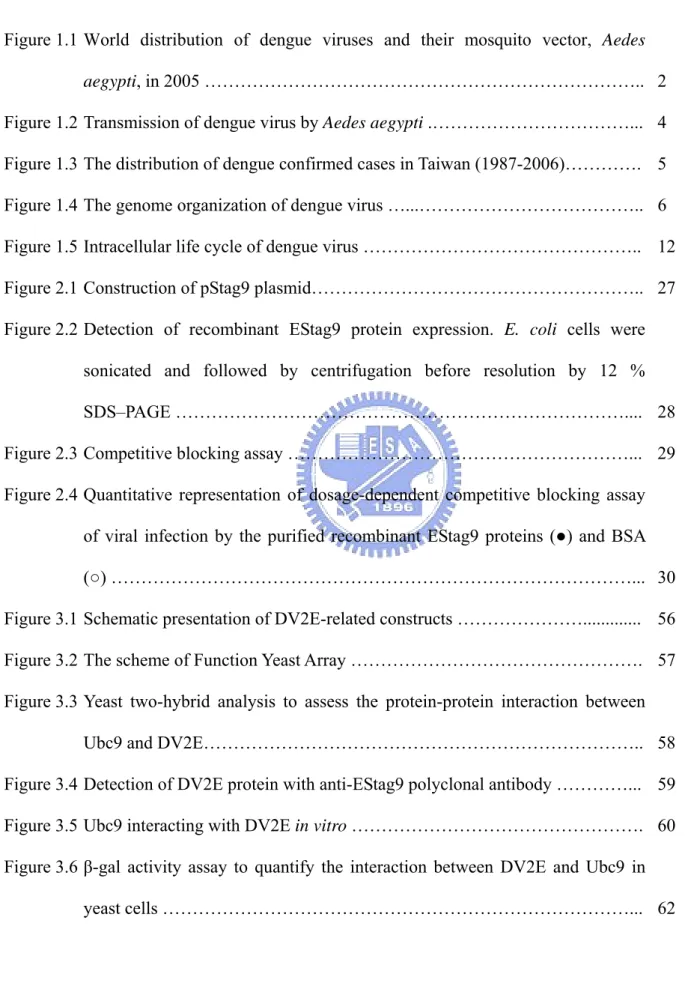
In 2005, dengue is the most important mosquito-borne viral disease affecting humans; its global distribution is comparable to that of malaria, and an estimated 2.5 billion people live in areas at risk for epidemic transmission (Figure 1.1) (CDC, 2007). Each year, tens of millions of cases of DF occur and, depending on the year, up to hundreds of thousands of cases of DHF. The case-fatality rate of DHF in most countries is about 5%, but this can be reduced to less than 1% with proper treatment. Most fatal cases are among children and young adults (CDC, 2007).
Figure 1.1 World distribution of dengue viruses and their mosquito vector, Aedes aegypti, in 2005 (CDC, 2007).
1.2 Clinical spectrum of dengue infection
significant illness, mild fever to life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The classic form of Dengue fever is a non-fatal febrile illness for older children and adults of about 5 to 7 days duration associated with sudden onset, extreme malaise, and pain of the muscles, back, limbs and eyes; rash is common as are other non-specfic constitutional symptoms such as nausea, vomiting and headache [George and Lum, 1997]. Manifestations of severe dengue include frank hemorrhage leading to shock through blood loss (dengue fever with hemorrhage), sudden increased vascular permeability leading to intravascular hypovolemia with or without frank hemorrhage, and severe encephalopathy with hepatitis. The classification of severe dengue has been complicated by the variation in clinical picture, for which the underlying pathophysiology may be different [George and Lum, 1997].
Patients with DHF may have fever lasting 2 to 7 days and a variety of nonspecific signs and symptoms, of which the most common manifestations are skin hemorrhages such as petechiae, purpura, or ecchymoses, but may also include epistaxis, bleeding gums, hematemesis, and melena. DHF patients develop thrombocytopenia and hemoconcentration, the latter as a result of the leakage of plasma from the vascular compartment, and the condition of these patients may rapidly evolve into dengue shock syndrome (DSS), which, if not immediately corrected, can lead to profound shock and death. Advanced warning signs of DSS include severe abdominal pain, protracted vomiting, marked change in temperature (from fever to hypothermia), or change in mental status (irritability or obtundation). Early signs of DSS include restlessness, cold clammy skin, rapid weak pulse, and narrowing of pulse pressure and/or hypotension. DHF/DSS can occur in children and adults, and the fatality rates among those with DSS may be as high as 44%. (CDC Division of Vector-Borne Infectious Disease, DVBID).
1.3 Transmission and infection with dengue virus
Dengue virus is transmitted by Aedes mosquitoes and causes the diseases mostly in tropical and subtropical regions worldwide. A number of Aedes (Stegomyia) mosquito species may act as
vectors including Ae. aegypti, Ae. albopictus, Ae. polynesiensis, and some members of Ae.
scutellaris group. From a public health standpoint, the urban endemic/epidemic cycle is the most
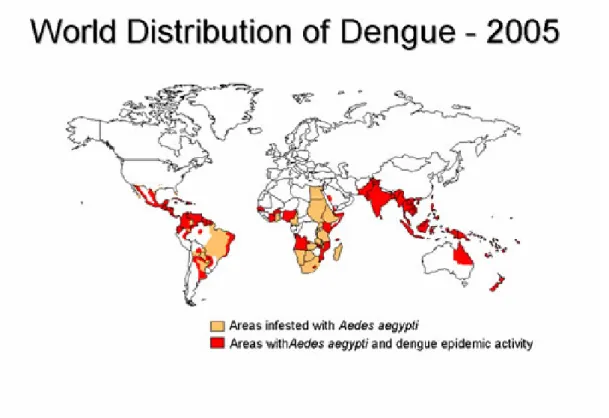
important transmission cycle. The viruses are maintained in an Ae. aegypti-human-Ae. aegypti cycle, with periodic epidemic occurring at 3 to 5 year intervals. Humans are infected with dengue virus by the bite of an infective Aedes mosquito [Gubler et al., 1979]. Adult Ae. aegypti mosquitoes are unobtrusive, prefer to rest indoors and feed on humans during daylight hours. The female mosquitoes often disrupt the feeding process at slightest movement and return to the same or a different person to continue feeding moments later. The Ae. aegypti females may thus feed on several persons during a single blood meal and transmit dengue virus to multiple persons within a short period of time [Gubler et al., 1979; Gubler et al., 1981; Gubler et al., 1984]. This behavior makes Ae. aegypti an efficient epidemic vector (Figure 1.2).
Figure 1.2 Transmission of dengue virus by Aedes aegypti. (CDC, 2007)
1.4 Epidemiology
The dengue fever may occur endemically or as epidemics. Within countries the virus spreads along transporation routes; between countries it appears first in seaports or airport cities. Epidemic dengue is most often described in setting where most or all of the population are non-immune. Outbreaks are often explosive with a majority of patients being older children and adults. Attack rates may be high, sometimes 80 to 90 percent, but more commonly, 40 to 50
percent of the population [Halstead, 1997]. A dengue epidemic requires the presence of the vector mosquito (Aedes aegypti), the virus, and a large number of susceptible human hosts. Outbreaks may be explosive or progressive, depending on the density and susceptibility of the vector, the strain of dengue virus, the immune level in the human population, and the amount of vector-human contact.
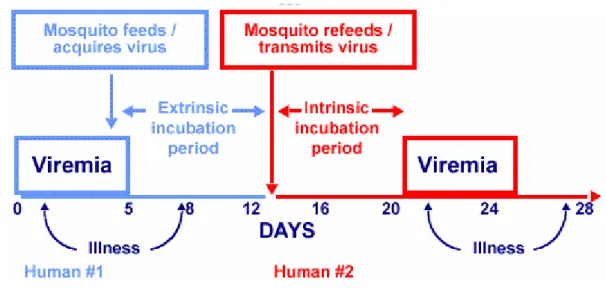
The dengue fever at Taiwan had several epidemics between 1901 and 1987. According the statistics of Centers for Disease Control R.O.C., there was outbreak at Kaohsiung County in 1987. Since them, the infections of dengue virus serotypes 1 to 4 have also been reported, and the imported cases have increased in recent years. It is suggested that Taiwan now has become a high dangerous region of dengue infection.
Figure 1.3 The distribution of dengue confirmed cases in Taiwan (1987-2006) (Centers for Disease Control R.O.C.)
1.5 Molecular biology of dengue viruses
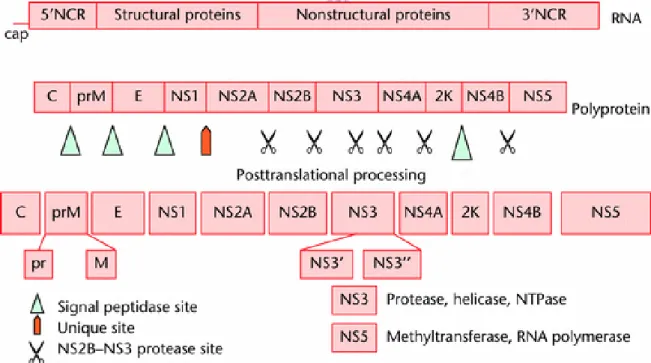
single-stranded RNA genome of 10,723 nucleotides (New Guinea C strain), having a type 1 cap at the 5' end, but lacking a poly(A) tract at the 3' end [Westaway et al., 1985; Rice et al., 1986; Brinton, 1986.; Westaway, 1987; Chambers et al., 1990]. The genomic RNA is of positive-strand polarity, having a single open reading frame that encodes a polyprotein of 3,391 amino acids, which is processed into three structural and at least seven nonstructural proteins so far identified. The gene order is 5'-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4BNS5-3', where C, prM, and E are the structural proteins and NS1 through NS5 represent the nonstructural proteins. The processing of the polyprotein precursor occurs cotranslationally as well as posttranslationally and is performed by either the host signalase in association with the membranes of the endoplasmic reticulum or the viral protease(s) [Chambers et al., 1990].
Figure 1.4 The genome organization of dengue virus. (Gubler, D. J., Centers for Disease Control and Prevention, Fort Collins, Colorado, USA)
1.5.1 Capsid (C) protein
assembly to ensure specific encapsidation of the viral genome. The mature form of DEN C protein is a highly basic protein of 12 kDa after removal of the C-terminal hydrophobic signal sequence. When the polyprotein is processed, the function of C-terminal region for capsid protein is serving as a signal sequence, the capsid protein then anchors into the ER membrane and thus translocates prM into the lumen of the endoplasmatic reticulum. Subsequently, this signal sequence is cleaved by the host cell signalases liberating the N-terminus of prM whereas C remains closely associated with the ER membrane promoting viral assembly. The membrane-associated capsid protein mediates the viral assembly by coordinated interaction with the E-prM heterodimer in the ER. The immune viral particles containing C protein and genomic RNA that forms as the nucleocapsid (NC) are then budding into the lumen of the ER [Wang et al., 2002]. It was reported that the capsid protein is also found in the nucleus and can possibly interact with hnRNP K, suggesting that C protein may play a role in regulation of the dengue lifecycle by controlling apoptosis [Chang et al., 2001].
1.5.2 Envelope (E) protein
The virus attachment and entry are mainly dependent on the envelope (E) protein, the major glycoprotein on the flavivirusparticle [Chambers et al., 1990; Monath and Henize, 1996]. The E protein forms an oligomer with the small membrane (M) protein and constitutes most of the accessible virion surface [Lee et al., 2000]. This reflects that the E protein is essential for membrane fusion and mediates the binding to host, it is also the primary antigen that inducing protective immunity and the major antigen for virus neutralization [Rice et al., 1996; Roehrig, 1997]. Therefore, the protein directly affects the host range, cellular tropism and in part, the virulence of DEN virus [Monath and Henize, 1996].
Based on the crystallography data of the tick-borne encephalitis flavivirus E protein, Rey et al. [Rey et al., 1995] noted that each E-protein monomer is folded into three distinct structural domains, domain I, II, and III. The domain I in the central structure of E protein is the antigenic
domain that carries the N glycosylation site. Structural domain II of the E protein is suggested to be responsible for pH-dependent fusion of the viral E protein and the endosomal membrane during uncoating. Structural domain III is reported to play the important role for flavivirus binding to host cells [Rey et al., 1995]. The structural domain III of E protein contains an immunoglobulin-like constant domain and is postulated to form the receptor-binding site for the virus particles.
1.5.3 Membrane (M) and pre-membrane (prM) protein
The flavivirus particle consists of a nucleocapsid core, which is surrounded by an ER-derived lipid bilayer containing E and prM/M, the structural proteins that were synthesized as a polyprotein [Mukhopadhyay et al., 2005]. The prM will be processed to the mature M protein late in secretion in the trans Golgi compartment by furin [Stadler et al., 1997]. Maturation of processed from prM to M protein is necessary to expose the E receptor binding domain and thus for virus infectivity [Heinz and Allison, 2003]. The prM is suggested to protect the E protein from pH-induced reorganization and premature fusion during the secretion of E proein [Guirakhoo et al., 1991; Guirakhoo et al., 1992; Zhang et al., 2003], and the prM protein is possibly to serve as a chaperone for proper E folding and assembly [Heinz and Allison, 2003]. It was reported that regulated efficiency of cleavage of prM/M is important for viral replication [Keelapang et al., 2004].
1.5.4 Non-structural 1(NS1) protein
NS1 is the first non-structural protein in the DEN polyprotein, following E protein and preceding the NS2A protein. It is a 46 kD glycoprotein, with two glycosylated asparagines, and 12 cysteines that form 6 disulfide bridges. Although it does not contain a hydrophobic membrane spanning region, the NS1 is translocated into the lumen of the ER during translation, where it dimerizes and stays membrane associated. The double stranded RNA corroborating evidence
showed that NS1 is essential for viral replication by an unknown mechanism [Muylaert et al., 1997; Flamand et al., 1999; Lindenbach and Rice, 1999]. Additionally, when the NS1 is exported through the Golgi excretory pathway, NS1 can be detected both outside the plasma membrane of infected cells and anchored into the membrane by a glycosyl-phosphatidylinositol (GPI). It has been suggested that the antibodies against NS1 in a DEN infected patient may bind to the NS1 protein both on the cell surface and activate GPI-mediated signaling in the infected cell. The binding of anti-NS1 antibodies is possibly enhancing viral replication or disease pathology [Jacobs et al., 2000]. NS1 is also excreting in a soluble hexameric form from mammalian cells but not from mosquito cells [Flamand et al., 1999]. Soluble NS1 is found in the blood of DEN infected patients [Young et al., 2000; Alcon et al., 2002] and the NS1 blood levels is reported to correlate with disease severity [Libraty et al., 2002; Avirutnan et al., 2006]. Soluble NS1 in the blood is suggested to contribute to DEN pathology by activating complement [Avirutnan et al., 2006], inducing auto-immune antibodies [Lin et al., 2003] or accumulating in hepatocytes in the liver [Alcon-LePoder et al., 2005]. Vaccination studies used to target and reduce free NS1 circulating in the blood have shown different results, some studies showed protection against an intracerebral challenge with DEN [Costa et al., 2005], but other studies did not show protection [Timofeef et al., 2004; Calvert et al., 2006].
1.5.5 NS3 protein
NS3 is a 67 to 70-kDa protein of 618 to 623 amino acids that is highly conserved among flaviviruses. The NS3 is proposed to have two functions in viral replication: serine protease and helicase. A region near the N-terminus of NS3 exhibits sequence and structural homology to the active domain of trypsin related serine protease [Bazan and Fletterick, 1989]. In combination with NA2B, it is required for proteolytic processing at the dibasic site of many viral proteins. The C-terminus of flaviviruses NS3 is suggested to be involved in several functions including RNA helicase [Gorbalenya et al., 1989], RNA-stimulated NTPase activity [Wengler and Wengler,
1991; Wallner et al., 1993], and the capping and methylation [Wengler and Wengler, 1993].
1.5.6 NS2A, NS2B, NS4A, and NS4B protein
NS2A, NS2B, NA4A, and NS4B are small non-structural proteins. All four proteins are poorly conserved in sequence but exhibit conserved hydrophobicity profiles among flaviviruses. The evidence suggesting that they are membrane-associated proteins (Chambers T. J., 1990). The functions of these four proteins remain largely undefined. NS2A (18 to 22-kDa of 218 to 231 amino acids) is reported to be required for the C-terminal processing of NS1 [Flagout and Lai, 1989]. NS2B (13 to 15-kDa of 130 to 132 amino acids) is suggested to be involved in the protease function of the NS2B-NS3 complex and essential for protease activity of the complex [Flagout et al., 1993]. The functions for NS4A (16.0 to 16.4-kDa of 149 to 150 amino acids) and NS4B (27 to 28-kDa of 248 to 256 amino acids) have not yet been identified. They may be involved in membrane localization of NS3-NS5 replication complex via protein-protein interaction, since the NS3-NS5 complex is reported to weakly associate with the membrane in spite of its hydrophilic characteristic [Chambers et al., 1990; Wengler et al., 1990].
1.5.7 NS5 protein
The NS5 protein consists of at least three important enzymatic functions which are essential for viral propagation in flaviviruses [Khromykh et al., 1998; Hanley et al., 2002]. The N-terminal region of NS5 protein represents the active domain of S-adenosyl-L-methionine dependent methyltransferase (SAM)(amino acids 1-320), which possess the methyl transferase and guanylyl transferase activities responsible for capping and methylating at the positive strand genomic RNA on its 5’ terminus [Egloff et al., 2002]. The C-terminal domain encodes the RNA dependent RNA polymerase (residues 420-900) responsible for synthesizing the double stranded replicative intermediate RNA template and also plus(+) strand RNA genomic RNA [Bartholomeusz and Thompson, 1999; Egloff et al., 2002; Nomaguchi et al., 2003]. The RNA dependent RNA
polymerase activity of this domain has been demonstrated for several other flaviviruses including West Nile virus, Kunjin virus, Hepatitis C viruses (HCV) and BVDV [Tan et al., 1996; Khromykh et al., 1998; Steffens et al., 1999; Guyatt et al., 2001]. The RNA dependent RNA polymerase has an essential GDD motif. In Flavivirus, it is shown that the mutations on this motif would result the virus non-replicative [Khromykh et al., 1998; Ranjith-Kumar et al., 2001].
1.6 Dengue virus replication
The intracellular replication cycles of the flaviviruses are very similar. Infection with the dengue virus is introduced into the host by the mosquito. The virus enters the permissive host cell via receptor-mediated endocytosis (RME), and fusion of viral and vesicular membranes allows the nucleocapsid entrying into cytoplasm and uncoating the viral genome. The translation of positive-strand viral RNA (vRNA) and viral proteins then begin and the structural protein capsid or core (C), premembrane (prM), and envelope (E) proteins, along with viral RNA, are assembled into progeny virions, and the virions are transported through the Golgi compartment and secreted.
It is generally accepted that DEN gains entry to its target cell by receptor-mediated endocytosis (RME). A number of different mammalian cell receptors have been proposed, including heparan sulfate [Chen et al., 1997; Hilgard and Stockert, 2000; Germi et al., 2002; Lin et al., 2002a], heat shock protein 70 (Hsp70) and Hsp90 [Valle et al., 2005], GRP78/BiP [Jindadamrongwech et al., 2004], CD14 [Chen et al., 1999], and 37-kDa/67-kDa high affinity laminin receptor [Thepparit and Smith, 2004], as well as DC-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN) [Navarro-Sanchez et al., 2003; Tassaneetrithep et al., 2003; Lozach et al., 2005] and liver/lymph node-specific ICAM-3-grabbing nonintegrin [Tassaneetrithep et al., 2003]. Although it is suggested that the mononuclear phagocyte lineage cells like monocytes, macrophages, and dendric cells are the primary targets in vivo [Jessie et al., 2004], DEN has been reported to be capable of infecting
numerous human cells, including dendritic cells (DCs), monocytes/macrophages, B cells, T cells, endothelial cells, hepatocytes, and neuronal cells, as well as a number of cell lines used for viral propagation in vitro [Anderson, 2003].
Figure 1.5 Intracellular life cycle of dengue virus. [Clyde et al., 2006]
1.7 Viral pathogenesis
The pathogenesis of dengue fever is not understood completely, and the sites of dengue replication in humans have not been well characterized. The main pathophysiologic features of DHF/DSS are (1) increased vascular permeability resulting in loss of plasma from the vascular compartment, leading to hemoconcentration and shock; and (2) a disorder of the homeostasis involving vascular changes, thrombocytopenia, and coagulopathy (WHO, 1997). Several hypotheses have attempted to explain DHF/DSS findings, but the lack of an animal model for DHF/DSS has made it difficult to clarify the steps involved in DHF/DSS pathogenesis. There is no treatment for these diseases and immunization may provide a relastic approach for controlling dengue infection [Jimenez and Lopes da Fonseca, 2001]. Although considerable research has
been directed towards the development of a safe, effective DEN vaccine since the middle of 20th century, no approved product is presently available. Several approaches have been taken to the expression of recombinant dengue E protein to develop as the vaccine candidate; unfortunately there is no effective vaccine for DEN available in spite of years of effort to develop live attenuated, inactivated whole virion and subunit vaccines based on E. coli, vaccinia virus and baculovirus expression system.
Thesis Objectives
Dengue viruses have reemerged as an increasingly important public health threat. Unfortunately, there is still no effective commercial DEN vaccine available in spite of years of effort to develop live attenuated, inactivated whole virion vaccines. Moreover, the exact internalization and trafficking pathways taken by flavivirus to gain access into host cells still remains unclear. Among the structural proteins, the envelope protein of flaviviruses has been demonstrated to play a crucial role in mediating virus-host cellular receptors interaction [Heinz et al., 1994; Helenius, 1995; Rey et al., 1995; Crill et al., 2001; Thullier et al., 2001], and it is also the primary antigen that induces protective immunity and hence the major antigen for virus neutralization. Therefore, the goals of thesis aimed to:
1. Expression of recombination E protein in order to test for the functionality of the receptor binding region of the truncated DV2E and to provide a potentially economic antigen source for subunit vaccine, and study the function of E protein.
2. Investigate the pathogenesis of dengue virus through the protein-protein interaction between host cellular protein and DV2E.
Chapter I
Blocking the dengue virus 2 infections on BHK-21 cells with purified
recombinant dengue virus 2 E protein expressed in Escherichia coli
Introduction
Dengue viruses are human pathogens that have reemerged as an increasingly important public health threat. Currently, there is only supportive treatment for those patients who infected by DEN. The treatments such as immunization are being developed in the hope of providing a realistic approach for controlling dengue infection [Jimenez and Lopes da Fonseca, 2001]. Unfortunately, there is still no effective commercial DEN vaccine available in spite of years of effort to develop live attenuated, inactivated whole virion vaccines.
Among the structural proteins, the E protein is the major structural protein on the surface of the mature dengue virions. The E protein forms oligomers with the small membrane (M) protein and constitutes most of the accessible virion surface [Lee et al., 2000]. This reflects the fact that the E protein is essential for membrane fusion and mediates binding to host cells. E protein is consisted of 495 amino acids with a molecular weight of about 60 kDa. It has been suggested that E protein has the major role responsible for host cell attachment and entry [Chambers et al., 1990; Monath and Henize, 1996]. It is also the primary antigen that induces protective immunity and hence the major antigen for virus neutralization. Therefore, the protein directly affects the host range, cellular tropism, and in part, the virulence of DVs [Monath and Henize, 1996].
Several approaches, based on Escherichia coli, vaccinia virus, Pichia yeast, and baculovirus expression systems [Delenda et al., 1994; Staropoli et al., 1996; Staropoli et al., 1997; Wei et al., 2003], have been taken to achieve the expression of recombinant DV E protein for the
development of subunit vaccine candidates, but the yields of recombinant E protein have been low and/or the procedures were not economically sound [Delenda et al., 1994; Staropoli et al., 1996; Staropoli et al., 1997; Wei et al., 2003]. In order to study the structure and function of the E protein and to provide a potentially economic antigen source for subunit vaccine development, it is set out to express E proteins in E. coli using expression vector pcDNA3 to produce recombinant proteins fused with in vivo expression tag sequences for purposes of identification and purification.
Here is report the functional expression of a recombinant E protein of DEN-2 virus strain PL046. This recombinant E protein has a C-terminal deletion of the hydrophobic region and a C-terminal addition of the S-tag peptide sequence that allows the application of a simple purification procedure by S-protein–agarose and antibody detection. Initially, majority of the expressed recombinant E proteins were in the form of inclusion body. The inclusion body could then be denatured by urea for isolation and purification procedures. After the removal of urea using dialysis treatment, the purified proteins still retained the receptor-binding function demonstrated by the ability of competitive blocking wild-type virus infection on host cell BHK-21.
Methods and Materials
Cells and viruses
The dengue-2 virus strain PL0146 is a gift from Dr. Yi-Ling Lin (IBMS, Academia Sinica). BHK-21 cell were cultured at 37oC, 5% CO
2 in MEM medium (Gibco 41500-034)
supplemented with 0.22% of sodium bicarbonate and 10% of fetal bovine serum (FBS)(Gobco). C6/36 cells were growth at 28oC in MEM medium (Gibco 41500-034) supplemented with 0.11% of sodium bicarbonate and 10% of FBS.
E. coli BL21 (DE3) were used for plasmid replication and expression in bacteria.
Construction of plasmids
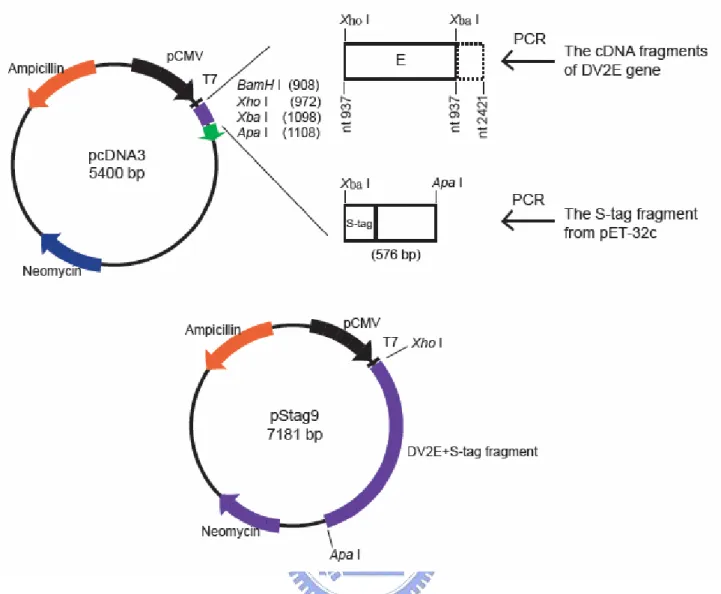
The cDNA fragment containing the DV2 E gene was kindly provided by Dr. Yi-Ling Lin (IBMS, Academia Sinica, Taipei). The E gene without the sequence of the C-terminal 93 amino acids was then cloned into the expression vector pcDNA3 and named pTru11E3. Two restriction sites were introduced to flank the truncated E gene sequence by PCR. The 5’ primer (5’ TTTCTCGAGGACAATGCGTTGCATAGG 3’) introduced a 5’ end XhoI site, while the 3’ primer (5’ AAATCTAGACTCAAGCATTTGGCCGATAGA 3’) introduced an XbaI site. The amplified fragments were digested with XhoI and XbaI enzymes and introduced into the pcDNA3 expression vector also restricted with XhoI and XbaI enzymes. To introduce the S tag sequence into pTru11E3, additional PCR was employed. The S sequence of pET-30a vector (Novagen) was amplified by PCR. The 3’ primer (5’ TTTGGGCCCACTACGTGAACCATCACC 3’) contained ApaI site and the 5’ primer (5’ TTTTCTAGACTGGTGCCACGCGGTTCT 3’) contained XbaI site. There is a stop codon before the sequence of 3’ primer. The amplified fragments containing the S sequence were then restricted with XbaI and ApaI and introduced into pTru11E3. The resulting plasmid, pStag9, contains the N-terminal 402 amino acid residues of DV2 E protein (nucleotides 937–2124 of
DV-2 genome) with an in-frame S tag fused at C-terminus as shown in Fig. 2.1. A thrombin cleavage site along with spacer sequences has also been included and situated between E gene and S tag sequence.
Expression and purification of recombinant protein
Escherichia coli BL21(DE3) were transformed with pStag9 and grown in LB broth at 37 oC
until OD600 reached 0.4. After additional 3 h incubation, the cells were harvested and centrifuged for 10 min at 5000 rpm. The pellets were resuspended with 1mM Tris–HCl (pH 8.0) containing 1mM PMSF and sonicated. The centrifuged cell lysates were re-suspended with inclusion body solubilization buffer (50mM Tris–HCl, pH 8.0, 1mM EDTA, 100mM NaCl, 8M urea, and 1mM PMSF) before incubation for 1 hour on ice. Removal of the urea was achieved by dialysis overnight in dialysis buffer (20mM Tris–HCl, pH 7.0, 150mM NaCl, and 0.1% Triton X-100).
Purification of recombinant proteins was performed by affinity purification using S-protein agarose (Novagen) following the instruction provided by the manufacturer. The S-protein agarose was washed with 10 volumes of binding/wash buffer before the sample was applied to the S-protein agarose. This was then followed by washing the agarose with 10 volumes of binding/wash buffer. The elution buffer (20mM Tris–HCl, pH 7.0, 150mM NaCl, 0.1% Triton X-100, and 3M magnesium chloride) was applied to elute the bound proteins. The eluted fractions were analyzed by SDS–PAGE. The collected proteins, named EStag9, were then subjected to dialysis treatment to remove the salt and urea. The concentrations of the purified EStag9 proteins were determined by Protein Assay Kit (Bio-Rad).
Western blot
Samples were subjected to a 12% SDS polyacrylamide gel and electrophoretically transferred onto nitrocellulose membranes (PROTRAN, Schleicher & Schuell). The membranes were then
washed in TBS buffer (Tris-buffered saline, 10mM Tris, pH 8.0 and 150mM NaCl) containing 5% non-fat dried milk at room temperature before the first antibody was added to the reaction mixture. The first antibody was either rabbit anti-DV2 E domain III polyclonal antibody (provided by Dr. Wen Chang IMB, Academia Sinica, Taipei) or anti-S tag antibodies conjugated with horseradish peroxidase protein (HRP). The reaction was then incubated overnight at 4 oC. The membranes were then washed three times in TBST buffer (Tris buffered saline, 10mM Tris, pH 8.0, 150mM NaCl, and 0.05% Tween 20) before the addition of horseradish peroxidase-conjugated goat anti-rabbit IgG or anti-mouse IgG (Chemicon international) in 1:10,000 dilutions as the second antibody when required. The reaction was performed in TBS buffer containing 5% non-fat dried milk and incubated at room temperature for 1 hour. The membranes were then washed with TBST buffer and the proteins were detected by the LumiGLO system (Kirkegaard and Perry Laboratories).
Competitive Blocking Assay (CBA)
BHK-21 cells were passaged at 4x105 cells per well in 6-well plates and incubated at 37 oC with 5% CO2 for 48 h. Serial dilution of the purified EStag9 proteins or BSA in MEM without
FBS was added to 0.5 ml of DV-2 PL046 strain in the amount of 80–100 pfu/well. The mixtures were mixed gently and added onto the BHK-21 cells in 6-well plates and then incubated at 37oC with 5% CO2 for 1 h. After aspiring the supernatant, 1:1 mixture of MEM and 2%
methylcellulose were added to the well and incubated at 37oC with 5% CO
2 for 5 days. The
medium was aspired before the cells were fixed with 3.7% formaldehyde. After 30 min, the solution was removed and the cells were stained with 1% crystal violet in 3.7% formaldehyde. The plates were washed with 3.7% formaldehyde before the plaque numbers were scored.
Results
Construction of the recombinant DV-2 E gene
The first 1206 nucleotides of the DV-2 (strain PL046) E gene coding region from the cDNA clone (provided by Dr. Yi-Ling Lin, IBMS, Academia Sinica, Taipei) were amplified by PCR. Restriction enzyme sites of XhoI and XbaI were introduced separately into the 50 and 30 of the PCR product to facilitate subsequent cloning into pcDNA3, an E. coli/mammalian expression shuttle vector. The S tag region of pET-30a was then subcloned into the XbaI and ApaI sites of the recombinant plasmid and was in frame with the sequence of the truncated E gene sequence. The resulting plasmid was named pStag9 (Fig. 2.1). The encoded recombinant protein is predicted to have 402 amino acids from E gene and a C-terminal S tag peptide and spacer sequence along with a thrombin cleavage site in between. This created a recombinant protein with 482 amino acids named EStag9. Its mass is predicted to be 53 kDa if there is no modification on the protein.
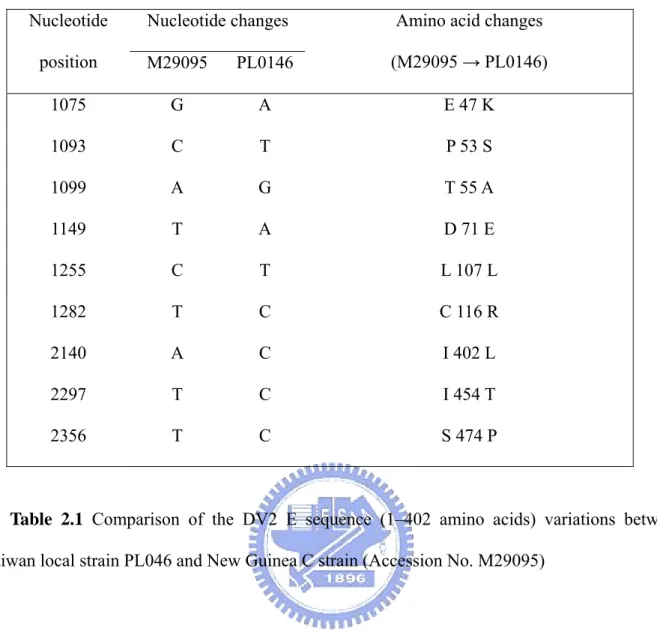
Sequence analysis of the recombinant E gene
The recombinant expression clone of the DV2 E gene of strain PL046 was subjected to sequence analysis. There are nine variations on the cDNA sequence in comparison to the DV2 strain New Guinea C (Accession No. M29095). As shown in Table 2.1, the mutation on nucleotide 1255 (amino acid 107) is a silent mutation, the rest are missense mutations. Those mutations are located at amino acid residues 47, 53, 55, 71, 107, 116, 402, 454, and 474 of DV-2 E proteins. According to the reported crystal structure [Rey et al., 1995; Modis et al., 2003], residue 47 is in domain I, residues 53, 55, 71, 107, and 116 in domain II, and the rest in the transmembrane domain.
Production and purification of recombinant DV-2 E protein in E. coli cells
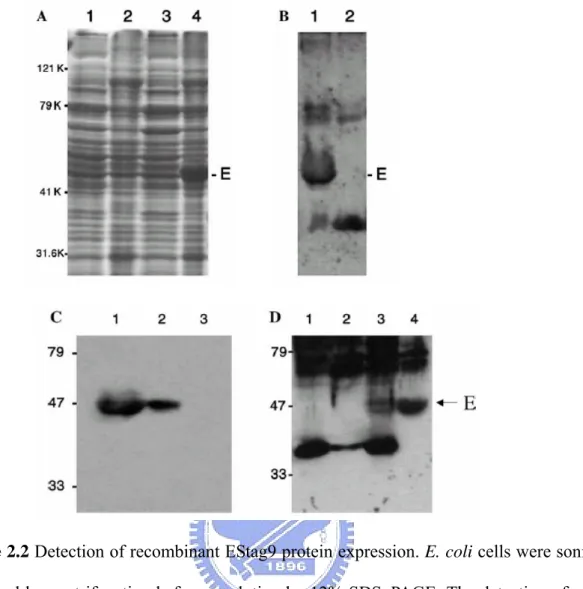
The pStag9 construct was transformed into E. coli strain BL21(DE3). The total cellular proteins from E. coli transformants were analyzed by SDS–PAGE with Coomassie blue staining (Fig. 2.2A) or by Western blot (Figs. 2.2B–D) using antibody against the S-tag sequence or against domain III of E proteins (provided by Dr. Wen Chang, IMB, Academia Sinica, Taipei). As shown in Fig. 2.2, panel A, a dominant band corresponding to the predicted size of the recombinant protein can be detected in the pellet from cells transformed with the pStag9 but not from cells transformed with the control vector pcDNA3 alone. No dominant band was detected in the supernatant of cell lysates (Fig. 2.2). Hence, the expressed proteins were in the form of inclusion bodies in E. coli cells.
Solubilization of the recombinant EStag9 proteins
In order to obtain the protein in soluble form, the pellets of cell lysates containing inclusion body were denatured in 8M urea following sonication treatments to the E. coli cells. After the purification with S-protein agarose, the EStag9 proteins then underwent dialysis to removed urea. The S-protein agarose (Novagen) can bind the 15 amino acid S tag peptides fused in frame at the C-terminus of EStag9. After elution, elutes in fraction of 0.5 ml were analyzed by SDS–PAGE and stained with Coomassie blue and assessed by Western blotting with anti-E domain III polyclonal antibodies. The resulted products were quantitated by Protein Assay Kit (Bio-Rad). The overall yield is 3 μg purified protein for 100 milliliter of E. coli culture.
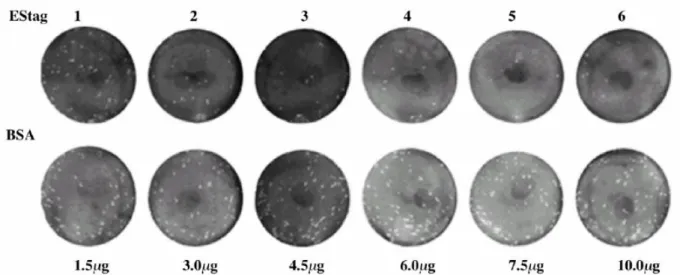
The competitive blocking assay assessing the biological function of EStag9 proteins
cells, the purified proteins were serial diluted and mixed with virions of DV2 PL046 strain. If the purified EStag9 can still bind host cells, it will compete with the DV2 virions for host cell surface receptors. This shall reduce the frequency of BHK-21 cells infected by wild-type virions and shall result in the reduction of the number of plaques formed. Since every plaque represents an infection event, the number of plaques in an assay plate indicates the numbers of successful virion infection events. As shown in Figs. 2.3 and 2.4, the plaque number was significantly reduced by the addition of the purified EStag9 proteins to the virions. Addition of BSA did not exhibit the same effect. Fig. 2.4 shows that the number of plaques formed on BHK-21 cells decreased as the amount of EStag9 proteins increased. This indicated that the purified EStag9 proteins could effectively block the plaque formation by competing with DV2 virions for the infection of host cells. As shown in Fig. 2.4, the addition of 3 μg of EStag9 proteins inhibited more than 50% PFU (plaque forming unit) compared to no Estag9 proteins addition. Addition of equal amount of BSA reduced 20% of the PFU under conditions tested. Addition of 10 μg of EStag9 protein reduced the PFU down to less than 10% of that of wild type, while the addition of BSA still reduced only 20%. This result indicates that the receptor binding function of the purified recombinant E protein is still retained.
Discussion
Analysis of the sequence of the cDNA clone of DV2 E gene (strain PL046) showed that there are nine single nucleotide variations compared with the DV-2 strain New Guinea C (Accession No. M29095). Those variations may arise from strain diversity or the process of cDNA construction. The crystal structure of the first 394 residues of DV2 E protein homodimer has been solved [Modis et al., 2003] and multiple lines of evidence indicated that the structure of E protein is conserved across the Flaviviridae [Rey et al., 1995]. According to their results, the flavivirus E protein can be divided into three distinct structural domains besides the transmembrane region. For DV E protein, domain I consists of amino acid residues 1–51, 132–192, and 280–295. Amino acid residues 52–131 and 193–279 are domain II and amino acid residues 296–394 are domain III. Hence, the mutation on residue 47 is in the domain I. Mutations on residues 53, 55, 71, 107, and 116 are in domain II. The last three are in the transmembrane domain at residues 402, 454, and 474. There is no variant in the domain III region, which is characterized by an immunoglobulin-like structure, and has been hypothesized to be the receptor-binding domain of the E proteins [Rey et al., 1995; Roehrig et al., 1998]. According to the model of Rey et al. [Rey et al., 1995], those mutations in each region seem to cluster together in space. Among those mutations, the one at residue 107 is a silent mutation, which is part of the fusion peptide structure [Modis et al., 2003]. The region from amino acids 100 to 108 between the anti-parallel strands c and d of domain II in TBE virus is almost completely conserved among Flaviviruses [Allison et al., 2001]. The cd loop region has been suggested to be directly involved in the interaction with target membrane during fusion and has been hypothesized to function as an internal fusion peptide at low pH [Allison et al., 2001]. The function of fusion peptide is critical to the successful infection and propagation of virions, hence, any mutation affecting its function will have detrimental effect. Therefore, it is not surprising to find a silent mutation at this location.
There is no variation in the domain III region. Monoclonal antibody recognizing the domain III could neutralize target cell infection [Thullier et al., 1999] and could strongly block virus adsorption [Crill et al., 2001]. This suggests that interactions between this region and target cells could mediate virus entry [Thullier et al., 2001]. Mutations in this region may affect the entry and/or infection of the virus. Hence, it is not surprising that no found variations in this area. Previous reports have shown that DV E proteins expressed in E. coli form inclusion bodies [Mason et al., 1990; Sugrue et al., 1997], which makes the recovery of the recombinant protein difficult. Even after the removal of the transmembrane domain, it was still largely insoluble in the bacterial cytoplasm [Sugrue et al., 1997]. This is consistent with our data that the expressed EStag9 proteins were presented in the precipitated fraction of cell lysate (Fig. 2.2). It is a possibility that over-expression of the E protein in E. coli facilitates the formation of inclusion body. Here, I have overcome this issue by denaturing the cell lysates containing protein inclusion bodies in 8M urea, and then refolded the proteins by dialysis to remove urea. I have tried to express the full length E gene in E. coli at the beginning, but no protein production was detected (data not shown). Truncation of the C-terminal hydrophobic region allows the over expression of the E protein in E. coli. To facilitate the purification process, a 15-amino-acid S-tag peptide was fused to the C-terminal of the truncated recombinant E proteins, which allow affinity purification by S-protein agarose (Novagen). The recombinant peptide also contains a thrombin cleavage site between the truncated E protein and S-tag. Therefore, the S-tag can be removed easily by digestion treatment to the recombinant proteins with biotinylated thrombin and then capture the cleaved S-tag with streptavidin agarose. This procedure allows the recovery of the purified recombinant proteins rapidly and easily.
The key issue of expressing the recombinant E protein in E. coli is whether the function and structure could be retained. Most studies used monoclonal antibodies to recognize the structural epitopes to determine the structural integrity [Staropoli et al., 1996; Staropoli et al., 1997; Kelly et al., 2000]. By using competitive assay on viral infections, it was directly tested the relevant
biological function of the purified EStag9 proteins. It have shown that the recombinant EStag9 proteins, when mixed with the dengue virions, could inhibit plaque formations of host cells caused by dengue viral infections up to greater than 90%, presumably by competing with wild type virions for host cell viral receptor binding. This suggests that purified and dialyzed EStag9 proteins have retained the structure, at least partially in the region possessing the functions for cell surface binding and entry, hence maintain the key function of our concern. Interestingly enough, BSA could also reduce 20% of PFU regardless of the amount of proteins added under our experimental conditions. Expressing proteins in E. coli is still the most economic and convenient method to produce large amount of viral proteins for research and application purposes. However, formation of inclusion body has always been a common issue. Here is reported that by removal of the denaturing urea in dialysis, the purified recombinant E proteins could still compete with wild type viral particles for infection on host cells.
Nucleotide changes Nucleotide
position M29095 PL0146
Amino acid changes (M29095 → PL0146) 1075 G A E 47 K 1093 C T P 53 S 1099 A G T 55 A 1149 T A D 71 E 1255 C T L 107 L 1282 T C C 116 R 2140 A C I 402 L 2297 T C I 454 T 2356 T C S 474 P
Table 2.1 Comparison of the DV2 E sequence (1–402 amino acids) variations between
Figure 2.1 Construction of pStag9 plasmid: the DV-2 E gene sequence from nucleotide 937
to 2142 (full-length E gene is from 937 to 2421) was amplified by PCR and inserted to pcDNA3 expression vector with the addition of an XhoI site and ATG at the 5' end and an XbaI site at the 3' end. This truncated E sequence was then fused at the 3' an S-tag fragment amplified from the pET-30c (appendix 1 and 2) and cloned into the XbaI and ApaI sites introduced by PCR onto the S-tag fragment.
Figure 2.2 Detection of recombinant EStag9 protein expression. E. coli cells were sonicated
and followed by centrifugation before resolution by 12% SDS–PAGE. The detection of protein expression was by (A) SDS–PAGE and with Coomassie blue staining. Total proteins were from supernatant (lanes 1 and 3) and pellet (lanes 2 and 4). Lanes 1 and 2 were mocks and lanes 3 and 4 were lysates from E. coli transformed with pStag9. (B) Western blotting with an anti-DV2 domain III antibody (provided by Dr. Wen Chang IMB, Academia Sinica, Taipei). Lane 1, E. coli lysates from cells transformed with pStag9; lane 2, mock. (C) Western blotting with an anti-S tag antibody. Lane 1, cell lysates of E. coli transformed with pStag9; lane 2, proteins purified by S-protein agarose from E. coli transformed with pStag9; and lane 3, mock. (D) Western blotting with an anti-DV2 domain III antibody. Lane 1, mock; lane 2, the supernatant of E. coli lysates from cells transformed with pStag9; lane 3, total E. coli cell lysates of cells transformed with pStag9; and lane 4, proteins purified from E. coli cells transformed with pStag9. The bands corresponding to EStag9 are indicated by E or an arrow by the side.
Figure 2.3 Competitive blocking assay. Purified EStag9 proteins and BSA were serial diluted
with medium without FBS and mixed with DV-2 virions before the addition to BHK-21 cells growing in 6-well plates. After absorption for 1 h at 37 oC with 5% CO2; the media were aspired
and the cells overlaid with agarose. After incubation for 5 days at 37 oC, 5% CO2, cells were
Figure 2.4 Quantitative representation of dosage-dependent competitive blocking assay of
viral infection by the purified recombinant EStag9 proteins (●) and BSA (○). Proteins were serial diluted with medium without FBS and mixture with DV2 strain PL046.
Chapter II
The Type 2 Dengue Virus Envelope Protein Interacts with Small Ubiquitin-like
Modifier-1(SUMO-1) Conjugating Enzyme 9 (Ubc9)
Introduction
Yeast two-hybrid technology
The yeast two hybrid (Y2H) system, first developed by Stan Fields and coworkers [Bartel et al., 1993a; Bartel et al., 1993b; Fields, 1993; Fields and Sternglanz, 1994], is a powerful technique for identifying novel protein-protein interactions [Fields et al., 1989]. The system involves the expression of chimeric proteins and their subsequent interactions within the yeast cells. The interaction is detected by the expression of a reporter gene that changes the phenotype of the recipient yeast cell. Unlike the in vitro biochemical methods, Y2H can detect in vivo interactions. Moreover, neither protein purification nor antibody production is required for this methodology [Topcu and Borden, 2000].
The Y2H system provides a sensitive method to detect relatively weak and transient protein-protein interactions. Such interactions may not be biochemically detectable, but may be critical for the proper functioning of complex biological systems [Guarente et al., 1993; Estojak et al., 1995]. It also allows one to screen a library of activation domain fusions or preys for the binding partners of one’s favorite protein expressed as a DNA binding domain fusion or bait, and it can be used to pinpoint protein regions mediating the interactions [Ito et al., 2002].
Sumolyation
other proteins. Conjugation of ubiquitin and ubiquitin-related proteins (Ublps) to cellular target proteins is involved in many aspects of eukaryotic gene expression by regulating the signaling for degradation and/or modifying the functions of target proteins [Vashavsky, 1997; Hershko and Ciechanover, 1998; Melchior, 2000; Yeh et al., 2000; Hay, 2001; Muller et al., 2001; Schwartz and Hochstrasser, 2003; Seeler and Dejean, 2003]. Unlike ubiquitin, conjugation of SUMO does not typically lead to degradation of the substrate and instead it causes alterations in function or changes in intracellular localization [Wilson and Rangasamy, 2001]. Three SUMO paralogues have been reported in mammalian cells and they are known as SUMO-1, -2, and -3 [Lapenta et al., 1997; Kamitani et al., 1998].
It is believed that conjugation and de-conjugation of SUMO to other proteins happens in a process similar to ubiquitination, which involves an E1 activating enzyme, an E2 conjugating enzyme, and an E3 target specificity enzyme. An inactive SUMO is converted to its active form by removal of its last four amino acids to expose at the C-terminus two essential glycine residues, which then form a thioester bond with a cysteine of the SUMO-activating E1 enzyme (SAE1-SAE2). Consequently, it was transferred to a conjugating E2 enzyme (Ubc9) and finally passed to the ε-amino group of specific lysine residues on the target proteins [Jensen et al., 2004]. Some evidence has suggested that Ubc9 can itself bind specifically to substrates presenting a consensus SUMO modification motif, ψKxE (ψ represent the hydrophobic resides, K is lysine, x is any residue, and E is glutamic acid). Recently, structural analysis has revealed that Ubc9 can recognize this sequence directly [Hay, 2001; Benier-Villamor et al., 2002; Lin et al., 2002b; Lin et al., 2002c; Tatham et al., 2003]. Ubc9 binding may play an important role in substrate recognition as well as in substrate modification [Sampson et al., 2001]. In recent years, growing numbers of viral proteins have been found to conjugate with SUMO-1 [Muller and Dejean, 1999; Hofmann et al., 2000; Rangasamy and Wilson, 2000a; Rangasamy et al., 2000b; Adamson and Kenney et al., 2001; Ahn et al., 2001; Endter et al., 2001; Xu et al., 2001; Gravel et al., 2002; Saitoh et al., 2002; Spengler et al., 2002; Lethbridge et al., 2003]. There are currently six known
sumoylated viral proteins, distributed among three DNA viral families: Adenoviridae, Papillomaviridae, and Hepesviridae [Rangasamy and Wilson, 2000a; Rangasamy et al., 2000b; Endter et al., 2001; Gravel et al., 2002; Saitoh et al., 2002; Lethbridge et al., 2003]. All six viral proteins are products of early genes with important regulatory roles in viral transcription or replication. In addition, some other viral proteins have been shown to interact directly with components of the sumoylation system. For examples, the Vaccinia Virus early protein E3L and the Tula Hantavirus Nucleocapsid protein (TULV-N) both interact with SUMO-1 [Rogan and Heaphy, 2000; Kaukinen et al., 2003], while the Mason-Pfizer Monkey Virus (MPMV) Gag protein interacts with Ubc9 [Weldon et al., 2003]. The Hantaan Virus Nucleocapsid protein (HTNV-N) interacts with both SUMO-1 and Ubc9 [Maeda et al., 2003], and the Epstein Barr Virus nuclear antigen 3C (ENBA-3C) interacts with SUMO-1 and SUMO-3 [Lin et al., 2002b].
In this study, I have identified Ubc9 as one host protein interacting with DV2E through the yeast-two-hybrid-based “Functional Yeast Array” (Level Biotechnology Inc., Taipei, Taiwan,). It was also demonstrated that DV2E could interact with Ubc9 in in vitro pull down assay. Furthermore, the mutagenesis result showed that residues K51 and K241 on DV2E were critical for the interaction with Ubc9. It was observed that DV2E-EGFP affected the distribution of Flag-Ubc9, making it concentrate toward the nuclear membrane instead of evenly distribute predominantly in whole nucleus. And these two proteins were co-localized near the cytoplasmic side of the nuclear membrane. In addition, over-expression of Ubc9 could reduce DV2 infection in BHK-21 cells in plaque assay, suggesting that Ubc9 may interfere with dengue viral propagation such as involving in the host defense system to prevent virus infection.
Methods and Materials
Cells and viruses
BHK-21 cells were cultured at 37oC, 5%CO2 in MEM medium supplemented with 0.22% of sodium bicarbonate and 10% of FBS. C6/36 cells were grown at 28oC in MEM medium
supplemented with 0.11% of sodium bicarbonate and 10% of FBS.
Mammalian 293 cells were cultured at 37oC with 5% CO2 in MEM medium (Gibco) supplemented with 4mM of L-glutamine, 1.5g/L of sodium bicarbonate, 4.5g/L of glucose, and 10% of fetal bovine serum (FBS) (Gibco).
C6/36 cells were growth at 28oC in MEM medium (Gibco 41500-034) supplemented with 0.11% of sodium bicarbonate and 10% of FBS.
Saccharomyces cerevisiae yeast strain LY001 and LY002 used for screening were provided
by Level Biotechnology Inc., Hsi-Chih, Taipei, Taiwan. Strain L40 (MATa ade2 his3 leu2 trp1
LYS2::lexAop-HIS3 URA3::lexAop-lacZ) was used for assessing the result of Functional Yeast
Array by yeast-two-hybrid.
E. coli BL21 (DE3) were used for plasmid replication and expression in bacteria.
Construction of plasmids
All the DV2E-related constructs in this study were shown in Fig. 3.1.
A recombinant plasmid containing E protein sequence, pStag9, was used as the template to amplify the DV2E DNA fragment by PCR. The forward strand primer, 5’ TTTCTCGAGGACAATGCGTTGCATAGG 3’, introduced a 5’ end Xho I site and the reverse strand primer, 5’ AAAGGTACCCTAAAGCATTTGGCCGATAGA 3’, introduced a stop codon and a Kpn I site. The amplified fragments were digested with Xho I and Kpn I and ligated to pLB1.0 vector (Level Biotechnology Inc., Hsi-Chih, Taipei, Taiwan) at the Xho I and Kpn I sites. The resulted plasmid encoding a recombinant protein contained the Lex A sequence at the
N-terminus alone with the N-terminal 88% of the DV2E protein (nucleotides 937~2142 of DV 2 annotated according to NGC strain) and was named pLexA-DV2E, which can express the DV2E in LY002 yeast cells. The DNA fragment containing DV2E sequence from pLexA-DV2E was introduced into the pBTM116 vector at the restriction sites Xho I and Kpn I to construct pBTM-D2E. For the yeast-two-hybrid, the pACT2-SUMO1 and pACT2-Ubc9 were used as the preys while the pBTM116-MST3 and pBTM116-DAXX were used as the negative and positive baits, respectively. The plasmid pStag9 was again used as template to construct the pDV2E-EGFP, containing the sequence encoding E protein with in-frame fusion of the EGFP at the C-terminal end. The forward strand primer, 5’ TTTCTCGAGGACAATGCGTTGCATAGG 3’, introduced a 5’ end Xho I site and the reverse strand primer, 5' AAAGGTACCCAAGCATTTGGCCGATAGAA 3', introduced a Kpn I site to the DV2E sequence amplified by PCR. The amplified DV2E fragments were digested with Xho I and Kpn I, and ligated to pEGFP-N2 vector (BD Biosciences) in frame with the sequence of EGFP to generate pDV2E-EGFP. The resulted construct expresses a protein containing the N-terminal 88% of the DV2E protein with the EGFP sequence.
The cDNA of E gene was then cloned into expression vector pcDNA3 by PCR amplification, and the construct was named pDEΔ94, which also containing the sequence of the last 66 amino acids of prM and the truncated E gene. The positive strand primer 5’ TTTCTCGAGTGGGAATGGGACTGGAGA 3’ introduced a 5’ end Xho I site and the negative strand primer 5’ AAATCTAGACTCAAGCATTTGGCCGATAGA 3’ introduced an Xba I site. The amplified fragments were digested with Xho I and Xba I site, and were introduced into pcDNA3 vector. To introduce a His tag sequence, two primers: 5’
TTTGGGCCCACTACGTGAACCATCACC 3’ containing Apa I site, and 5’
TTTTCTAGACTGGTGCCACGCGGTTCT 3’ containing Xba I site were used for PCR amplification using pET-30b vector (Novagen) as template to obtain fragment containing the His tag sequence. The PCR products were then restricted with Xba I and Apa I site and introduced
into pDEΔ94. The resulted plasmid pDEΔ94-pta15 contains the 3’ end of prM (nucleotides 734~936) and the N-terminal 88 % of DV2E (nucleotide 937-2142) protein along with a His-tag at C-terminus (nucleotides 937~2142). The pACT2-SUMO1, pACT2-Ubc9, pBTM116-MST3, pBTM116-DAXX, and pFlag-Ubc9, and pGEX-4T-Ubc9 were from the collection of Dr. Hsiu-Ming Shih (IBMS, Academia Sinica, Taipei, Taiwan). The pACT2 carries a GAL4 activation domain and a LEU marker while pBTM116 contains a DNA-binding domain and a
TRP marker. The pFlag-Ubc9 carries a FLAG tag fused to Ubc9 gene in pCMV-Tag2a vector,
and can express the fusion protein in mammalian cells. The pGEX-4T-Ubc9 carries the GST-Ubc9 fusion protein and can over-express it in E. coli.
Rapid Screening by Functional Yeast Array
The Functional Yeast Array system (Level Biotechnology Inc., Hsi-Chih, Taipei, Taiwan) is derived from the yeast-two hybrid technology. The procedure is a rapid, high-through-put screening based on the automated 96-well plate liquid handling. This system contains about 1200 full-length cDNA of known human genes, each fused to the VP16 trans-activation domain, as the preys. Those genes are divided into 5 different groups known as modules according to their functions. These five modules are Apoptosis, Cell Interaction, Transcription Factor, Cancer Related, and Signal Transduction. The plasmids containing the preys have special design to restrict the growth of cells to reduce the false positive (Level Biotechnology Inc.) [Gietz et al., 2002; Ito et al., 2002; Knudsen et al., 2002; Mouradian, 2002; Ranish et al., 2003]. The Functional Yeast Array screening utilizes a recombinant LexA-DV2E construct which contains the DV2E gene fragment cloned into pLB-1.0 vector (Level Biotechnology Inc.) as the bait to against module preys. For this study, it was chosen three sets of modules, Apoptosis, Cell Interaction and Signal Transduction, totally about 500 individual clones, as preys for the procedure. As shown in Fig. 3.2, the Trp- Lys-LY001 yeast strain (Level Biotechnology Inc.) expressing LexA-DV2E was mated with the Leu- LY002 yeast (Level Biotechnology Inc.)
![Figure 1.5 Intracellular life cycle of dengue virus. [Clyde et al., 2006]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8617829.191247/23.892.124.787.266.704/figure-intracellular-life-cycle-dengue-virus-clyde-et.webp)