行政院國家科學委員會專題研究計畫 成果報告
Bcl-2 基因過度表現對於物質 P 誘發膀胱功能亢進的影響
計畫類別: 個別型計畫 計畫編號: NSC92-2314-B-002-334- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學醫學院泌尿科 計畫主持人: 余宏政 共同主持人: 鄭劍廷 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 11 月 2 日
中文摘要 物質 P 可以誘發過動膀胱是因其透過 ICAM 與活性氧之作用。這些作用可以受抗氧化 物質補充而改善。我們以誘發 Bcl-2 portein 的結果發現可以改善之。同時也可以減低 發炎、過度收縮與細胞凋亡現象。此二篇相關文章已發表於 Am J Physiol 與 J Physiol 期刊。 Abstract
We explored whether substance P (SP) via neurokinin (NK) receptor facilitates bladder afferent signaling and reactive oxygen species (ROS) formation in bladder connected with neurogenic inflammation. We evaluated ROS activity and cystometrograms as well as pelvic nervous activity in anesthetized rat bladder with SP stimulation. Our results showed endogenous SP via NK1, not NK2 receptor in mediating a micturition reflex. Increased SP
by electrical stimulation of pelvic nerve or exogenous SP from intra-arterial or spinal route can facilitate myogenic and neurogenic bladder contractions. Furthermore, exaggerated SP release caused an increase in ROS amounts in the bladder and whole blood with the mechanism of increased mast cell degranulation, intercellular adhesion molecule (ICAM) expression, and leukocytes adhesion, a primary source of ROS in the inflamed bladder. Treatment with NK1 receptor antagonist or ROS scavengers reduced the bladder ICAM
expression and ROS amounts, and ameliorated the hyperactive bladder response. Our study indicates that the mechanism by which SP participates in the neurogenic bladder may be complicated by its proinflammatory activity and its ability to stimulate ROS generation. Introduction
The tachykinins substance P (SP), neurokinin A (NKA), and neurokinin B (NKB) belong to a family of neuropeptides that are widely distributed in the mammalian central and peripheral afferent nervous systems and produce their biological actions by activating three distinct receptor types, termed NK1, NK2, and NK3 receptors [1]. These afferents
commonly innervate smooth muscle, submucosal layers, and blood vessels of visceral organs [2-4]. Upon release from sensory afferents, SP and NKA, via NK1 and NK2
receptors, act on smooth muscle or blood vessels to regulate visceral motility and blood flow [1,2]. Recent evidence suggests that endogenous tachykinins may play a role in visceral inflammation, hyperreflexia and hyperalgesia [4,5]. For example, in the rat urinary bladder, SP may be more relevant than NKA for the mediation of plasma protein extravasation and inflammatory response [6], whereas NKA is an important mediator of smooth muscle contraction [2]. In addition, tachykinins may lead to expression of adhesion molecules by endothelial cells, chemotaxis and activation of immune cells, mucus secretion and water absorption/secretion in the pulmonary, gastrointestinal and genito-urinary tract [6-8].
The mammalian bladder is richly innervated by capsaicin-sensitive, SP-containing afferent fibers [9,10]. Afferents signaling mechanical and chemical environments in the bladder evoke sacrolumbar reflexes in lumbar sympathetic neurons to increase bladder capacity, or elicit parasympathetic-bladder efferent excitation to trigger a normal micturition reflex [11-13]. However, the bladder capacity and/or the micturition reflex may be altered in a number of pathophysiologic conditions, such as interstitial cystitis, cyclophosphamide-induced cystitis, and irritants induced hypersensitivity [14]. The
mechanisms responsible for bladder hyperactivity may vary from condition to condition and are likely complicated. Bladder biopsies in some patients diagnosed with interstitial cystitis have shown increased density of SP-containing fibers and NK1 receptors [15-17].
Furthermore, administration of SP is known to cause bladder inflammation [2,7,18], and generation of reactive oxygen species (ROS) by inflammatory cells [19,20]. Thus, SP may contribute either a direct or an indirect role in neurogenic inflammation and in regulation of bladder motility in various clinical conditions. Nonetheless, whether ROS generated by SP-reacted inflammatory cells contributes to bladder hyperactivity remains to be determined.
Our objective in the present study was to clarify the contribution and the possible mechanism of SP in bladder hyperreflexia. We showed that bladder hyperactivity caused by pelvic nerve stimulation is associated with an increased SP level in bladder. We had also used NK receptor antagonists and free-radical scavengers to test the role of SP-induced and ROS-induced hyperactivity, respectively. Our results clearly showed that SP via NK1
receptor activation enhances micturition reflex and ROS release from the inflammatory cells, and consequently leads to a hyperactive bladder.
Materials and Methods Drugs
The drugs/chemicals used are listed in Table 1. The drugs were prepared and stored at -70°C, and subsequent dilutions of the drugs were made in saline on the day of the experiments.
Surgery
Adult female Wistar rats weighing 220-240 g were anesthetized with subcutaneous urethane (1.2 g/kg), which is well known to anesthetize yet permit full reflex bladder contractions [12,13]. The maintenance of deep anesthesia was determined by the persistence of miotic pupils as judged from frequent inspection and by the lack of heart rate and arterial blood pressure (ABP) fluctuations in the absence of visceral stimuli [12]. An experiment was terminated when the baseline mean ABP was below 90 mmHg. The animal care and experimental protocol were in accordance with the guidelines of the National Science Council of the Republic of China (NSC, 1997). All efforts were made to minimize both animal suffering and the number of animals used throughout the experiment. At the end of each experiment, the animals were sacrificed by an intravenous potassium chloride injection.
PE-50 catheters were placed both in the left femoral artery for measurement of ABP and in the left femoral vein for administration of anesthetics when needed. The ABP was recorded on a polygraph (Gould RS3400) with a transducer (P23 1D, Gould-Statham, Quincy, MA). A length of stretched PE-10 tubing was inserted just above the bifurcation of the aorta from the right femoral artery, and was used for injection of various drugs. Body temperature was kept at 36.5-37°C by an infrared light and was monitored with a rectal thermometer.
Experimental models and protocols
We used a transcystometric model (via cannulation of bladder dome) mimicking normal micturition of the bladder to evaluate the role of SP in bladder micturition reflex and bladder hyperactivity [12,21]. Briefly, the urinary bladder was exposed through a midline incision of the abdomen, and urine was emptied by application of light pressure. A
PE-50 T-tube was inserted through the apex of the bladder dome. The bladders were filled by continuous infusion of 0.9% saline (0.15 ml/min) at room temperature and were allowed to drain/micturate repeatedly via the urethra.
The change in bladder pelvic afferent nervous activity (PANA), bladder pelvic efferent nervous activity (PENA), ABP, and various parameters measured by cytometerogram can be determined before and after intra-arterial (i.a) or intrathecal (i.t) administration of various chemicals. All chemicals were injected through the i.a catheter in a volume of 1 ml/kg (0.20-0.25 ml) and were followed by 0.1 ml of heparinized saline. For
i,t administration, a catheter (Portex, Hythe, Kent, UK) was inserted through the
atlanto-occipital membrane for 8.5 cm, in such a way that the tip of the catheter was placed just above the lumbosacral enlargement, as described previously [21]. Chemicals were given i.t at a volume of 20 µl and were followed by 30 µl of saline.
In the first part of study, the animals were divided into 3 groups; the first group (group 1) was used to test the role of endogenous SP in bladder micturition reflex by administration of NK receptor antagonists and the second and third groups were used to test the effect of exogenous SP and SP+NK receptor antagonists on the bladder hyperactivity. SP was given at concentrations of 0.1 to 10 µg and 5µg for i.a and i.t administration, respectively. A plasma SP level from iliac vein was in a range from 35-1200 ng/ml 10 min following i.a injection. To verify the SP activity via NK1 receptor, we injected SP (group 2),
SP along with a NK1 receptor antagonist CP96,345 (group 3a) or a NK2 receptor antagonist
SR48968 (group 3b). The NK receptor antagonists were given at a dose of 250-500 µg/rat via i.a route and 5 µg-250 µg/rat via i.t route. The amounts used were less than those (up to 5,000 µg/kg via i.v route and up to 250 µg/kg via i.t route) in previous studies [22,30,31]. Cystometrogram for measurement of bladder response
The bladder catheter was connected via a T-tube to a pressure transducer (P23 1D, Gould-Statham), and the IVP was recorded continuously on a Gould polygraph (RS3400, Gould, Cleveland, OH). The following parameters of bladder responsiveness were also measured: the threshold volume (infused volume at the point preceding a micturition reflex), the number of active contractions (>15 mmHg), micturition volume (volume of expelled urine collected in a preweighted tube), residual volume (fluid amount remaining following a bladder contraction; infused volume calculated from infusion rate minus micturition volume), bladder capacity (residual volume plus micturition volume), intercontraction interval (the time lag between two micturition cycles), and basal pressures.
Recording of PANA and PENA
Multifiber PANA and PENA were measured simultaneously in eight rats. The two left pelvic nerve branches attached to the urinary bladder surface were isolated and were simultaneously recorded by placement of the intact nerve fibers in parallel with two pairs of thin, bipolar stainless steel electrodes [12]. The electrical signals were amplified 20,000-fold, filtered (high-frequency cutoff at 3,000 Hz and low-frequency cutoff at 30 Hz) with an AC preamplifier (Grass model P511, Valley View, OH), continuously recorded on magnetic tape, and displayed on a Gould oscilloscope (1604). The amplified signals (spikes) were transformed by a window discriminator 121 (World Precision Instruments, Sarasota, FL) and analyzed with an impulse counter (Gould integrator amplifier, 13-4615-70), which was set to count the total number of spikes per sec [12,13]. The background activity, which
could be caused by the nerve contact with electrodes, nerve damage during handling, and the equipment itself, was excluded from the window discriminator by adjustment of the threshold voltage [13].
Nerve fiber with PANA was confirmed by its ability to show increased activity in response to small increments of IVP by saline infusion via T-tube. Nerve fiber with PENA had minimal activity until a threshold volume/pressure in the bladder was reached which produced a bursting discharge causing a micturition contraction (Fig. 1) [12,13,32].
Electric stimulation of pelvic nerve
The next study was intended to determine whether nerve activity causing bladder hyperactivity was accompanied with release and/or elevation of SP in bladder. Electric current of square wave pulses with pulse duration of 0.05 ms was applied from a stimulator (Grass S88, Quincy, MA) through a stimulus isolation unit (Grass SIU5B) and a constant current unit (Grass CCU1A).
Six rats were first given the solution of atropine (1 mg/ml/kg) i.a to provide an effective inhibition of muscarinic (parasympathetic) effect. One branch of pelvic nerve was dissected from left major pelvic ganglion to urinary bladder and was identified by a bladder contraction elicited by electrical stimulation [12]. Electric stimulation of urinary bladder with different frequency (1 to 10 Hz) was performed for 5 min and bladder response including IVP and number of active contractions was recorded. Blood samples withdrawn from the bladder outflow (iliac vein) were obtained for SP level determination before and during each period of stimulation.
Measurement of SP
The measurement of plasma levels of SP in iliac vein was performed as described previously [33]. The study was intended to determine whether pelvic nerve activity results in elevation and release of SP in bladder. Briefly, supernatant from plasma samples was diluted with the same volume of buffer A (RIK-BA-1, Peninsula Laboratory). Then each sample was passed slowly through a C18 Sep-Pak column (RIK-SEPCOL-1, Peninsula Laboratory). The column was washed with 9 ml of buffer A and eluted with 3 ml of buffer B (RIK-BB-1, Peninsula Laboratory). The eluted samples were dried by vacuum centrifugation and stored at −70°C for later analysis. A SP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) was used for detecting the SP level. Each sample was dissolved in 1% hydrochloride, diluted to a suitable concentration with enzyme immunoassay buffer, and assayed in duplicate. The SP, which was linked to acetylcholine esterase as a tracer, and rabbit SP antiserum were added to the sample and incubated in the assay plate at 4°C for 18 h. Then the wells were rinsed five times with washing buffer. Ellman's reagent was added for development of the plates in each well. After development, these plates were read at 410 nm, and SP levels were calculated.
Detection of ROS production in urinary bladder following exogenous SP administration SP is capable of causing intracellular ROS generation and release by inflammatory cells and, perhaps, other types of cells [19,20]. Thus, we want to examine whether ROS generated following exogenous SP administration contributes to bladder hyperactivity. The ROS generation in response to SP stimulation was measured in whole blood (obtained from the femoral artery) and bladder by a chemiluminescence (CL) detection method as described previously [25]. In addition, the possible cellular source of ROS in the urinary bladder was examined by the fluorescence emitted after dichlorofluorescin (DCFH)
diacetate infusion [34].
A change in the bladder surface during the filling and micturition states of the transcystometric model may affect the measurement of ROS. Thus, an isovolumetric model allowing minimal bladder surface change was adopted for measurement of ROS generation from the bladder in vivo. Eighteen rats were used and grouped (described below) in this part of the experiment. To establish an isovolumetric condition, we inserted one PE-50 tube into the bladder through the urethra and tied it in place by a ligature around the urethral orifice [35]. The catheter was connected to a separate pressure transducer and an infusion pump via a T-tube connector. Transurethral filling (0.15 ml/min) of 0.9% saline into the urinary bladder via the urethral catheter was done until rhythmic bladder contractions occurred. The infusion was then stopped, and the bladder was maintained under constant-volume conditions by ligation of the ureteres bilaterally.
The method for detection of ROS from the organ surface after [2-methyl-6- (4-methoxyphenyl)-3,7-dihydroimidazo-(1,2-a)-pyrazin-3-one hydrochloride (MCLA)] (i.a., 0.2 mg/ml/h) administration was adapted [25] for demonstration of ROS production in the hyperactive bladder. The rat was maintained on a respirator (tidal volume: 1.0-1.5 ml; rate, 80-90 cycles/min; inspiratory pressure, 20-30 cm H2O) and a circulating water pad at 37°C
during photon detection. For excluding photon emission from sources other than the urinary bladder, the animal was housed in a dark box with a shielded plate. Only the bladder window was left unshielded and was positioned under a reflector, which reflected the photons from the exposed bladder surface onto the detector area. The MCLA-enhanced CL signal from the bladder surface was measured continuously before and during SP (10 µg) administration by use of a CL Analyzing System (CLD-110, Tohoku Electronic Industrial Co., Sendai, Japan).
Cellular origin of ROS in bladder following SP administration
We conducted DCFH diacetate tissue staining to determine the cellular origin of ROS in the bladder following SP administration. DCFH diacetate is a stable nonfluorescent compound that can diffuse into cells, is hydrolyzed to DCFH, and is thereby trapped within the cells [34]. DCFH is oxidized by ROS to yield dichlorofluorescein (DCF), a fluorescent molecule [34].
Ten minutes after SP (10 µg) i.a administration, the bladder was filled transurethrally with 5 µM DCFH diacetate (Sigma, St. Louis, MO) and 1 µM propidium iodide (Sigma) in a volume of 0.5 ml saline for additional 30 min and then washed out by saline. The bladder was removed, cut and embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Inc. Torrance, CA). Next, frozen sections (10 µm in thickness) were obtained and presence of cellular fluorescence intensity in tissue sections was examined by Leica fluorescence microscopy (DMRD, Leica Microsystems Wetzlar GmbH, Wetzlar, Germany).
In addition, a portion of the urinary bladder was cut and fixed in 10% neutral buffered formalin solution, dehydrated in graded ethanol, and embedded in paraffin. Five-µm sections of bladders were stained with hematoxylin & eosin (H&E) and Giemsa, and were evaluated for the extent of inflammatory cell accumulation, and number of mast cell present [36].
We have detected formation of ROS in bladder in SP-treated rats. Next, we performed studies to determine the role of ROS on the bladder hyperactivity. Before SP stimulation, the baseline parameters of ABP, IVP, and ROS amounts were recorded for 30 min as a control value. After 10 min of SP stimulation, the rats (n=18 in total) were grouped and treated with saline (n=2), CP96,345 (500 µg, n=4), SR48968 (500 µg, n=2), SOD (500 U per rat, n=4), FC4S (250 µg, n=3), or C3 (250 µg, n=3). The three anti-oxidants (SOD,
fullerene-FC4S, or fullerene-C3) were used to test whether the SP-induced bladder
hyperactivity can be ameliorated by free-radical scavengers. The ABP and cystometrogram were monitored simultaneously for 60 min. After measurement, the bladder tissues and leukocytes isolated from 4 ml of whole blood were collected and frozen in −70°C for immunoblotting analysis.
Immunoblot analysis for ICAM, and β–actin
The immunoblotting method was described previously [25]. We measured the amounts of ICAM, and β–actin in bladder tissues and leukocytes of SP-treated rats. For protein analysis, bladder samples and leukocytes were homogenized with a prechilled mortar and pestle in extraction buffer, which consisted of 10 mM Tris-HCl (pH 7.6), 140 mM NaCl, 1 mM PMSF, 1% NP-40, 0.5% deoxycholate, 2% β-mercaptoethanol, 10 µg/ml pepstatin A, and 10 µg/ml aprotinin. The mixtures were homogenized completely by vortexing and kept at 4°C for 30 min. The homogenate was centrifuged at 12,000 ×g for 12 min at 4°C, the supernatant was collected, and protein concentration was determined by BioRad Protein Assay (BioRad Laboratories, Hercules, CA, USA). Antibodies raised against intercellular adhesion molecule (ICAM, Cat.# AF583, R&D systems, Inc., Minneapolis, MN, USA), and β–actin (Cat.# A5316, Clone AC-74, Sigma, Saint Louis, MI, USA) were used. Both of these antibodies cross-react with respective rat antigens.
SDS-PAGE was performed on 12.5% separation gels in the absence of urea, and was stained with Coomassie brilliant blue. Proteins on the SDS-PAGE gels, each lane containing 30 µg of total protein, were transferred to nitrocellulose filters. The immunoreactive bands were detected by incubation with the antibody described above, followed by secondary antibody-alkaline phosphatase, and finally with NBT and 5-bromo-4chloro-3-indolyl phosphate, toluidine salt (Roche Diagnostic GmbH, Mannheim, Germany) stock solution for 30 min at room temperature.
Data acquisition and statistical analysis
All nervous activity (PANA and PENA) was expressed as number of spikes per sec. We analyzed the PANA and the frequency of bursting PENA before and after drug treatment. All data are presented as mean ± standard error of the mean (SEM). Data were subjected to analysis of variance, followed by Duncan‘s multiple-range test for assessment of the differences among groups. Student’s paired t-test was used for detecting differences between control and drug treatment. P<0.05 was considered to indicate statistical significance.
Results
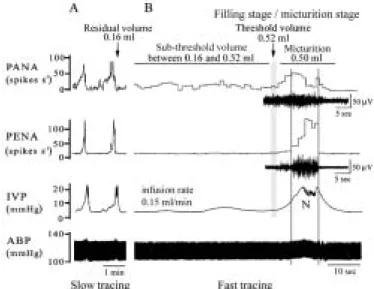
The PANA and PENA, IVP, and ABP in rats with normal micturition reflex were recorded simultaneously. The IVP was gradually increased upon accumulation of saline in the urinary bladder, causing activation of PANA. The frequency of PANA over time was progressively increased by the increase of bladder filling volume. When a threshold volume
(about 0.42-0.62 ml, mean 0.5 ml) was reached to evoke a micturition reflex, the PANA quickly reached its peak (i.e., enhanced PANA), and then a bursting efferent discharge (PENA) lasted for 7-15 sec was detected (Fig. 1). The bursting PENA can simultaneously trigger an active neural reflex-mediated bladder contraction, as shown by a quick rise in IVP. The ABP was slightly and transiently elevated (for about 15 to 35 sec) in accordance with the abrupt rise in IVP and the neurogenic (N) bladder contraction (Fig. 1).
Endogenous SP via NK1 receptor participates a normal micturition reflex
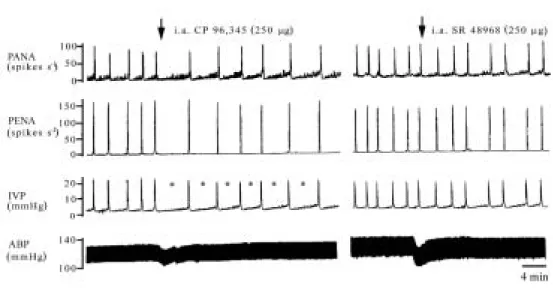
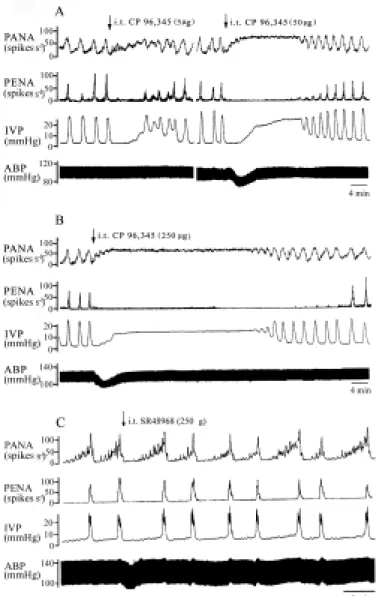
As shown in Fig. 2 (left panel), i.a administration of CP96,345 (250 µg) significantly decreased the frequency of PANA over time, the frequency of bursting PENA, and active contractions (P<0.05), and increased bladder capacity (Table 2, P<0.05). This result suggested that i.a CP96,345 exerts a partial inhibition on afferent neurotransmission of micturition reflex to increase the bladder capacity. A more significant effect of CP96,345 was detected when it was given by the i.t route. The mean frequency of PANA over time increases after the i.t CP96,345 administration, while the peak frequency of PENA dropped to zero, implicating that lumbosacral spinal NK1 receptor blockade completely inhibited the
afferent and efferent neural transmission of micturition reflex. All of the inhibition in micturition parameters is displayed as a function of dose (Fig. 3 and Table 2). The abrupt and dose-dependent hypotensive response to i.t administration of the NK1 antagonist,
indicating CP96,345 leakage into the systemic circulation. By contrast, i.a or i.t administration of SR48968 had no influence on the micturition reflex (Table 2), suggesting that SP via mainly the NK1 receptor, not the NK2 receptor, mediates the afferent micturition
pathway in the lumbosacral spinal cord (Fig. 2, right panel, and Fig. 3C). Pelvic nerve stimulation enhances bladder hyperactivity and SP release
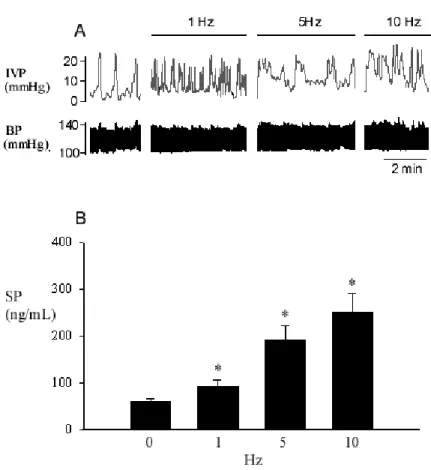
In rats, an increase in frequency (1-10 Hz) of pelvic nerve stimulation significantly enhanced the duration of bladder contraction, decreased intercontraction intervals (1.6±0.4 min, 0.6±0.2 min, 0.4±0.1 min, and 0.3±0.1 min at frequency of 0 Hz, 1 Hz, 5 Hz, 10 Hz, respectively), and, at the same time, increased the release of SP to the bladder outflow (59±8 ng/mL, 94±14 ng/mL, 190±30 ng/mL, and 254±36 ng/mL at frequency of 0 Hz, 1 Hz, 5 Hz, 10 Hz, respectively) (Fig. 4). The data clearly showed that bladder hyperactivity caused by pelvic nerve stimulation is associated with an increased SP level in a dose-dependent manner in bladder. Furthermore, we suggest that in addition to SP, NKA and other substances are released as well from sensory pelvic afferents during electric stimulation and consequently lead to a prolonged bladder contraction.
Exogenous SP facilitates micturition reflex in bladder hyperreflexia
As shown in Fig. 5A, at residual volume status immediately after micturition, no significant PANA and PENA were observed, and the IVP remained at the basal level when saline infusion was stopped. Approximately 5-10 sec after i.a administration of SP (0.1-5 µg), the bladder revealed an elevated IVP, an enhanced PANA and PENA in a dose-dependent manner (Fig. 5B, fast tracing).
At the filling state, the bladder revealed increased PANA by mechanical distension of bladder afferents. Shortly after i.a administration of SP (10 µg), a bursting PENA followed by a prolonged tonic bladder contraction (Fig. 5C) lasted for approximately 10 min. Ten min after SP stimulation, the bladder remained hyperactive. This was shown by an increased frequency of active contractions (number of active contractions, n.c.) (P
<0.05), and decreases in bladder capacity (P<0.05, Table 2). Our results further indicated that i.a administration of SP can initiate the micturition reflex response in bladders containing a subthreshold amount (residual volume) of fluid, e.g. by activating NK receptors in both smooth muscle and sensory neurons and increasing the afferent discharge (PANA) to the central nervous system to result in a hyperactive bladder. A similar result was reported previously [35].
Similarly, i.t SP administration also induced bladder hyperactivity (Fig. 6). This was evidenced by an increased number of active contractions, decreased bladder capacity, and an elevation of baseline pressure (Table 2). The increased baseline pressure may be due to an increased release of tachykinins and other substances by continuing input of motor impulses to the smooth muscle through the pelvic parasympathetic nerves, which is brought about by micturition reflex afferents. These data suggest that increased levels of SP in the rat lumbosacral spinal cord also play a role in inducing bladder hypersensitivity via an afferent pathway.
Increased ROS formation from whole blood and bladder surface following exogenous SP administration
As shown in Fig. 7A, the SP-treated whole blood samples showed an increase in ROS formation in a dose-dependent manner (78±20, 336±67, 560±105, 1,035±213, 1,201±196, and 1,108±230 counts/10 sec at doses of 0, 0.1, 1, 10, 50, and 100 µg of SP, respectively). The source of whole blood ROS came from leukocytes, not erythrocytes and plasma (Fig. 7C). Fig. 7B shows that co-incubation with the NK1-receptor antagonist
CP96,345 (50 µg) prevented SP-induced ROS generation from whole blood, which was reduced from 1,065±210 counts/10 sec to 97±18 counts/10 sec in response to treatment with 50 µg SP. By contrast, SR48968 had no significant inhibitory effect on SP-induced ROS generation (1,065±210 counts/10 sec vs. 899±177 counts/10 sec, P>0.05). Co-incubation with free-radical scavengers also significantly reduced the SP-induced ROS generation (SOD, 220±51 counts/10 sec; C3, 430±89 counts/10 sec; FC4S, 190±56
counts/10 sec; P<0.05). The magnitude of the reduction exhibited by free-radical scavengers was slightly lower than that exhibited by CP96,345, suggesting that direct blockade of upstream NK1 receptor activation by a long-lasting effect of non-peptide
CP,96,345 is more efficient than scavenging downstream ROS activity by free-radical scavengers.
Blood samples for ROS generation from rats subjected to i.a. SP treatment were examined. As shown in Fig. 7D, the value of ROS in whole blood increased significantly from 101±18 counts/10 sec of control to 2,198±365 counts/10 sec after SP stimulation. This increase was reduced somewhat by simultaneous treatment with CP96,345 (756±134 counts/10 sec), SOD (897±168 counts/10 sec), C3 (1,312±260 counts/10 sec), or FC4S
(1,032±185 counts/10 sec), but not by SR48968 (2250±368 counts/10 sec). Inhibition of bladder hyperactivity by free-radical scavengers
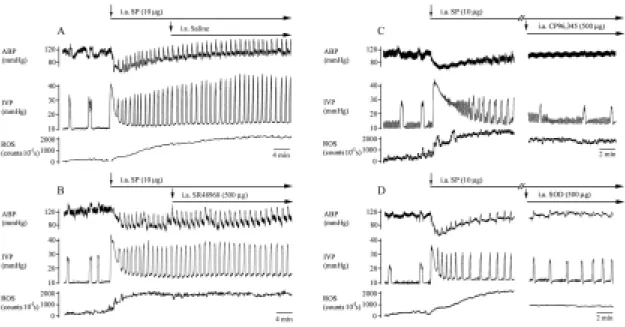
During isovolumetric condition without SP stimulation, the frequency of bladder contractions was 3.4±0.5 n.c./10 min, and the basal level of bladder ROS was maintained around 140±25 counts/10 sec. I.a SP stimulation significantly reduced the ABP (from 124±5 to 78±6 mmHg, P < 0.05), increased the frequency of active contractions (7.3±1.7 n.c./10 min, P < 0.05) and the bladder ROS generation (Fig. 8A, from 140±25 before SP
stimulation to 2,560±345 counts/10 sec after 10 min of SP, P < 0.05). 10 min after SP stimulation, i.a saline had no effect on SP induced response. The response of hyperactive bladder and increased ROS generation could be maintained > 30 min.
Pretreatment of NK receptor antagonist is well known to reduce tachykinins induced hyperactivity [2,6,9,21], therefore, for evaluation of possibly pharmacotherapeutic potential of agents on SP induced hyperactivity, we administered the test agents 10 min after SP stimulation. Ten min after SP stimulation, i.a administration of CP96,345 significantly decreased the frequency of bladder contractions (to 3.4±0.8 n.c./10 min, P < 0.05) and reduced, in part, ROS generation from the bladder surface (right panel of Fig. 8C, from 2,705±453 counts/10 sec to 1,266±320 counts/10 sec, P < 0.05). The i.a application of SOD (right panel of Fig. 8D), C3, and FC4S also significantly decreased the frequency of
bladder contractions (to 4.5±1.1 n.c./10 min by SOD, 4.9±1.2 n.c./10 min by C3, and
4.2±1.0 n.c./10 min by FC4S) and ROS generation (to 1,556±345 counts/10 sec by SOD,
1,956±455 counts/10 sec by C3, and 1,705±345 counts/10 sec by FC4S). However,
SR48968 had no effects on SP induced hyperactivity and ROS production (Fig. 8B). These data showed that ROS plays, in part, a role in SP-induced bladder hyperactivity.
Cellular origin of ROS in SP-induced bladder inflammation
Sections from the bladder mucosa and smooth muscle taking 40 to 45 min after SP administration revealed infiltration of leukocytes and presence of several degranulated mast cells (Fig. 9). Sections of urinary bladders from control animals did not exhibit any signs of inflammation at the end of the experiments.
We used DCFH diacetate and PI under a fluorescence microscope for localization of ROS production and cell viability in bladder tissue, respectively. We found that green fluorescence of DCF was present mainly in neutrophils in and around vessels (Fig. 9F) and in the submucosal layer (Fig. 9G,H) of the bladder. In a similar location of the green fluorescence of DCF, there was red fluorescence of PI (Fig. 9F-H), indicating the presence of ROS-induced cellular damage. Neither green DCF fluorescence nor red PI fluorescence was observed in the control bladders (Fig. 9E).
NK receptor antagonist or SOD on ICAM expression in SP-treated bladder and leukocytes
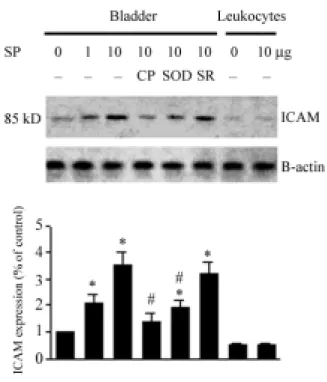
The expression of ICAM, and β-actin in the bladder and leukocytes after SP treatment was assessed by immunoblotting with antibodies against ICAM, and β-actin (Fig. 10). Expression of ICAM was detected in control bladder and leukocytes. The expression of ICAM in bladder tissues was dose-dependently increased by SP stimulation (control, 1.0±0 fold; 1 µg SP, 2.0±0.3 fold; 10 µg SP, 3.5±0.5 fold). However, the enhanced ICAM expression was significantly inhibited by CP96,345 (from 3.5±0.5 fold to 1.3±0.3 fold) or SOD (from 3.5±0.5 fold to 1.9±0.3 fold). SR48968 had no effect on enhanced ICAM expression (3.5±0.5 fold vs. 3.1±0.6 fold). Expression of ICAM in leukocytes was unaffected by SP stimulation.
Discussion
The current study demonstrates for the first time the involvement of ROS generation in SP-mediated bladder hyperreflexia. SP is a sensory neuropeptide present both in small myelinated A-delta fibers and in small-diameter unmyelinated C fibers [9]. Once released from bladder afferent nerves and the sacral spinal cord, SP is involved in the
mechanoreceptor-mediated micturition reflex. In rats, systemic administration of capsaicin for depletion of SP had resulted in urine retention or an increased volume/pressure threshold for micturition, implicating an excitatory role of SP in the afferent micturition pathway [9,36]. We demonstrated that i.a administration of exogenous SP could initiate the micturition reflex response in bladders containing a subthreshold amount (residual volume) of fluid. The SP-mediated micturition reflex or bladder hyperactivity is mainly through NK1 receptors. We further showed that exogenous or excessive SP stimulation also resulted
in increased ROS generation. Application of NK1 receptor blockade or free-radical
scavengers could inhibit micturition pathway facilitation and SP-mediated ROS generation and, subsequently, ameliorate SP-induced bladder hyperactivity.
In our study, the use of the cystometrogram and electrophysiological recording techniques in urethane-anesthetized rats provides valuable tools for analyzing the neural control of the urinary bladder [13,32,36]. Efficient bladder voiding is triggered primarily by the cumulative afferent activities from the bladder mechanoreceptors during bladder distension, which subsequently elicits parasympathetic bladder efferent excitation to evoke a micturition reflex when a threshold volume/pressure is reached [12,13]. A bladder hyperreflexia or hyperactivity indicate a state when micturition reflex response is initiated even when bladders containing a subthreshold amount (residual volume) of fluid. A normal bladder contraction is coordinated by efferent purinergic, cholinergic, muscarinic, and nicotinic (somatic) elements [13]. In addition, several neuropeptides (e.g., SP and NKA) have been demonstrated in the rat urinary bladder, yet their roles in normal micturition and bladder hyperactivity have to be further elucidated [2].
Stimulation by exogenous SP or NK receptor agonists has been shown to induce bladder hyperactivity [2,9,21]. Maggi and co-workers [9] demonstrated facilitation of reflex micturition by intravesical administration of [β-Ala8
] neurokinin A (NK2 receptor agonist),
suggesting a peripheral site of action via smooth-muscle contraction in the bladder. SP can also be a centrally excitatory neurotransmitter producing a slow excitatory postsynaptic potential in the neurons of the spinal cord, and mediating a dorsal root C-fiber reflex to the dorsal-horn neurons, leading to bladder hyperreflexia (Fig. 3 and Table 2). Thus, SP exerts effects on both the peripheral bladder and the central spinal cord to facilitate the micturition pathway and consequently lead to bladder hyperactivity. The SP-mediated hyperactivity can be inhibited by i.a or i.t administration of CP96,345 (NK1 receptor antagonist), but not
of SR48968 (NK2 receptor antagonist).
It is also known that a peripheral release of tachykinins determines a set of responses (loosely defined as neurogenic inflammation) that includes vasodilatation, plasma protein extravasation, smooth muscle contraction, stimulation of afferents, and inflammation [6]. To further complicate matters, both sensory neurons and immune cells can express and release tachykinins, which may also contribute to neurogenic inflammation in the bladder. SP (0.3-1 µM) is able to induce, in a dose-dependent manner, secretion of various cytokines (e.g., IL-1, IL-6, TNF-α) from cultured lymphocyte-enriched mononuclear cells isolated from human peripheral blood [37]. In addition, SP has been shown to cause a pro-inflammatory change in tissues, such as degranulation of mast cells and leukocyte adhesion to the venular endothelium [18] by a mechanism of SP enhanced ICAM expression in the bladder tissue (Fig. 10). The possible involvement of SP and its
NK1 receptor in pathophysiologic changes of bladder inflammation has been underscored
by a recent study showing that mice deficient in NK1 receptor have dramatically reduced
antigen-induced cystitis [38].
As a consequence of SP-immune and -inflammatory cell interaction, a variety of substances, such as histamine, cytokines and reactive oxygen species (ROS) are released [8,18,20,37]. In whole blood and leukocytes incubated with SP, ROS activity was displayed in a dose-dependent manner and SP-induced ROS release and ICAM expression are inhibited by CP96,345, not SR48968, confirming a mediating role of the NK1 receptor in
triggering ROS generation in leukocytes. We further showed that SP-induced ROS generation in bladder as well as bladder hyperreflexia can be partly ameliorated by CP96,345 or, noticeably, by free-radical scavengers. It is not totally unexpected that SP-induced ROS formation may contribute to the myogenic and neural hyperactivity. ROS are known to be involved in changes in muscle tone, vascular smooth muscle strip contraction [39,40], and increased neural activity/conduction velocity in vitro by mechanisms such as alterations in membrane conductance, calcium homeostasis, calcium-dependent processes, as well as eicosanoid and nitric oxide metabolism [40-42].
In summary, our studies provided direct evidence that SP participates the micturition reflex response in bladders, by activating NK receptors to facilitate the afferent pathway. Increased SP stimulation may enhance the afferent discharge (PANA) to the central nervous system to result in a hyperactive bladder. On the other hand, increased nerve activity by other means may result in increased release of SP, further complicate bladder hyperactivity. Our study indicates that the mechanism by which SP participates in the neurogenic bladder may be complicated by its proinflammatory activity and its ability to stimulate ROS generation.
References
1.Maggio, J.E. Tachykinins. Annu. Rev. Neurosci. 11:13-21, 1988.
2. Maggi, C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 26:911-944, 1995. 3. Sharkey, K. A., R. G. Williams, M. Schultzberg, and G. J. Dockray. Sensory SP innervation of the urinary bladder: possible site of action on capsaicin in causing urine retention. Neurosciences 10:861-868, 1983.
4. Chahl, L.A. Antidromic vasodilation and neurogenic inflammation. Pharmacol. Ther. 37:275-300, 1988.
5. Lu, B., M. Figini, C. Emanueli, P. Geppetti, E. F. Grady, N. P. Gerald, J. Ansell, D. G. Payan, C. Gerard, and N. Bunnett. The control of microvascular permeability and blood pressure by endopeptidase. Nature Med. 3:904-907, 1997.
6. Lecci, A., S. Giuliani, M. Tramontana, F. Carini, and C.A. Maggi. Peripheral actions of tachykinins. Neuropeptides 34:303-13, 2000.
7. Abelli, L., V. Somma, C.A. Maggi, D. Regoli, M. Astollfi, M. Parlani, P. Rovero, B. Conte, and A. Meli. Effects of tachykinins and selective tachykinin receptor agonists on vascular permeability in the rat lower urinary tracts: evidence for the involvement of NK1 receptors. J Auton Pharmacol. 9: 253-260, 1989.
8. Saban, M.R., R. Saban, D. E. Bjorling, and M. Haak-Frendscho. The involvement of leukotrienes, TNFα, and the LFA1/ICAM-1 interaction in substance P-induced granulocyte infiltration. J. Leukocyte. Biol. 61: 445-451, 1997.
primary sensory neurons in the urinary bladder and urethra. In Nervous Control of the Urogenital System. 1993, Ed. Maggi, C.A. pp. 383-422. Chur, Switzerland: Harwood Publisher.
10. Su, H. C., J. Wharton, J. M. Polak, P. K. Mulderry, M. A. Ghatel, G. Gibson, G. Terenghi, J. F. B. Morrison, J. Ballesta, and S. R. Bloom. Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunochemistry. Neuroscience 18:727-747, 1986.
11. De Groat, W. C. Autonomic Failure. In: A Textbook of Clinical Disorders of the Autonomic Nervous System, Fourth Edition, edited By C.J. Mathias & R. Bannister Oxford University Press, 1999, p. 151-165.
12. Chien, C. T., H. J. Yu, Y. J. Cheng, M. S. Wu, C. F. Chen, and S. M. Hsu. Reduction in renal haemodynamics by exaggerated vesicovascular reflex in rats with acute urinary retention. J. Physiol. 526:397-408, 2000.
13. Chien, C. T., H. J. Yu, T. B. Lin, and C. F. Chen. Neural mechanisms of impaired micturition reflex in rats with acute partial bladder outlet obstruction. Neuroscience 96: 221-230, 2000.
14. Hanno, P. M., J. R. Landis, Y. Matthews-Cook, J. Kusek, and L. J. R. Nyberg. The diagnosis of interstitial cystitis revised: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J. Urol. 161:553-557, 1999.
15. Hohenfellner, M., L. Nunes, R. A. Schmidt, A. Lampela, J. W. Yhuroff, and E. A. Tanagho. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J. Urol. 147:587-591, 1992.
16. Pang, X., J. Marchand, G. R. Sant, and T. C. Theoharides. Increased number of SP-positive fibres in interstitial cystitis. Br. J. Urol. 75:744-750, 1995.
17. Marchand, J. E., G. R. Sant, and R. M. Kream. Increased expression of substance-P receptor-encoding mRNA in bladder biopsies from patients with interstitial cystitis. Br. J.
Urol. 1998, 81:224-228.
18. Suzuke, H., S. Miura, Y. Y. Liu, M. Tsuchiya, and H. Ishii. Substance P induces degranulation of mast cells and leukocyte adhesion to venular endothelium. Peptides 16:1447-1452, 1995.
19. Tanabe, T., H. Otani, K. Mishima, R. Ogawa, and C. Inagaki. Mechanisms of oxyradical production in substance P stimulated rheumatoid synovial cells. Rheumatol.
Int.16:159-167, 1996.
20. Brooks, A. C., Whelan, C. J., and W. M. Purcell. Reactive oxygen species generation and histamine release by activated mast cells: modulation by nitric oxide synthase inhibition. Br. J. Pharmacol. 128:585-90, 1999.
21. Lecci, A., S. Giuliani, C. Garret, and C. A. Maggi. Evidence for a role of tachykinins as sensory transmitters in the activation of micturition reflex. Neuroscience 54:827-837, 1993.
22. Montier, F., A. Carruette, S. Moussaoui, D. Boccio, and C. Garret. Antagonism of substance P and related peptides by RP 67580 and CP-96,345, at tachykinin NK1 receptor sites, in the rat urinary bladder. Eur. J. Pharmacol. 251:9-14, 1994.
23. Schmidt, A. W., S. Mclean, and J. Heym. The substance P receptor antagonist CP-96,345 interacts with Ca2+ channels. Eur. J. Pharmacol. 219:491-492, 1992.
antagonist of the neurokinin A (NK2) receptor. Life Sci 50:PL101-106, 1992.
25. Chien, C. T., P. H. Lee, C. F. Chen, M. C. Ma, M. K. Lai, and S. M. Hsu. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J. Am. Soc. Nephrol. 12:973-982, 2001.
26. Dugan, L. L., D. M. Turetsky, C. Du, D. Lobner, M. Wheeler, C. R. Almli, C. K. Shen, T. Y. Luh, D. W. Choi, and T. S. Lin. Carboxyfullerenes as neuroprotective agents.
Proc. Natl. Acad. Sci. USA 1997, 94:9434-9439.
27. Yu, C., J. B. Bhonsle, T. Canteenwala, J. P. Huang, J. Shiea, B. J. Chen and L. Y. Chiang. Novel water-soluble hexa(sulfobutyl)fullerenes as potent free radical scavengers.
Chem. Lett. 465-466, 1998.
28. Skatchkov, M. P., D. Sperling, U. Hink, A. Mulsch, D. G. Harrison, I. Sindermann, T. Meinertz, and T. Munzel. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production.
Biochem. Biophys. Res. Commun. 254:319-324, 1999.
29. Nishida, A., H. Kimura, M. Nakano, and T. Goto. A sensitive and specific chemiluminescence method for estimating the ability of human granulocytes and monocytes to generate O2−. Clin. Chim. Acta 179:177-182, 1989.
30. Kawatani, M., G. Matsumoto, L.A. Birder, H. Fukuoka, M. Yoshiyama, A. Misaki, and S. Suzuki. Intrathecal administration of NK1 receptor antagonist, CP96345, inhibits the micturition reflex in the rat. Regul. Pept. 46:392-395, 1993.
31. Maggi, C.A., A. Lecci, C. Garret, and S. Giuliani. Spinal effects of selective NK-1 and NK-2 receptor antagonists on bladder motility in anesthetized rats. Regul. Pept. 46:389-391, 1993.
32. Steers, W. B., and W. C. De Groat. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J. Urol. 140:864-871, 1988.
33. Chen, L. W., C. F. Chen, and Y. L. Lai. Chronic activation of neurokinin-1 receptor induces pulmonary hypertension in rats. Am. J. Physiol. 276:H1543-H1551, 1999.
34. Suematsu, M., H. Suzuki, H. Ishii, S. Kato, T. Yanagisawa, H. Asako, M. Suzuki, and M. Tsuchiya. Early midzonal oxidative stress preceding cell death in hypoperfused rat liver. Gastroenterology 103:994-1001, 1992.
35. Maggi, C.A., S. Giuliani, Santicioli, P., Abelli, L., Regoli, D., and A. Meli. Further studies on the mechanisms of the tachykinin-induced activation of micturition reflex in rats: evidence for the involvement of the capsaicin-sensitive bladder mechanoreceptors. Eur. J.
Pharmacol. 136:189-205, 1987.
36. Cheng, C. L., Ma, C. P., and W. C. Effects of capsaicin on micturition and associated reflexes in rats. Am. J. Physiol. 265:R132-R138, 1993.
37. Cuesta, M.C., L. Quintero, H. Pons, and H. Suarez-Roca. Substance P and calcitonin gene-related peptide increase IL-1 beta, IL-6 and TNF alpha secretion from human peripheral blood mononuclear cells. Neurochem. Int. 40:301-306, 2002.
38. Saban, R, M. R. Saban, N. B. Nguyen, B. Lu, C. Gerard, N. P. Gerard, and T. G. Hammond. Neurokinin-1 (NK-1) receptor is required in antigen-induced cystitis. Am. J.
Pathol. 156:755-780, 2000.
39. Rodriguez-Martinez, M. A., E. C. Garcia-Cohen, A. B. Baena, R. Gonzalez, M. Salaices, and J. Marin. Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. Br. J.
Pharmacol. 125:1329-1335, 1998.
40. Bauer, V., R. Sotnikova, J. Machova, S. Matyas, V. Pucovsky, and M. Stefek. Reactive oxygen species induced smooth muscle responses in the intestine, vessels and airways and the effect of antioxidants. Life Sci. 65:1909-1917, 1999.
41. Zhong, Z., H. D. Connor, M. Yin, N. Moss, R. P. Mason, H. Bunzendahl, D. T. Forman, and R. G. Thurman. Dietary glycine and renal denervation prevents cyclosporin A-induced hydroxyl radical production in rat kidney. Mol. Pharmacol. 56:455-463, 1999. 42. Horakova, L., and S. Stolc. Antioxidant and pharmacodynamic effects of pyridoindole stobadine. Gen. Pharmacol. 30:627-638, 1998.
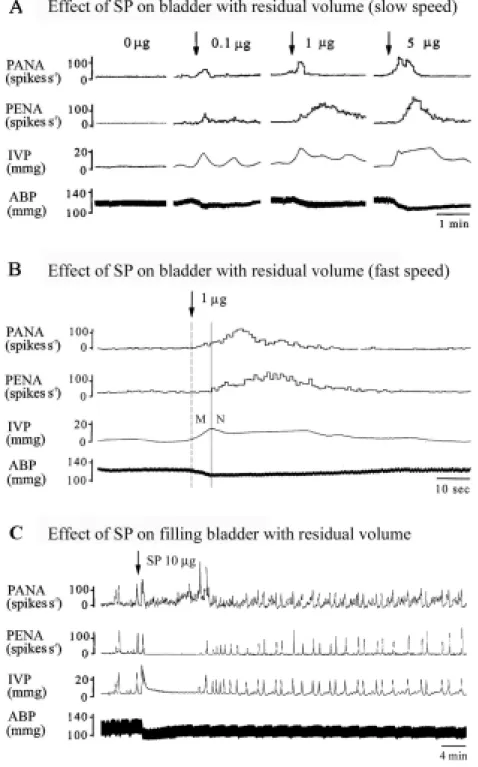
Fig. 1 Typical demonstration of saline infusion (0.15 ml/min) into the urinary bladder evoking normal micturition. A: Simultaneous recordings of pelvic afferent nervous activity (PANA) and pelvic efferent nervous activity (PENA), intravesical pressure (IVP), and arterial blood pressure (ABP) at a slow chart speed. B: At a fast chart speed, accumulated urine in the urinary bladder gradually enhances mechanoreceptor-dependent PANA. Up to a threshold volume, the enhanced PANA evokes a bursting type of PENA and triggers a series of fluctuating bladder contractions to expel the urine. A mild increase in ABP defined as a vesicovascular reflex is associated with a micturition reflex. The conditions of measured residual volume, sub-threshold volume, threshold volume, and micturition volume are indicated. Studies were carried out by use of a transcytometric bladder model.
Fig. 2 Effects of i.a CP96,345 and SR48968 on the micturition parameters on
ABP, IVP, PANA, and PENA. The bladder was continuously filled with normal saline at 0.1 ml/min. The frequency of PANA over time was progressively increased by the increase of bladder filling volume. When a threshold volume was reached, the frequency of PANA over time reached to its maximal value, and then a peak frequency of PENA and sharp rise in IVP was detected. Note that CP96,345 increased the intercontraction interval (i.e., decreased the frequency of active contractions) and decreased the peak frequency of PENA (left panel). By contrast, i.a administration of SR48968 (250 µg) did not influence any parameters of the micturition reflex (right panel). The asterisks (*) indicate i.a CP96,345 significantly elongated the value of intercontraction interval as compared to the control value.
Fig. 3 Inhibitory effects of i.t low (A, left panel), median (A, right panel), or
high dose of CP96,345 (B) on the micturition parameters. Bladder contraction was induced by continuous infusion of saline into the bladder. I.t CP96,345 markedly depressed the peak frequency of PENA-mediated micturition, although the increased frequency of PANA over time by overdistended bladder was noted. Because of inhibition of peak frequency of bursting PENA-mediated micturition and of accumulated urine volume, a prolonged and enhanced frequency of PANA over time occurred in the overdistended bladder (A, right and B). The inhibitory effect of CP96,345 on the micturition reflex was displayed in a dose-dependent manner. SR48968 i.t. (C panel) had no effects on the micturition parameters.
Fig. 4 Effect of pelvic nerve stimulation on bladder activity and plasma SP
concentration from the bladder outflow. Graded increases in frequency of pelvic nerve stimulation increased the duration of bladder contractions and decreased the intercontraction intervals (A). The increased frequency of electric stimulation also enhanced the SP release into the iliac vein (B). * P < 0.05 when compared to the control level.
Fig. 5 Excitatory effects of i.a. SP on the micturition parameters on arterial
blood pressure (ABP), intravesical pressure (IVP), pelvic afferent nervous activity (PANA), and pelvic efferent nervous activity (PENA) at residual volume. At the stage of residual volume without saline infusion, SP increases the response in PANA, PENA, and IVP and lowers ABP in a dose-dependent manner (A). SP triggers both myogenic (M) bladder contraction and PANA and PENA mediated neural (N) bladder contraction (B). At residual volume with continuous saline infusion, SP produces initially a tonic contraction followed, during its relaxation phase, by a series of
rhythmic contractions (micturition reflex), which persisted even when the tonic contraction had returned to resting values (C). Note that an increased frequency of micturition (or shortened intercontraction interval) after SP stimulation is observed.
Fig. 6 Effects of i.t saline and SP on the micturition parameters on ABP, IVP,
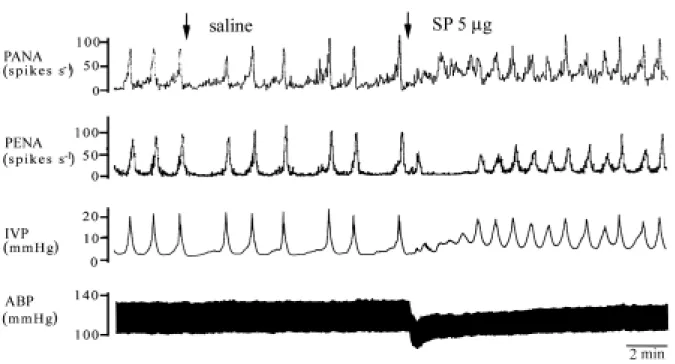
PANA, and PENA. Bladder contraction was induced by continuous infusion of saline into the bladder. I.t saline has no effects on each micturition parameter. Note that i.t SP evoked a systemic vasodilation, and a hyperactive bladder, including a high frequency of IVP, PANA, and PENA and an elevation of baseline pressure.
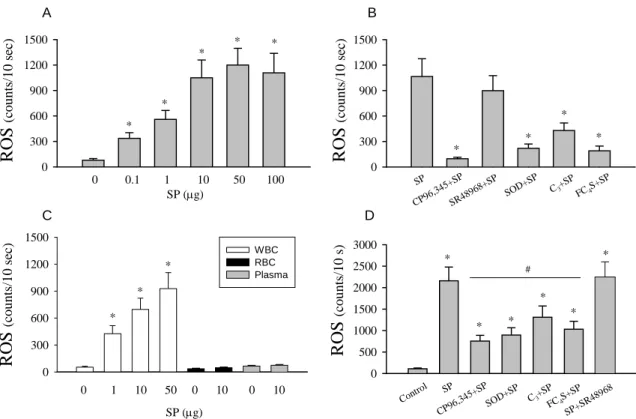
SP (µg) ROS (c ou nts/1 0 sec) 0 300 600 900 1200 1500 0 0.1 1 10 50 100 * * * * * ROS (c ou nts/1 0 sec) 0 300 600 900 1200 1500 * * * * SP CP96 ,345+ SP SR48 968+ SP SOD+ SP C3+S P FC4S+ SP ROS (c ou nts/10 s) 0 500 1000 1500 2000 2500 3000 SP CP96 ,345+ SP SOD+ SP C3+S P FC4S+ SP Contr ol * * * * * # * SP+S R489 68 RO S (c ou nts/1 0 sec) 0 300 600 900 1200 1500 WBC RBC Plasma * * * 0 1 10 50 0 10 0 10 SP (µg) A B C D
Fig. 7 SP-induced ROS generation in whole blood. SP (from 0 to 100 µg)
induced whole-blood ROS formation in a dose-dependent manner (A). The maximal value of SP-induced ROS was attained at 50 µg. The primary ROS sources come from leukocytes (WBC), not from erythrocytes (RBC) or plasma (B). The effect of SP (50 µg) can be prevented by co-incubation with by CP96,345, SOD, C3, and FC4S, but not
by SR48968 (C). The whole-blood ROS obtained from rats with i.a SP treatment (10 µg/per rat in a volume of 0.2 ml saline) was also increased significantly (D). These enhanced ROS can be reduced somewhat by simultaneous treatment with CP96,345, SOD, C3, or FC4S injection (D), but not by SR48968. * P < 0.05 when compared to
respective control value, # P<0.05 when compared to the SP-induced ROS value without drug treatment.
Fig. 8 In vivo response of SP-induced bladder hyperactivity and bladder ROS
generation in an isovolumetric model. I.a SP injection induced significant vasodilation and bladder hyperactivity, as well as bladder ROS generation (A). Saline or SR48968 had no effect on SP-induced bladder hyperactivity and ROS production (A&B). Treatment with CP96,345 reduced the frequency of micturition and decreased, in part, ROS generation in the bladder (right panel of C). SOD injection decreased ROS generation in the bladder and also reduced the frequency of micturition (right panel of D).
Fig. 9 Mast cells and leukocytes in the control (A, 400×) and SP-induced
inflamed urinary bladder (B, 400×). Mast cells and leukocytes primarily occurred in the vicinity of the blood vessels in the SP-treated bladder, but not in the control
bladder. Note that some leukocytes adhered to the endothelium of one vessel, where mast cell degranulation was found (C and D). We used DCFH diacetate and PI under a fluorescence microscope for localization of ROS production and cell viability in bladder tissue. We found that green fluorescence of DCF was apparent in the vessels (F) and in the submucosal layer (G and H), where numerous cells with red fluorescence (PI staining) were found (G and H), suggesting oxidative damage. Neither green nor red fluorescence was observed in the control bladder (E).
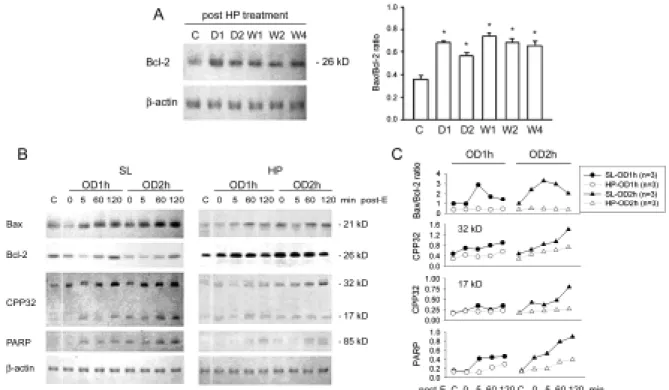
Figure 10. Apoptosis-related protein expression in OD/E bladders. Western blot
analysis with specific antibodies to Bax, Bcl-2, CPP32, PARP, and β-actin of homogenates of rat bladder subjected to HP treatment and OD/E induced injury. A: Bcl-2 was significantly upregulated in HP rats. The elevation persisted for at least 4 weeks (bar chart). B (left panel): In SL-OD bladders, the expression of Bcl-2 appeared to be decreased, but gradually returned to its pre-overdistension level 120 min post emptying. Note the increased expression of Bax, CPP32 (32 kD proenzyme and 17 kD cleaved product), and PARP in SL-OD2h rats. B (right panel): Note the markedly increased Bcl-2 in HP and HP-OD rats. The expression of Bax and, to a much lesser degree, of CPP32 and PARP was slightly increased in HP-OD rats. Equal protein loading was displayed by β-actin. C: Mean changes in apoptosis-related protein expression are displayed. Note the increased Bax/Bcl-2 ratio in SL-OD rats. The ratio remains low in HP-OD rats. * P<0.05 when compared to control value.
Fig. 11 Western blot analysis with specific antibodies to ICAM, and β-actin of
homogenates of rat bladders and leukocytes subjected to SP stimulation. Note the dose-dependently increased expression of ICAM (85 kD) after SP (1 and 10 µg) stimulation. The expression of ICAM appeared to be decreased after CP96,345 (CP, 500 µg) and superoxide dismutase (SOD, 500 µg) treatment, but not by SR48968 (SR, 500 µg). In leukocytes, SP had no effect on ICAM expression. Equal protein loading was displayed by β-actin. Lower panel is the mean data by densitometry.
Table 1. Drugs/chemicals used in this experiment
=========================================================================================================================== Drugs/Chemicals Name Source Function Reference __________________________________________________________________________________________________________________________________________ SP [Sar9, Met (O2)11]-Substance P Peninsula Lab., Belmont, CA, U.S.A. A neuropeptide released
from sensory nerves
22
CP96,345 (S)-N-methy-N-[4-(4-acetylamino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)-buyl]benzamide
Pfizer Central Research, Groton, CT, U.S.A. NK1 receptor antagonist 23
SR48968 (S)-N-methy-N-[4-(4-acetylamino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)-buyl]benzamide
Sanofi Recherche, Montpellier, France NK2 receptor antagonist 24
SOD Superoxide dismutase Sigma, St. Louis, MO, U.S.A. A free-radical scavenger 25
C3 Hexacarboxylic acid Dr. T.Y. Luh, Department of Chemistry,
National Taiwan University, Taiwan
A free-radical scavenger with C60 fullerene structure
26
FC4S Hexa(sulfobutyl)fullerenes Dr. L.Y. Chiang, Center for Condensed Matter Sciences, National Taiwan University, Taiwan
A free-radical scavenger with C60 fullerene structure
27
Lucigenin N,N'-dimethyldiacridinium Sigma, St. Louis, MO, U.S.A. A chemiluminescence probe
for superoxide anion
28
MCLA 2-Methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo-[1,2-a]-py razin-3-one-hydro-chloride
TCI-Ace, Tokyo Kasei Kogyo Co. Ltd., Tokyo, Japan
A chemiluminescence probe for superoxide anion
29
Table 2. Effects of intra-arterial or intrathecal administration of substance P, CP96,345 and SR48968 on micturition parameters in urethane
anesthetized rats.
Drugs Frequency IVP BC MV RV BP
i.a. SP 10 µg before 3.5±0.4 26.0±2.7 0.61±0.10 0.50±0.07 0.11±0.02 7.5±1.8 n=6 after 8.6±1.5* 31.1±3.2* 0.33±0.08* 0.21±0.06* 0.12±0.02 14.4±2.9* i.t SP 10 µg before 3.9±0.6 28.1±2.0 0.56±0.11 0.56±0.11 0.14±0.02 7.9±1.6 n=5 after 7.3±1.8* 29.2±3.5 0.30±0.06* 0.30±0.04* 0.10±0.02 13.6±2.7* i.a CP96,345 250 µg before 4.4±0.9 23.4±2.0 0.62±0.11 0.50±0.07 0.12±0.03 6.9±1.4 n=6 after 2.4±0.4* 24.0±2.3 0.89±0.16* 0.75±0.10* 0.14±0.02 7.1±1.4 i.t CP96,345 5 µg before 3.7±0.5 24.0±3.0 0.60±0.11 0.50±0.09 0.12±0.03 6.8±1.3 n=6 after 1.9±0.2* 25.1±2.9 DI DI DI 16.7±3.0* i.t CP96,345 50 µg before 4.0±0.6 23.9±3.0 0.62±0.10 0.48±0.09 0.13±0.03 7.2±1.4 n=6 after 0.9±0.1* 24.5±3.0 DI DI DI 17.5±3.5* i.t CP96,345 250 µg before 4.1±0.6 25.0±3.1 0.65±0.10 0.52±0.09 0.13±0.02 7.4±1.6 n=6 after 0.2±0.1* 25.4±2.9 DI DI DI 15.9±3.2* i.a SR48968 250 µg before 4.2±0.5 23.6±2.4 0.62±0.10 0.44±0.07 0.17±0.03 8.0±1.9 n=6 after 4.5±0.7 23.9±2.5 0.64±0.10 0.47±0.07 0.16±0.03 8.4±2.1 i.t SR48968 250 µg before 4.0±0.6 24.6±2.6 0.59±0.10 0.40±0.08 0.18±0.03 7.7±1.8 n=6 after 4.4±0.7 25.0±2.8 0.61±0.11 0.43±0.09 0.17±0.04 8.1±2.0 --- Frequency: number of active contractions (> 15 mmHg) during 10-min period, IVP: intravesical pressure (mmHg) during micturition, BC: bladder capacity (ml), MV: micturition volume (ml), RV: residual volume (ml), BP: baseline pressure (mmHg). Results are expressed as mean ± standard error of the mean. Comparisons are made before and after during administration: * P<0.05.