國立交通大學生物科技學系
碩士論文
黃芩素經由泛素-蛋白分解體降解路徑抑制
survivin 蛋白的表現
Inhibition of survivin protein expression by
ubiquitin-proteasome degradation pathway
following baicalein
研究生: 黃厚巽
指導教授: 趙瑞益 教授
黃芩素經由泛素-蛋白分解體降解路徑
抑制
survivin 蛋白的表現
Inhibition of survivin protein expression by
ubiquitin-proteasome degradation pathway following baicalein
研究生: 黃厚巽 Student: Hou-Syun Huang
指導教授: 趙瑞益 Advisor: Jui-I Chao
國立交通大學生物科技學系
碩士論文
A Thesis
Submitted to Department of Biological Science and Technology
College of Biological Science and Technology
National Chiao Tung University
in partial Fulfillment of the Requirements
for the Degree of
Master
In
Biological Science and Technology
July 2010
Hsinchu, Taiwan, Republic of China
致謝
時間過的真快,在趙老師實驗室經歷了兩年的學習與訓練,到如今即將畢 業了。學業上在老師的提攜以及學長姐的帶領下、同學間的教學相長,對於實 驗研究有更進一步的了解與體認,並且增進了自己面對困難、解決問題的能力。 在這段時間以來與實驗室夥伴們的學習相處下,我也學到更多待人處事的經 驗,這是教科書上學不到的東西。在趙老師的指導下,使我學到做研究應有的 邏輯觀念與學術素養,並且讓我有機會嘗試實驗室以往沒有的實驗技術和方 法。實驗室的兩大支柱,惠芳學姊以及光凱學長,在我遭遇問題時挺身而出, 指點迷津讓問題迎刃而解;並且在分生達人的啟蒙下,學習到的分生實驗與知 識上讓我獲益良多。生技公司上班的和欽學長,讓我了解在業界所需具備的能 力以及所應抱持的態度。在剛開始對實驗室不熟悉的階段,阿良跟真宜學姐從 養細胞開始總是不厭其煩地一步一步帶領我走入研究的世界。在實驗室共患 難、同進退的同學們,靜怡、繼慶跟勝壹兩年來的相處下,度過了許多美好的 歡笑時光,也遭遇了許多困難與挫折。遇到困難時大家總是互相討論、幫助我 解決問題以及面對當前的挑戰;遭遇挫折以及心情低落時,他們給我陪伴安慰 以及背後的支持;閒暇之虞,出遊散心以及互相打嘴砲也成為我美好的回憶。 實驗室的美女學妹團,婷婷、淳淳、阿簡和阿蓮,有了她們的加入使得原本枯 燥乏味的實驗生活更顯多采多姿。我們的助理白雲大哥,成為我們實驗室的得 力助手。還有昱耀、旻翰、Tammy 與玉梅有著年輕的活力,他們的認真的學習態 度也是我效訪的對象。還有張家靖老師家與曾慶平老師家無數幫助過我的學長 姐和同學們協助以及指導我解決實驗上許多的問題與困難。天天守候在外的大 溝、耳洞跟皮皮,每天忠實陪伴著我們,在心情低落時,他們就像老朋友一樣 安慰著你,陪你走到車棚為單調的生活帶來一絲樂趣。最後要歸功於我的家人, 辛苦的從小供給我唸書到如今,他們是我最大的後盾,讓我沒有後顧之憂的完 成碩士學位,並且感謝這些日子以來所有幫助過我的每一個人。Contents
Contents
...
I
中文摘要
...
VI
Abstract
...
VII
1. Introduction
...
1
1.1. Flavonoids and anticancer activities... 1
1.2. Apoptosis pathways ... 2
1.3. Baicalein and apoptosis ... 3
1.4. Baicalein and cell cycle progression... 3
1.5. Baicalein and tumorigenesis inhibition ... 4
1.6. Survivin and cancer ... 5
1.7. Survivin and cell division ... 6
1.8. Survivin and apoptosis... 7
1.9. Survivin and cancer therapy ... 7
1.10. Ubiquitin-proteasome system ... 8
1.11. Survivin and bladder cancer ... 9
1.12. Oxaliplatin and cancer therapy ... 10
2. Materials and methods
...
12
2.1. Reagents and Antibodies ... 12
2.2. Cell culture... 12
2.3. Cell viability assay... 13
2.4. Time-lapse of living cell image analysis ... 13
2.5. Annexin V/PI apoptosis assay ... 14
2.6. Western blot... 14
2.7. Reverse transcription-polymerase chain reaction (RT-PCR) ... 15
2.8. Quantitative real-time PCR... 16
2.9. Immunoprecipitation... 17
2.10. Immunofluorescence staining and confocal microscopy... 18
2.11. Statistical analysis... 19
3. Results
...
20
3.1. Baicalein induces cytotoxicity and proliferation inhibition in bladder cancer cells ... 20
3.2. Baicalein inhibits survivin protein expression but not altered gene expression in bladder cancer cells ... 20
3.3. Baicalein reduces the protein stability of survivin in bladder cancer cells.. 21
degradation in bladder cancer cells... 22
3.5. Baicalein treatment increases ubiquitination of survivin... 22
3.6. Co-treatment of baicalein and oxaliplatin enhances the cytotoxicity and survivin protein inhibition in bladder cancer cells ... 23
4. Discussion ...24
5. Conclusion ...27
List of figures
Fig. 1. Effect of baicalein on the cell viability in BFTC905 bladder cancer cells... 49
Fig. 2. Time-lapse observation of baicalein-induced cancer cell death... 50
Fig. 3. Effect of baicalein on the protein levels of survivin in bladder cancer cells... 51
Fig. 4. Effect of baicalein on the survivin mRNA level in bladder cancer cells by
RT-PCR analysis. ... 52
Fig. 5. Effect of baicalein on the survivin mRNA level in bladder cancer cells by
real-time PCR ... 53
Fig. 6. Stability of survivin protein in the baicalein-treated in bladder cancer cells .. 54
Fig. 7. Effect of MG132 (a proteasome inhibitor) on the baicalein-inhibited survivin
... 55
Fig. 8. Effect of MG132 on the apoptosis level in the baicalein-treated BFTC905 cells
... 56
Fig. 9. Ubiquitination of survivin in baicalein-treated BFTC905 cells. ... 57
Fig. 10. Effects of baicalein on the co-expression of survivin and ubiquitin proteins in
bladder cancer cells... 58
Fig. 11. Effect of co-treatment with baicalein and oxaliplatin on the cell viability in
bladder cancer cells... 59
cancer cells... 60
Fig. 13. Proposed model of survivin down-regulation by baicalein in human bladder
cancer cells... 61
Appendix 1. Function of survivin in mitosis ... 62
中文摘要
Survivin 是一種抗細胞凋亡的蛋白,具有促進癌細胞存活與增生的功能。黃 芩素會誘發癌細胞生長停止及促進細胞凋亡的作用,然而黃芩素調控survivin 蛋 白表現的機制仍然不清楚。在本研究中,我們探討BFTC905 人類膀胱癌細胞處 理黃芩素後,survivin 的角色與調控機制。處理黃芩素明顯地抑制 BFTC905 細 胞中survivin 蛋白的表現。雖然如此,利用反轉錄聚合酶鏈反應與即時聚合酶鏈 反應分析,發現黃芩素並不會影響survivin mRNA 的表現量。利用蛋白質合成抑 制劑cycloheximide 處理後,會促進黃芩素減少 survivin 蛋白的表現量。相反地, 處理一種蛋白分解體的抑制劑 MG132,能部分回復黃芩素所抑制的 survivin 蛋 白表現,並且MG132 會降低黃芩素所誘發的細胞凋亡。有趣地,利用免疫沉澱 分析發現黃芩素會誘發survivin 蛋白進行泛素化作用,泛素化的 survivin 蛋白被 黃芩素大量誘發。此外,共同處理黃芩素與oxaliplatin 會加強對 BFTC905 細胞 的毒殺作用及抑制survivin 蛋白的表現。綜合以上結果,我們推測黃芩素抑制人 類膀胱癌細胞中survivin 蛋白表現,是經由泛素-蛋白分解體的降解路徑。Abstract
Survivn is an anti-apoptosis protein that plays the roles in promoting cancer cell
survival and proliferation. Baicalein has been shown to induce growth inhibition and
apoptosis in cancer cells; however, the regulation of survivin protein expression by
baicalein remains unclear. In this study, we investigated the role and regulation of
survivin following baicalein treatment in the BFTC905 human bladder cancer cells.
Treatment with baicalein markedly inhibited survivin protein expression in BFTC905
cells. Nevertheless, the survivin mRNA level did not alter with baicalein treatment
using reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR.
Treatment with a protein synthesis inhibitor (cycloheximide) increased the decrease
of survivin protein level in the baicalein-treated cells. In contrast, a specific
proteasome inhibitor (MG132) partially restored baicalein-inhibited survivin protein
expression. Moreover, MG132 can reduce apoptosis induction by baicalein.
Interestingly, baicalein induced the protein ubiquitination of survivin using
immunoprecipitation assays. The ubiquitinated survivin proteins were increased by
baicalein. Besides, co-treatment of baicalein and oxaliplatin enhanced the cytotoxicity
and survivin protein inhibition in BFTC905 cells. As a consequence, we suggest that
1. Introduction
1.1. Flavonoids and anticancer activities
Flavonoids are a group of polyphenolic compounds that exist in plants,
vegetables, and fruits (Havsteen, 1983). Flavonoids include flavonols, flavones,
isoflavones, flavanones, anthocyanidins, and flavanols (Manach et al., 2004; Scalbert
and Williamson, 2000). Intake of flavonoids has been shown to reduce risk of cancer,
inflammation and heart diseases (Havsteen, 2002; Middleton et al., 2000). Various
types of flavonoids, such as luteolin, quercetin, kaempferol and catechin, display
anticancer effects on growth inhibition and apoptosis (Brusselmans et al., 2005; Lee
et al., 2005a; Psahoulia et al., 2007; Spencer et al., 2003; Yin et al., 2001). It has been
shown that flavonoids can induce release of cytochrome c with activation of
caspase-3 and caspase-9 to promote apoptosis (Michels et al., 2005; Wang et al.,
1999). For example, epigallocatechin gallate (EGCG), the major catechin in tea, has
been shown to induce apoptosis in various cancers, including leukemia (Hibasami et al., 1996), prostatic cancer (Brusselmans et al., 2003; Chung et al., 2001), gastric cancer (Horie et al., 2005), colon cancer (Chen et al., 2003), and lung cancer (Yang et
1.2. Apoptosis pathways
Apoptotic features include cell membrane blebbing, cell shrinkage, chromatin
condensation and DNA fragmentation, finally ending with the engulfment by
macrophages or neighboring cells, thereby avoiding an inflammatory response in
surrounding tissues (Savill and Fadok, 2000). Apoptosis can be separated two major
pathways incliding extrinsic and intrinsic pathways. External apoptotic pathway is
initiated by death ligands binding to their receptors such as CD-95/fas receptor and
TNFα-receptor, which is followed by activation of initiator caspase-8 to induce the
downstream apoptotic pathway (Walczak and Krammer, 2000). Intrinsic factors or
intracellular stimuli such as DNA damage can mediate mitochondrial apoptotic
pathway to initiate the release of cytochrome c and SMAC/DIABLO for apoptosis
induction (Shi, 2002). Both extrinsic and intrinsic pathways lead to activation
caspase-3 for apoptotic induction (Scaffidi et al., 1998; Shi, 2002). The inhibitor of
caspase-activated deoxyribonuclease (CAD) can be cleaved by activated caspase-3 to
release CAD (Enari et al., 1998; Sakahira et al., 1998). Then CAD enters the nucleus
to degrade the chromosomal DNA and leading to DNA fragmentation and cell death
(Enari et al., 1998). The NF-κB is a transcriptional factor which activates various
antiapoptotic signals or proteins can promote cancer cell survival and tumorigenesis
(Francois et al., 2005; Nakano et al., 2006; Suh and Rabson, 2004).
1.3. Baicalein and apoptosis
Baicalein is a bioactive flavonoid extracted from root of Scutellaria baicalensis
or Scutellaria radix that contains anticancer activities (Bonham et al., 2005; Chao et
al., 2007; Ma et al., 2005). It has been found that baicalein induce apoptosis in a
variety of human cancer cells (Chao et al., 2007; Chen et al., 2000; Kuntz et al., 1999;
Lee et al., 2005b; Ma et al., 2005; Pidgeon et al., 2002; Wang et al., 2009). Baicalein
induces cancer cell death which is associated with regulating CDK1 kinase and
survivin in bladder cancer cells (Chao et al., 2007). Moreover, baicalein induces
apoptosis by decreasing Bcl-2 and increasing p53 and Bax human in lung cancer cells
(Leung et al., 2007). In addition, NF-κB-regulated anti-apoptotic genes including
Bcl-2 and Bcl-XL have been shown that they are suppressed by baicalein (Lee et al.,
2005b; Pidgeon et al., 2002).
1.4. Baicalein and cell cycle progression
The regulation of cell cycle progression is regulated by cyclin dependent kinase
with cyclins and negatively regulated by CDK inhibitors (CDKIs) (Schwartz and
Shah, 2005; Shapiro, 2006). Baicalein has been shown to reduce cyclin D proteins to
mediate cell cycle arrest in breast, lung and prostate cancer cells (Lee et al., 2005b;
Pidgeon et al., 2002). Furthermore, baicalein declines the protein expression of cyclin
B1 and CDK1 protein levels in lung cancer cells (Lee et al., 2005b; Leung et al.,
2007). Down-regulation of CDK1 and Cyclin B1 by baicalein is involved in the
regulation of S phase progression (Leung et al., 2007). Baicalein suppresses CDK 2/4
expression and inhibits of the expression of p21 and p27 in prostate cancer cells
(Pidgeon et al., 2002). Baicalein also involves G1 and G2 arrest in association with
repression of CDK1, CDK2, cyclin D2 and cyclin A proteins, and with up-regulation
of cyclin E, p15, p53 and p21 (Hsu et al., 2001). In addition, baicalein can induce
G2/M arrest in leukemia cells (Roy et al., 2007). The levels of cyclin B1 and CDK1
are reduced for inducing G2/M arrest by baicalein in bladder cancer cells (Chao et al.,
2007). Moreover, baicalein arrested S and G2/M phase in breast cancer cells (Wang
et al., 2009). Accordingly, baicalein displays anticancer ability by mediating the
blockage of cell cycle progression in various cancer cell types.
1.5. Baicalein and tumorigenesis inhibition
(Bonham et al., 2005; Miocinovic et al., 2005). For example, baicalein displays the
ability to attenuate tumor growth in pancreatic tumor mouse model (Tong et al.,
2002). The antitumor effects of baicalein inhibits tumorigenesis in C3H/HeN mice
implanted with murine bladder cancer cells (Ikemoto et al., 2004).
1.6. Survivin and cancer
The survivin gene is located on the human 17q25 chromosome that expresses a
16.5-kDa protein (Ambrosini et al., 1997). Survivin belongs to the smallest member
of the IAP (inhibitor of apoptosis proteins) family (Deveraux et al., 1998), which
contains a single 76-amino of the characteristic zinc finger baculovirus–inhibitor of
apoptosis repeat (BIR) domain that is essential for the caspase-inhibitory function
(Altieri, 2003; Ambrosini et al., 1997; Li et al., 1998). Survivin, it is homodimeric,
arranged through hydrophobic surface of the BIR domain of each survivin monomer
(Chantalat et al., 2000; Verdecia et al., 2000). Survivin, a unique inhibitor of
apoptosis, expressed in embryonic and fetal organs in the developmental stages but
undetectable in normal adult tissues (Ambrosini et al., 1997). Furthermore, survivin is
selectively expressed in transformed cells and in most human cancers including lung,
breast, pancreatic, and colon carcinomas, soft tissue sarcomas, brain tumors,
Chakravarti et al., 2002; Grossman et al., 1999; Islam et al., 2000; Kappler et al.,
2004; Kawasaki et al., 1998; Monzo et al., 1999; Satoh et al., 2001; Tanaka et al.,
2000). Additionally, three isoforms of survivin have been found in human cells
including survivin‐2β, survivin‐∆Ex3, and survivin‐3β (Mahotka et al., 1999).
1.7. Survivin and cell division
Survivin is one of chromosome passenger proteins that regulates cell division
(Ambrosini et al., 1997). The conserved mitotic complex of aurora‐B, INCENP,
borealin, and survivin is essential for chromosome movements during mitosis, proper
spindle checkpoint surveillance, and execution of cytokinesis (Lens et al., 2006).
Appendix 1 shows that survivin can control motosis progression (Mita et al., 2008).
Chromosome passenger proteins are required to target the complex to kinetochores,
correct misaligned chromosomes, properly form the central spindle and complete
cytokinesis for equal and complete cell division (Gassmann et al., 2004; Honda et al.,
2003; Wheatley et al., 2001). Survivin localizes to kinetochores at metaphase,
transfers to the central spindle midzone at anaphase, and accumulates in midbodies at
telophase (Vagnarelli and Earnshaw, 2004). Moreover, the phosphorylation of
survivin on Thr34 by CDK1-cyclin B1 has been reported with increased survivin
1.8. Survivin and apoptosis
Mammalian IAPs family including XIAP (X-linked IAP), c-IAP1, 2, NAIP
(neuronal apoptosis-inhibiting protein), and survivin specifically inhibit the apoptosis
(Miller, 1999; Salvesen and Duckett, 2002). XIAP, cIAPs, and NAIP have been
shown to inhibit the caspase-3, -7, and -9 proteins by binding specific regions of
caspases (Riedl and Shi, 2004). It has been shown that survivin inhibits caspase-3, -7,
and -9 but not caspase-8 (Shin et al., 2001; Tamm et al., 1998). However, survivin
also can mediate caspase-3-independent pathway (Banks et al., 2000).
Smac/DIABLO is a pro-apoptotic protein and participates in the activation of
caspase-9 (Srinivasula et al., 2000). Survivin has affinity with Smac/DIABLO to
inhibit apoptosis by antagonizing the pro-apoptotic ability of Smac/DIABLO (Altieri,
2003).
1.9. Survivin and cancer therapy
Survivin displays both cell division and anti-apoptosis that can promote
tumorigenesis. Survivin has been shown to promote angiogenesis (Blanc-Brude et al.,
2003; Conway et al., 2003; Kawasaki et al., 2001; O'Connor et al., 2000b; Tran et al.,
inhibit tumorigenesis and angiogenesis providing for cancer therapy. Antisense
oligonucleotides, siRNA and dominant-negative mutants of survivin are successfully
exploited to suppress survivin expression (Koul et al., 2006; Marusawa et al., 2003;
Nakao et al., 2006; Zhang et al., 2001). Small-molecule antagonist suppresses
survivin transcription by inhibiting promoter of survivin providing potential cancer
therapy (Nakahara et al., 2007). Additionally, survivin has been predicted response to
chemotherapy and radiotherapy in patients with bladder cancer (Als et al., 2007),
breast cancer (Hinnis et al., 2007), multiple myeloma (Nakagawa et al., 2006), and
lymphoma (Adida et al., 2000; Schlette et al., 2004; Watanuki-Miyauchi et al., 2005).
1.10. Ubiquitin-proteasome system
Ubiquitin-proteasome system (UPS) is an essential mechanism involved in
cellular process such as degradation, cell cycle regulation, antigen processing, signal
transduction and transcription (Boutillier et al., 1999; Nandi et al., 2006; Orlowski,
1999). One of UPS important function involves in ubiquitin-proteasome-dependent
degradation of proteins (Ciechanover et al., 1980). The target protein is labeled with
multiple ubiquitin moieties and degraded by the 26S proteasome (Ciechanover and
Schwartz, 1998; Laney and Hochstrasser, 1999). The 76-amino-acid ubiquitin
between the ubiquitin and lysine side chains in the target proteins. Free Ub is
recruited by the E1 (Ub-activating enzyme) and transferred to lysine residue of the E2
(Ub-conjugating enzyme) (Haas et al., 1982). E2 and substrate are bound by the E3
(Ub-protein ligases), which is responsible for substrate recognition. E3 involves that
ubiquitin is transferred to a lysine amino groups of the target protein, then E2 and E3
are released (Hershko et al., 1986). The cyclic transfer of more Ub to the first Ub attached to the substrate is by E4 (ubiquitin-chain elongation factor)(Hoppe, 2005).
Appendix 2 shows that ubiquitin-proteasome system (Donohue and Osna, 2003).
Degradation of survivin occurs by the ubiquitin-proteasome pathway at the G1 phase,
and it is stabilized when heat shock protein 90 (Altieri, 2004; Fortugno et al., 2003;
Zhao et al., 2000). It has been shown that flavonoids such as kaempferol and
quercetin enhance apoptosis by degradation of survivin in glioma cells (Siegelin et al.,
2008; Siegelin et al., 2009). Furthermore, indomethacin reduced half-life of survivin
and increased survivin ubiquitination (Chiou and Mandayam, 2007). In addition,
chlamydocin involved survivin degradation by proteasome in ovarian cancer cells
(De Schepper et al., 2003).
1.11. Survivin and bladder cancer
death in the year 2009 in the United States (Jemal et al., 2009). Survivin expression
improves our prediction of cancer recurrence and survival in bladder cancer patients
(Shariat et al., 2009). Survivin signaling pathways have been evaluated for
survivin-targeted therapy in bladder cancer (Shariat et al., 2007). Blockage of
survivin expression induces apoptosis and suppresses the growth of the tumor in
bladder cancer cells (Fuessel et al., 2006; Ku et al.). Moreover, detection of survivin
and its associated gene may provide an early biomarker of aggressive tumor behavior
in the bladder cancers (Salz et al., 2005).
1.12. Oxaliplatin and cancer therapy
Oxaliplatin, a clinical anticancer drug, is a third-generation platinum compound
that confers a different spectrum of activity compared with cisplatin (Hochster et al.,
2003; Ramanathan et al., 2003). Like cisplatin, oxaliplatin acts as an alkylating agent
on DNA, forming platinated intrastrand cross-linksbetween two adjacent guanine
bases or two adjacent guanine–adenine bases that result in the blockage of replication
and transcription (Fink et al., 1997). The combination has proven efficacy in
5-fluorouracil-resistant advanced disease and in previously untreated colorectal
cancer (Andre et al., 1998; Maindrault-Goebel et al., 1999). Combination of
therapy in recent years. For example, combination of cyclooxygenase-2 inhibitors and
oxaliplatin increases the growth inhibition and death in colon cancer cells (Lin et al.,
2005). Moreover, oxaliplatin can inhibit survivin protein expression in cancer cells
(Lin et al., 2005).
1.13. The purpose of this study
Our laboratory has provided that baicalein inhibited survivn protein expression
in human bladder cancer cells (Chao et al., 2007). However, the regulation and
mechanism of survivin expression after treatment with baicalein remains unclear. In
this study, the regulation of survivin protein expression is investigated following
baicalein treatment in the human bladder cancer cells. We provide that baicalein
reduces survivin protein expression mediated by the ubiquitin-proteasome pathway.
Moreover, the survivin protein expression is additionally inhibited by combination of
baicalein and oxaliplatin. Understanding the mechanism by which survivin regulates
baicalein-induced apoptosis may provide the identification of novel strategies for
2. Materials and methods
2.1. Reagents and Antibodies
Baicalein, oxaliplatin, Hoechst 33258, and 3-(4,5-dimethyl-thiazol-2-yl)
2,5-diphenyl tetrazolium bromide (MTT), and cycloheximide were purchased from
Sigma Chemical Co. (St. Louis, MO) Anti-survivin (FL-142 and D-8), goat
anti-rabbit IgG horseradish peoxidase, goat anti-mouse IgG horseradish peoxidase,
and the FITC (fluorescein isothiocyanate)-labeled goat anti-mouse IgG antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Anti-ubiquitin (Apu2) and anti-actin (C4) antibodies were purchased from Millipore
(Bedford, MA). MG132 (Carbobenzoxy-L-leucyl-L-leucyl-L-leucinal) were
purchased from Calbiochem (San Diego, CA). Baicalein was dissolved in DMSO,
and the concentration of DMSO was < 1 % in the control and drug-containing
medium.
2.2. Cell culture
The BFTC905 cell line was derived from human bladder papillary transitional
cell carcinoma of a Chinese female patient. The cells were cultured in complete
supplemented with 10 % fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/
ml streptomycin, and L-glutamine (0.03 %, w/v), and cells were incubated at 37 °C
and 5 % CO2.
2.3. Cell viability assay
BFTC905 cells were plated in 96-well plates at a density of 1 × 104 cells/well for
16–20 h and then treated with various concentrations of baicalein for 24 h in
RPMI-1640 medium. After the treatment, the cells were washed with PBS and were
recultured in RPMI-1640 medium for 2 day. The cells were incubated with 0.5 mg/ml
of MTT in RPMI-1640 medium for 4 h. The surviving cells converted MTT to
formazan, which generates a blue-purple color when dissolved in dimethyl sulfoxide.
The intensity of formazan was measured at 565 nm using a plate reader (VERSAmax,
Molecular Dynamics Inc., CA) for enzyme-linked immunosorbent assays. The cell
viability was calculated by dividing the absorbance of treated cells by that of the
control in each experiment.
2.4. Time-lapse of living cell image analysis
BFTC905 bladder cancer cells were plated at a density of 1.5 × 105 cells Petri
or without 60 μM baicalein by time-lapse observation under an optical phase contrast
microscope with an incubator system (Olympus, Tokyo, Japan).
2.5. Annexin V/PI apoptosis assay
BFTC905 bladder cancer cells were plated at a density of 5 × 105 cells Petri
60-mm Petri dish in complete medium for 16 to 20 h. Thereafter, the cells were
treated with or without 5 μM MG132 for 1 h, then were treated with or without 60
μM baicalein for 24 h. Apoptotic cells was performed using an Annexin-V-FITC
Apoptosis Detection Kit (BioVision, Mountain View, CA) according to the
manufacturer’s instructions. Then cells were collected and resuspended in 500 μl of
binding buffer, and added 5 μl of Annexin-V-fluorescein isothiocyanate (FITC) and 5
μl of propidium iodide (PI). Analyses were performed with a FACSCalibur flow
cytometer (Becton Dickinson, Sunnyvale, CA).
2.6. Western blot
At the end of drug treatment, the cells were lysed in the ice-cold whole cell
extract buffer containing the protease inhibitors. The lysate was vibrated for 30 min at
4 °C and centrifuged at 10,000 × rpm for 10 minutes. Protein concentration was
in samples were subjected to electrophoresis of using 12 % sodium dodecyl
sulfate-polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride
membranes and the membranes were blocked overnight at 4 °C using blocking buffer
(5 % non-fat dried milk in solution containing 50 mM Tris/HCl (pH 8.0), 2 mM
CaCl2, 80 mM sodium chloride, 0.05 % Tween 20 and 0.02 % sodium azide).
Thereafter, the membrane were incubated for 2 h at 25 °C with specific primary
antibodies followed by anti-rabbit or anti-mouse immunoglobulin G-horseradish
peroxidase conjugate secondary antibodies. The membranes were washed three times
for 10 min with TBS containing 0.05 % Tween 20. The blot was incubated with
enhanced chemiluminescence detection system (PerkinElmer Life Sciences) for 5 min
and then exposed to X-ray film. To verify equal protein loading and transfer, actin
was used as the protein loading control. The software of Un-Scan-It gel (Ver. 5.1,
Silk Scientific, Inc.) was adopted for semi-quantification of the intensity in each
band.
2.7. Reverse transcription-polymerase chain reaction (RT-PCR)
BFTC905 cells were plated at a density of 2 × 106 cells per 60-mm Petri dish inculture medium. Total cellular RNA was purified by Trizol reagent (Invitrogen,
determined by a spectrophotometer (Eppendorf, Hamburg Germany). cDNAs were
synthesized by SuperScriptTM III reverse transcriptase (Invitrogen) with oligo-dT12-18
primer (Invitrogen). Each reverse transcript was amplified with GAPDH as an
internal control. The following primer pairs were used for amplification: survivin,
forward primer: 5’-GGCATGGGTGCCCCGACGTTG-3’and reverse primer:
5’-CAGAGGCCTCAATCCATGGCA-3’; GAPDH, forward primer:
5’-CGGAGTCAACGGATTTGGTCGTAT-3’ and reverse primer:
5’-AGCCTTCTCCATGGTGGTGAAGAC-3’. RT-PCR was performed by a DNA
thermal cycler, Mastercycler gradient (Eppendorf, Hamburg Germany), 56 °C for 30
s, and 72 °C for 40 s; and 72 °C for 5 min. The PCR products were visualized on 1.2
% agarose gels with ethidium bromide staining under UV transillumination with a
digital camera system (DH27-S3, Medclub, Taoyuan, Taiwan).
2.8. Quantitative real-time PCR
Each real-time PCR was carried out in triplicate in a 25 μl volume using SYBR
Green qPCR Master Mix (Fermentas Life Sciences,St. Leon-Rot, Germany)
according to the manufacturer’s protocol. Primers sequences were as follows:
survivin 5’-ATTCGTCCGGTTGCGCTTTCC-3’ and
5’-GCGAGAAGATGACCCAGATC-3’ and 5’-GGATAGCAACGCCTGGATAG-3’.
The PCR conditions were for 10 min at 95 °C for initial denaturation, followed by 40
cycles of 95 °C for 15 s and 60 °C for 1 min in the ABI Prism 7000 Sequence
Detection System (Applied Biosystems, Foster City, CA). Relative gene expression
quantifications were calculated according to the comparative Ct method using β-actin
as an internal standard. The fold amplification of genes was respectively detected by
calculating the 2-∆∆Ct of the genes.
2.9. Immunoprecipitation
The PureproteomeTM Protein G Magnetic Beads (Millipore, Bedford, MA) were
mixed so that all of the beads are uniformly resuspended. The beads were plased into
a 1.5 ml microcentrifuge tubes, then tubes were removed into the Magna GrIP Rack
(Millipore). Then the storage buffer was removed with a pipette. The beads were
washed by adding 500 μl of PBS containing 0.1 % Tween® 20 surfactant and
vortexing vigorously for 10 seconds. The tubes were returned to the magnetic rack and allow the beads to adhere to the side. The buffer was removed with a pipette. The
washed beads were resuspend in 350 μl of PBS containing 0.1 % Tween 20 surfactant.
The survivin antibody was added to the resuspended beads with incubation at room
then the buffer was removed with a pipette. The beads were washed 3 times with 500
μl of PBS containing 0.1 % Tween 20 surfactant. After the last wash, the tubes were
removed from the rack and the cell lysates were added. According to the relative
protein expression of survivin in control and baicalein-treated samples, the total protein lysates were adjusted in immunoprecipitation analysis for equal survivin
protein expression of control and baicalein-treated sample. Then samples were
immobilized survivin antibody at 2–8 °C with continuous mixing overnight. The
tubes were placed into the magnetic rack, and then removed the sample with a pipette.
The beads were washed 3 times with 500 μl of PBS containing 0.1 % Tween 20
surfactant. After the last wash, the tube were removed from the magnetic rack and
added the sample buffer for western blot analysis.
2.10.
Immunofluorescence staining and confocal microscopy
To view the protein expression of survivin and ubiquitin after baicalein treatment,
the cells were subjected to immunofluorescence staining and confocal microscopy.
After fixation with 4 % paraformaldehyde solution, the cells were washed three times
with PBS, and non-specific binding sites were blocked in PBS containing 10 % FBS
and 0.3 % Triton X-100 for 1 h at 37 °C. Thereafter, the cells were separately
antibody in PBS containing 10 % FBS for 1 h at 37 °C, and washed three times with
0.3 % Triton X-100 in PBS. Then the cells were individually incubated with goat
anti-mouse Cy3 (1:200) and anti-rabbit FITC in PBS containing 10 % FBS for 1 h at
37 °C. The nuclei were stained with Hoechst 33258. The samples were examined
under a confocal microscope Fluoview 300 (Olympus, Tokyo, Japan).
2.11.
Statistical analysis
Data were analyzed using Student’s t test, and a p value of <0.05 was considered
3. Results
3.1. Baicalein induces cytotoxicity and proliferation inhibition in
bladder cancer cells
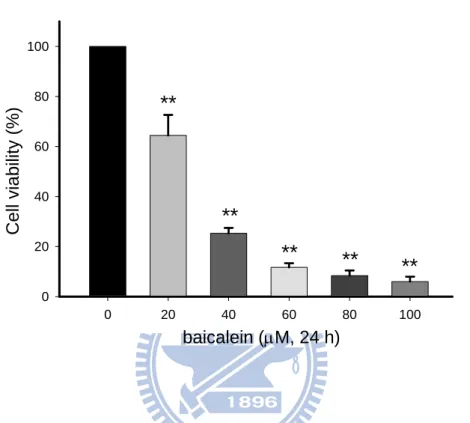
To examine the cytotoxicity and proliferation following baicalein treatment in
BFTC905 bladder cancer cells, the cells were analyzed by MTT assay. Treatment
with 20–100 μM baicalein for 24 h significantly reduced the cell viability via a
concentration-dependent manner in BFTC905 cells (Fig. 1). The value of IC50 (the
concentration of 50 % inhibition of cell viability) was around 30 μM. Moreover,
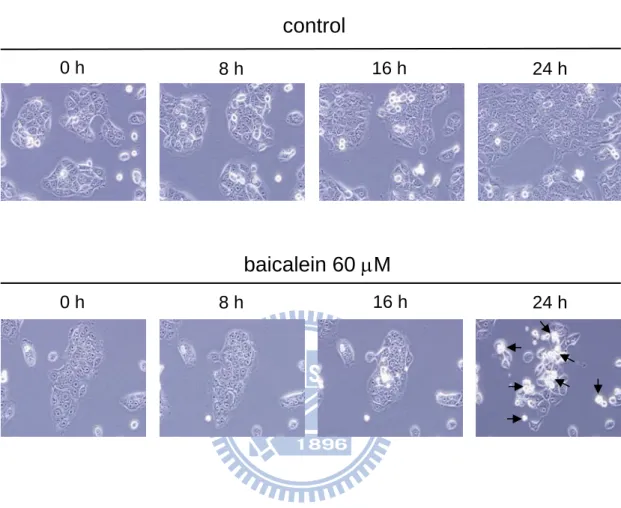
baicalein inhibited cell proliferation and induced cell death that can be observed by
time-lapse living cell morphology observation alteration. The arrows show that
baicalein induced the cell death at 24 h observation (Fig. 2). However, the untreated
cells clearly displayed the increase of cell proliferation and cell number at 24 h
observation (Fig. 2).
3.2. Baicalein inhibits survivin protein expression but not altered
gene expression in bladder cancer cells
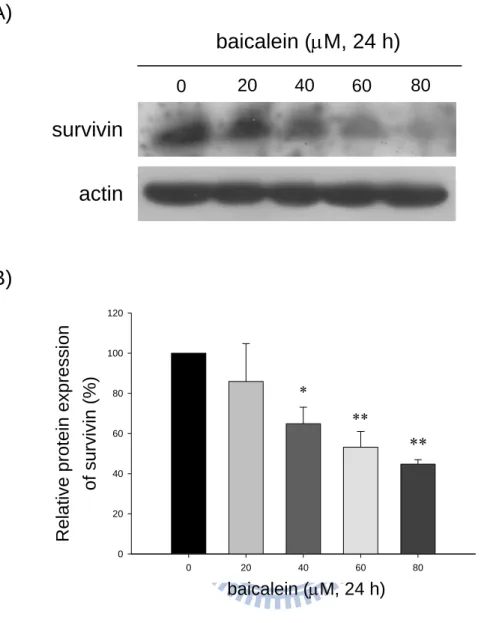
To study the effect of survivin protein expression by baicalein in BFTC905
protein levels of survivin were decreased by 20–80 μM baicalein for 24 h in
BFTC905 cells (Fig. 3A and 3B). The quantified data also shows that baicalein
significantly reduced survivin protein expression in BFTC905 cells (Fig. 3B).
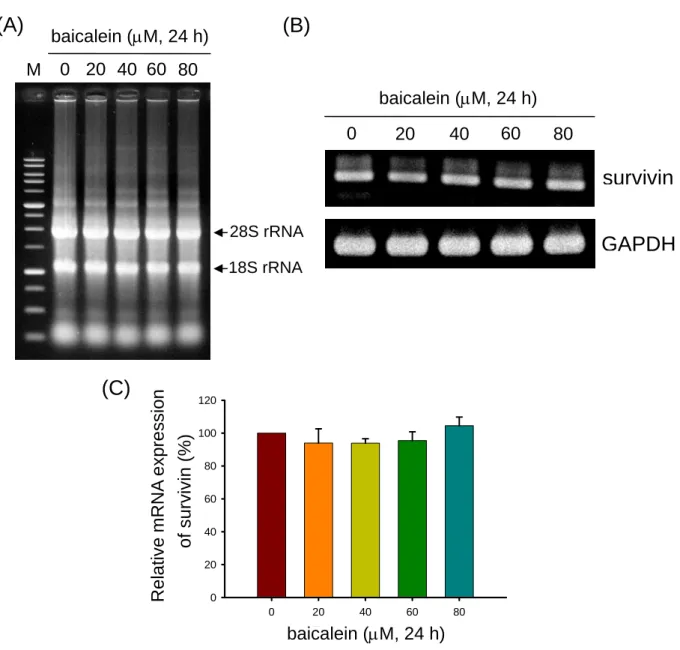
Moreover, we have further investigated the survivin expression on transcriptional
levels by reverse transcription-PCR and real-time PCR. The qualities of total RNA
extracts were presented by the contents of 28S rRNA and 18S rRNA (Fig. 4A).
However, baicalein did not alter the survivin mRNA expression (Fig. 4B and 4C).
The survivin mRNA expression in baicalein-treated cells was compared to the control
for 24 h by real-time PCR (Fig. 5A). The mRNA level of survvin was not statistically
altered by treatment with baicalein (Fig. 5B).
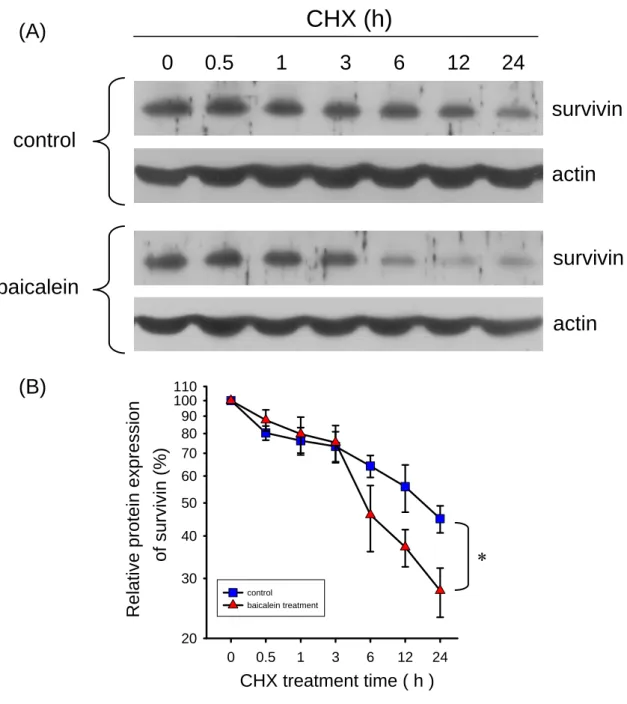
3.3. Baicalein reduces the protein stability of survivin in bladder
cancer cells
To further determine the effect of baicalein on the protein stability and half-life
of survivin proteins, a protein synthesis inhibitor (cycloheximide, CHX) was
examined on the effect of survivin protein expression. Treatment with 10 μg/ml CHX
for 24 h reduced around the half of total amount of survivin proteins (Fig. 6A).
However, treatment with CHX and baicalein, the survivin protein levels were
and baicalein almost completely blocked the survivin protein expression after 12 h
period (Fig. 6B).
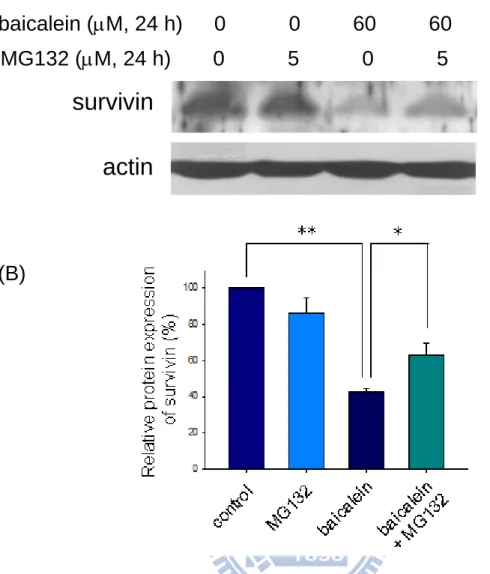
3.4. Proteasome inhibitor decreases baicalein-induced survivin
protein degradation in bladder cancer cells
To investigate the role of proteasome on baicalein-induced down-regulation of
survivin protein expression, MG132 (a proteasome inhibitor) was utilized in this
study. Treatment with 60 μM baicalein for 24 h significantly reduced survivin protein
expression (Fig. 7). Pre-treatment of BFTC905 cells with 5 μM MG132 potentially
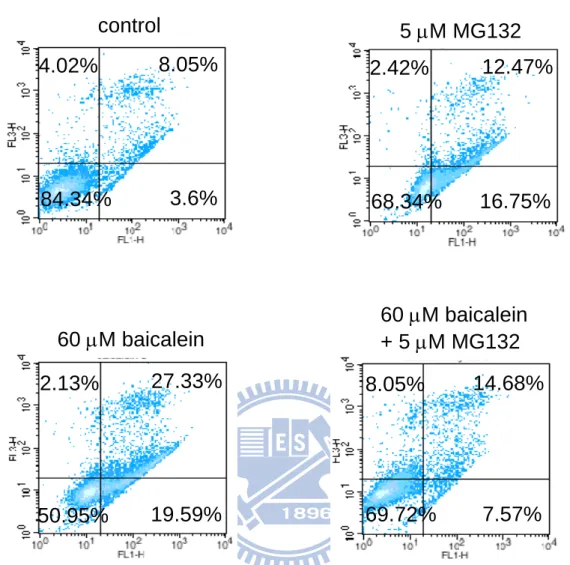
reversed survivin protein level in the baicalein-treated cells (Fig. 7). In annevin V/PI
apoptosis assay, baicalein increased higher apoptosis level than untreated sample in
BFTC905 cells (Fig. 8). Moreover, pre-treatment of MG132 potentially inhibited
apoptosis in the baicalein-treated BFTC905 cells (Fig. 8).
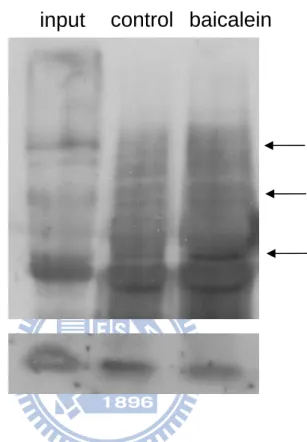
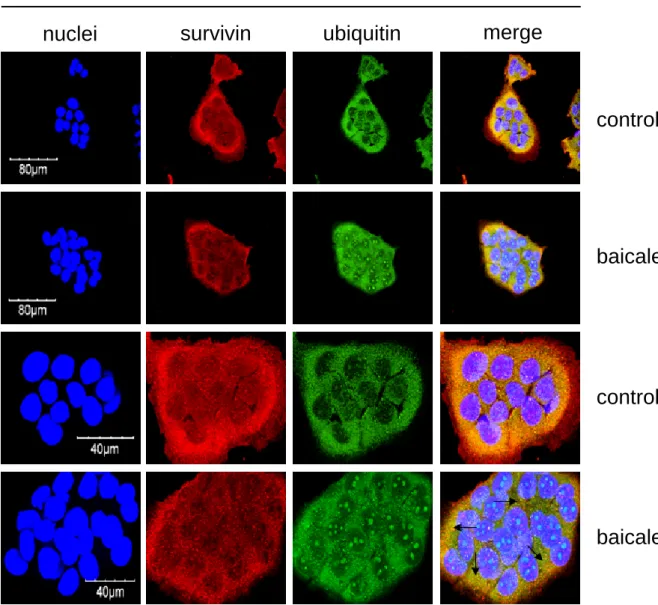
3.5. Baicalein treatment increases ubiquitination of survivin
We have determined the effect of baicalein on the protein ubiquitination of
survivin. As shown in Fig. 9, baicalein induced ubiquitinated survivin levels in
BFTC905 cells. To further confirm the ubiquitination of survivin proteins, the
microscopy. Baicalein reduced the intensity of red fluorescence (Cy3) of survivin
proteins. However, baicalein induced high intensity of green fluorescence of ubiquitin
proteins. The yellow color indicated that co-locolization of survivin and ubiquitin
(Fig. 9, arrows).
3.6. Co-treatment of baicalein and oxaliplatin enhances the
cytotoxicity and survivin protein inhibition in bladder cancer cells
As shown in Fig. 11, co-treatment with 10–50 μM baicalein and 1 μM
oxaliplatin for 24 h enhanced the cytotoxicity in BFTC905 cells. To study the
combination effect of baicalein and oxaliplatin on the survivin protein inhibition, the
cells were co-treated with baicalein and oxaliplatin followed by Western blot analysis.
Both of baicalein and oxaliplatin significantly reduced survivin protein expression
(Fig. 12A and 12B). Co-treatment with baicalein and oxaliplatin for 24 h enhanced
4. Discussion
Various types of flavonoids display anticancer effects on growth inhibition and
apoptosis (Brusselmans et al., 2005; Lee et al., 2005a; Psahoulia et al., 2007; Spencer
et al., 2003; Yin et al., 2001). In this study, baicalein significantly inhibited cell
viability in the human BFTC905 bladder cancer cells. We also found that baicalein
induced growth inhibition and cell death by time-lapse observation in BFTC905 cells.
Survivin has been demonstrated to inhibit apoptosis and to promote mitotic
progression in cancer cells (Ambrosini et al., 1997; Li et al., 1998). Interestingly,
baicalein significantly reduced survivin protein expression in BFTC905 cells.
However, baicalein did not influence the survivin mRNA expression by RT-PCR and
real-time PCR assays. As a consequence, we suggest that baicalein inhibits the
survivin expression on the alteration of protein level but not gene expression.
Ubiquitin-proteasome pathway is an essential mechanism participating in
cellular process. Proteasome degradation has also been shown to play an important
role in regulation of apoptosis and cell proliferation by indomethacin (Chiou and
Mandayam, 2007). Furthermore, kaempferol and quercetin enhanced apoptosis by
degradation of survivin in glioma cells (Siegelin et al., 2008; Siegelin et al., 2009). It
in a cell cycle-dependent manner (Zhao et al., 2000). We have shown that baicalein
inhibited survivin protein expression (Chao et al., 2007); however, suppression of
survivin on the post-translational level by baicalein has not been shown previously.
We have further determined the half-life of survivin by baicalein. Baicalein enhanced
the survivin protein degradation when the cells were co-treated with CHX. The data
indicates that baicalein induces the survivin protein instability in bladder cancer cells.
Furthermore, MG132 proteasome inhibitor prevented survivin protein degradation in
the baicalein-treated cells. In addition, MG132 can reduce the baicalein-induced
apoptosis. Baicalein also activated ubiquitination of survivin in bladder cancer cells.
Accordingly, our findings suggest that baicalein inhibits survivin protein expression
through the ubiquitin-proteasome pathway in human bladder cancer cells. We provide
a model of baicalein-induced down-regulation of survivin as shown in Fig. 13.
The stability of survivin resulted from the protein phosphorylation at Thr34 by
the mitotic kinase complex CDK1/cyclin B1 (O'Connor et al., 2000a; Wall et al.,
2003). Quercetin increases the survivin protein expression, which correlates with
raising the protein levels of cyclin B1 and phospho-CDK1 (Kuo et al., 2004). The
levels of cyclin B1 and CDK1 were reduced for inducing G2/M arrest by baicalein in
bladder cancer cells (Chao et al., 2007). The role of CDK1/cyclin B1 on the
XAF1-XIAP complex enhanced degradation of survivin protein (Arora et al., 2007).
Therefore, further investigations are required to determine the roles of XAF1-XIAP
and CDK1/cyclinB1 on the regulation of survivin protein expression following
baicalein in bladder cancer cells.
Combination of various anticancer agents may increase the efficiency of cancer
therapy (Hochster et al., 2003; Ramanathan et al., 2003; Rathkopf et al., 2009).
Previously, Oxaliplatin reduced survivin protein expression and induced apoptosis in
cancer cells (Lin et al., 2005). In this study, we have further examined the anticancer
effects of combination of baicalein and oxaliplatin on the cell viability and survivin
expression in human bladder cancer cells. Both baicalein and oxaliplatin significantly
induced cell death in BFTC905 cells. Interestingly, co-treatment of baicalein and
oxaliplatin additively decreased the levels of survivin proteins and increased
cytotoxicity in BFTC905 cells. Accordingly, the combination of baicalein and
oxaliplatin may increase anticancer effects on survivin inhibition and cancer cell
5. Conclusion
We have summerized that the down-regulation of survivin by baicalein is
mediated ubiquitin-proteasome degradation pathway in human bladder cancer cells
(Fig. 13). Our findings indicate that the blockage of survivin by baicalein may
provide the novel strategies for elevating the efficiency of cancer therapy in bladder
6. Reference
Adida, C., Berrebi, D., Peuchmaur, M., Reyes-Mugica, M., and Altieri, D.C. (1998).
Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet 351,
882-883.
Adida, C., Haioun, C., Gaulard, P., Lepage, E., Morel, P., Briere, J., Dombret, H.,
Reyes, F., Diebold, J., Gisselbrecht, C., et al. (2000). Prognostic significance of
survivin expression in diffuse large B-cell lymphomas. Blood 96, 1921-1925.
Als, A.B., Dyrskjot, L., von der Maase, H., Koed, K., Mansilla, F., Toldbod, H.E.,
Jensen, J.L., Ulhoi, B.P., Sengelov, L., Jensen, K.M., et al. (2007). Emmprin and
survivin predict response and survival following cisplatin-containing
chemotherapy in patients with advanced bladder cancer. Clin Cancer Res 13,
4407-4414.
Altieri, D.C. (2003). Survivin, versatile modulation of cell division and apoptosis in
cancer. Oncogene 22, 8581-8589.
Altieri, D.C. (2004). Coupling apoptosis resistance to the cellular stress response: the
IAP-Hsp90 connection in cancer. Cell Cycle 3, 255-256.
Andre, T., Louvet, C., Raymond, E., Tournigand, C., and de Gramont, A. (1998).
Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin
(FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and
5-fluorouracil regimen. Ann Oncol 9, 1251-1253.
Arora, V., Cheung, H.H., Plenchette, S., Micali, O.C., Liston, P., and Korneluk, R.G.
(2007). Degradation of survivin by the X-linked inhibitor of apoptosis
(XIAP)-XAF1 complex. J Biol Chem 282, 26202-26209.
Banks, D.P., Plescia, J., Altieri, D.C., Chen, J., Rosenberg, S.H., Zhang, H., and Ng,
S.C. (2000). Survivin does not inhibit caspase-3 activity. Blood 96, 4002-4003.
Blanc-Brude, O.P., Mesri, M., Wall, N.R., Plescia, J., Dohi, T., and Altieri, D.C.
(2003). Therapeutic targeting of the survivin pathway in cancer: initiation of
mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin
Cancer Res 9, 2683-2692.
Bonham, M., Posakony, J., Coleman, I., Montgomery, B., Simon, J., and Nelson, P.S.
(2005). Characterization of chemical constituents in Scutellaria baicalensis with
antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin
Cancer Res 11, 3905-3914.
Boutillier, A.L., Kienlen-Campard, P., and Loeffler, J.P. (1999). Depolarization
role of the ubiquitin/proteasome signalling pathway. Eur J Neurosci 11, 441-448.
Brusselmans, K., De Schrijver, E., Heyns, W., Verhoeven, G., and Swinnen, J.V.
(2003). Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid
synthase in intact cells and selectively induces apoptosis in prostate cancer cells.
Int J Cancer 106, 856-862.
Brusselmans, K., Vrolix, R., Verhoeven, G., and Swinnen, J.V. (2005). Induction of
cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty
acid synthase activity. J Biol Chem 280, 5636-5645.
Chakravarti, A., Noll, E., Black, P.M., Finkelstein, D.F., Finkelstein, D.M., Dyson,
N.J., and Loeffler, J.S. (2002). Quantitatively determined survivin expression
levels are of prognostic value in human gliomas. J Clin Oncol 20, 1063-1068.
Chantalat, L., Skoufias, D.A., Kleman, J.P., Jung, B., Dideberg, O., and Margolis,
R.L. (2000). Crystal structure of human survivin reveals a bow tie-shaped dimer
with two unusual alpha-helical extensions. Mol Cell 6, 183-189.
Chao, J.I., Su, W.C., and Liu, H.F. (2007). Baicalein induces cancer cell death and
proliferation retardation by the inhibition of CDC2 kinase and survivin
associated with opposite role of p38 mitogen-activated protein kinase and AKT.
Mol Cancer Ther 6, 3039-3048.
Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon
adenocarcinoma cells. Carcinogenesis 24, 1369-1378.
Chen, C.H., Huang, L.L., Huang, C.C., Lin, C.C., Lee, Y., and Lu, F.J. (2000).
Baicalein, a novel apoptotic agent for hepatoma cell lines: a potential medicine
for hepatoma. Nutr Cancer 38, 287-295.
Chiou, S.K., and Mandayam, S. (2007). NSAIDs enhance proteasomic degradation of
survivin, a mechanism of gastric epithelial cell injury and apoptosis. Biochem
Pharmacol 74, 1485-1495.
Chung, L.Y., Cheung, T.C., Kong, S.K., Fung, K.P., Choy, Y.M., Chan, Z.Y., and
Kwok, T.T. (2001). Induction of apoptosis by green tea catechins in human
prostate cancer DU145 cells. Life Sci 68, 1207-1214.
Ciechanover, A., Heller, H., Elias, S., Haas, A.L., and Hershko, A. (1980).
ATP-dependent conjugation of reticulocyte proteins with the polypeptide
required for protein degradation. Proc Natl Acad Sci U S A 77, 1365-1368.
Ciechanover, A., and Schwartz, A.L. (1998). The ubiquitin-proteasome pathway: the
complexity and myriad functions of proteins death. Proc Natl Acad Sci U S A 95,
2727-2730.
Conway, E.M., Zwerts, F., Van Eygen, V., DeVriese, A., Nagai, N., Luo, W., and
molecular mechanisms of hypoxia-induced up-regulation. Am J Pathol 163,
935-946.
De Schepper, S., Bruwiere, H., Verhulst, T., Steller, U., Andries, L., Wouters, W.,
Janicot, M., Arts, J., and Van Heusden, J. (2003). Inhibition of histone
deacetylases by chlamydocin induces apoptosis and proteasome-mediated
degradation of survivin. J Pharmacol Exp Ther 304, 881-888.
Devault, A., Cavadore, J.C., Fesquet, D., Labbe, J.C., Lorca, T., Picard, A., Strausfeld,
U., and Doree, M. (1991). Concerted roles of cyclin A, cdc25+ mitotic inducer,
and type 2A phosphatase in activating the cyclin B/cdc2 protein kinase at the
G2/M phase transition. Cold Spring Harb Symp Quant Biol 56, 503-513.
Deveraux, Q.L., Roy, N., Stennicke, H.R., Van Arsdale, T., Zhou, Q., Srinivasula,
S.M., Alnemri, E.S., Salvesen, G.S., and Reed, J.C. (1998). IAPs block apoptotic
events induced by caspase-8 and cytochrome c by direct inhibition of distinct
caspases. EMBO J 17, 2215-2223.
Donohue, T.M., Jr., and Osna, N.A. (2003). Intracellular proteolytic systems in
alcohol-induced tissue injury. Alcohol Res Health 27, 317-324.
Enari, M., Sakahira, H., Yokoyama, H., Okawa, K., Iwamatsu, A., and Nagata, S.
(1998). A caspase-activated DNase that degrades DNA during apoptosis, and its
Fink, D., Zheng, H., Nebel, S., Norris, P.S., Aebi, S., Lin, T.P., Nehme, A., Christen,
R.D., Haas, M., MacLeod, C.L., et al. (1997). In vitro and in vivo resistance to
cisplatin in cells that have lost DNA mismatch repair. Cancer Res 57,
1841-1845.
Fortugno, P., Beltrami, E., Plescia, J., Fontana, J., Pradhan, D., Marchisio, P.C., Sessa,
W.C., and Altieri, D.C. (2003). Regulation of survivin function by Hsp90. Proc
Natl Acad Sci U S A 100, 13791-13796.
Francois, S., El Benna, J., Dang, P.M., Pedruzzi, E., Gougerot-Pocidalo, M.A., and
Elbim, C. (2005). Inhibition of neutrophil apoptosis by TLR agonists in whole
blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB
signaling pathways, leading to increased levels of Mcl-1, A1, and
phosphorylated Bad. J Immunol 174, 3633-3642.
Fuessel, S., Herrmann, J., Ning, S., Kotzsch, M., Kraemer, K., Schmidt, U.,
Hakenberg, O.W., Wirth, M.P., and Meye, A. (2006). Chemosensitization of
bladder cancer cells by survivin-directed antisense oligodeoxynucleotides and
siRNA. Cancer Lett 232, 243-254.
Gassmann, R., Carvalho, A., Henzing, A.J., Ruchaud, S., Hudson, D.F., Honda, R.,
Nigg, E.A., Gerloff, D.L., and Earnshaw, W.C. (2004). Borealin: a novel
Cell Biol 166, 179-191.
Grossman, D., McNiff, J.M., Li, F., and Altieri, D.C. (1999). Expression and
targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest
Dermatol 113, 1076-1081.
Haas, A.L., Warms, J.V., Hershko, A., and Rose, I.A. (1982). Ubiquitin-activating
enzyme. Mechanism and role in protein-ubiquitin conjugation. J Biol Chem 257,
2543-2548.
Havsteen, B. (1983). Flavonoids, a class of natural products of high pharmacological
potency. Biochem Pharmacol 32, 1141-1148.
Havsteen, B.H. (2002). The biochemistry and medical significance of the flavonoids.
Pharmacol Ther 96, 67-202.
Hershko, A., Heller, H., Eytan, E., and Reiss, Y. (1986). The protein substrate
binding site of the ubiquitin-protein ligase system. J Biol Chem 261,
11992-11999.
Hibasami, H., Achiwa, Y., Fujikawa, T., and Komiya, T. (1996). Induction of
programmed cell death (apoptosis) in human lymphoid leukemia cells by
catechin compounds. Anticancer Res 16, 1943-1946.
Hinnis, A.R., Luckett, J.C., and Walker, R.A. (2007). Survivin is an independent
Cancer 96, 639-645.
Hochster, H., Chachoua, A., Speyer, J., Escalon, J., Zeleniuch-Jacquotte, A., and
Muggia, F. (2003). Oxaliplatin with weekly bolus fluorouracil and low-dose
leucovorin as first-line therapy for patients with colorectal cancer. J Clin Oncol
21, 2703-2707.
Honda, R., Korner, R., and Nigg, E.A. (2003). Exploring the functional interactions
between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell 14,
3325-3341.
Hoppe, T. (2005). Multiubiquitylation by E4 enzymes: 'one size' doesn't fit all. Trends
Biochem Sci 30, 183-187.
Horie, N., Hirabayashi, N., Takahashi, Y., Miyauchi, Y., Taguchi, H., and Takeishi,
K. (2005). Synergistic effect of green tea catechins on cell growth and apoptosis
induction in gastric carcinoma cells. Biol Pharm Bull 28, 574-579.
Hsu, S.L., Hsieh, Y.C., Hsieh, W.C., and Chou, C.J. (2001). Baicalein induces a dual
growth arrest by modulating multiple cell cycle regulatory molecules. Eur J
Pharmacol 425, 165-171.
Ikemoto, S., Sugimura, K., Kuratukuri, K., and Nakatani, T. (2004). Antitumor
effects of lipoxygenase inhibitors on murine bladder cancer cell line (MBT-2).
Islam, A., Kageyama, H., Takada, N., Kawamoto, T., Takayasu, H., Isogai, E., Ohira,
M., Hashizume, K., Kobayashi, H., Kaneko, Y., et al. (2000). High expression of
Survivin, mapped to 17q25, is significantly associated with poor prognostic
factors and promotes cell survival in human neuroblastoma. Oncogene 19,
617-623.
Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J., and Thun, M.J. (2009). Cancer
statistics, 2009. CA Cancer J Clin 59, 225-249.
Kappler, M., Bache, M., Bartel, F., Kotzsch, M., Panian, M., Wurl, P., Blumke, K.,
Schmidt, H., Meye, A., and Taubert, H. (2004). Knockdown of survivin
expression by small interfering RNA reduces the clonogenic survival of human
sarcoma cell lines independently of p53. Cancer Gene Ther 11, 186-193.
Kawasaki, H., Altieri, D.C., Lu, C.D., Toyoda, M., Tenjo, T., and Tanigawa, N.
(1998). Inhibition of apoptosis by survivin predicts shorter survival rates in
colorectal cancer. Cancer Res 58, 5071-5074.
Kawasaki, H., Toyoda, M., Shinohara, H., Okuda, J., Watanabe, I., Yamamoto, T.,
Tanaka, K., Tenjo, T., and Tanigawa, N. (2001). Expression of survivin
correlates with apoptosis, proliferation, and angiogenesis during human
colorectal tumorigenesis. Cancer 91, 2026-2032.
enhances TNF-induced apoptosis through modulation of nuclear factor-kappaB
signaling pathway in human glioma cells. Biochem Biophys Res Commun 350,
463-471.
Ku, J.H., Seo, S.Y., Kwak, C., and Kim, H.H. Cytotoxicity and apoptosis by survivin
small interfering RNA in bladder cancer cells. BJU Int.
Kuntz, S., Wenzel, U., and Daniel, H. (1999). Comparative analysis of the effects of
flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer
cell lines. Eur J Nutr 38, 133-142.
Kuo, P.C., Liu, H.F., and Chao, J.I. (2004). Survivin and p53 modulate
quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma
cells. J Biol Chem 279, 55875-55885.
Laney, J.D., and Hochstrasser, M. (1999). Substrate targeting in the ubiquitin system.
Cell 97, 427-430.
Lee, H.J., Wang, C.J., Kuo, H.C., Chou, F.P., Jean, L.F., and Tseng, T.H. (2005a).
Induction apoptosis of luteolin in human hepatoma HepG2 cells involving
mitochondria translocation of Bax/Bak and activation of JNK. Toxicol Appl
Pharmacol 203, 124-131.
Lee, H.Z., Leung, H.W., Lai, M.Y., and Wu, C.H. (2005b). Baicalein induced cell
Anticancer Res 25, 959-964.
Lens, S.M., Vader, G., and Medema, R.H. (2006). The case for Survivin as mitotic
regulator. Curr Opin Cell Biol 18, 616-622.
Leung, H.W., Yang, W.H., Lai, M.Y., Lin, C.J., and Lee, H.Z. (2007). Inhibition of
12-lipoxygenase during baicalein-induced human lung nonsmall carcinoma
H460 cell apoptosis. Food Chem Toxicol 45, 403-411.
Li, F., Ambrosini, G., Chu, E.Y., Plescia, J., Tognin, S., Marchisio, P.C., and Altieri,
D.C. (1998). Control of apoptosis and mitotic spindle checkpoint by survivin.
Nature 396, 580-584.
Lin, J., Hsiao, P.W., Chiu, T.H., and Chao, J.I. (2005). Combination of
cyclooxygenase-2 inhibitors and oxaliplatin increases the growth inhibition and
death in human colon cancer cells. Biochem Pharmacol 70, 658-667.
Ma, Z., Otsuyama, K., Liu, S., Abroun, S., Ishikawa, H., Tsuyama, N., Obata, M., Li,
F.J., Zheng, X., Maki, Y., et al. (2005). Baicalein, a component of Scutellaria
radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of
proliferation and induction of apoptosis in human myeloma cells. Blood 105,
3312-3318.
Mahotka, C., Wenzel, M., Springer, E., Gabbert, H.E., and Gerharz, C.D. (1999).
inhibitor survivin with different antiapoptotic properties. Cancer Res 59,
6097-6102.
Maindrault-Goebel, F., Louvet, C., Andre, T., Carola, E., Lotz, J.P., Molitor, J.L.,
Garcia, M.L., Gilles-Amar, V., Izrael, V., Krulik, M., et al. (1999). Oxaliplatin
added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as
second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur
J Cancer 35, 1338-1342.
Manach, C., Scalbert, A., Morand, C., Remesy, C., and Jimenez, L. (2004).
Polyphenols: food sources and bioavailability. Am J Clin Nutr 79, 727-747.
Marusawa, H., Matsuzawa, S., Welsh, K., Zou, H., Armstrong, R., Tamm, I., and
Reed, J.C. (2003). HBXIP functions as a cofactor of survivin in apoptosis
suppression. EMBO J 22, 2729-2740.
Michels, G., Watjen, W., Niering, P., Steffan, B., Thi, Q.H., Chovolou, Y.,
Kampkotter, A., Bast, A., Proksch, P., and Kahl, R. (2005). Pro-apoptotic effects
of the flavonoid luteolin in rat H4IIE cells. Toxicology 206, 337-348.
Middleton, E., Jr., Kandaswami, C., and Theoharides, T.C. (2000). The effects of
plant flavonoids on mammalian cells: implications for inflammation, heart
disease, and cancer. Pharmacol Rev 52, 673-751.
Trends Cell Biol 9, 323-328.
Miocinovic, R., McCabe, N.P., Keck, R.W., Jankun, J., Hampton, J.A., and Selman,
S.H. (2005). In vivo and in vitro effect of baicalein on human prostate cancer
cells. Int J Oncol 26, 241-246.
Mita, A.C., Mita, M.M., Nawrocki, S.T., and Giles, F.J. (2008). Survivin: key
regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin
Cancer Res 14, 5000-5005.
Monzo, M., Rosell, R., Felip, E., Astudillo, J., Sanchez, J.J., Maestre, J., Martin, C.,
Font, A., Barnadas, A., and Abad, A. (1999). A novel anti-apoptosis gene:
Re-expression of survivin messenger RNA as a prognosis marker in
non-small-cell lung cancers. J Clin Oncol 17, 2100-2104.
Nakagawa, Y., Abe, S., Kurata, M., Hasegawa, M., Yamamoto, K., Inoue, M.,
Takemura, T., Suzuki, K., and Kitagawa, M. (2006). IAP family protein
expression correlates with poor outcome of multiple myeloma patients in
association with chemotherapy-induced overexpression of multidrug resistance
genes. Am J Hematol 81, 824-831.
Nakahara, T., Takeuchi, M., Kinoyama, I., Minematsu, T., Shirasuna, K., Matsuhisa,
A., Kita, A., Tominaga, F., Yamanaka, K., Kudoh, M., et al. (2007). YM155, a
human hormone-refractory prostate tumor xenografts. Cancer Res 67,
8014-8021.
Nakano, H., Nakajima, A., Sakon-Komazawa, S., Piao, J.H., Xue, X., and Okumura,
K. (2006). Reactive oxygen species mediate crosstalk between NF-kappaB and
JNK. Cell Death Differ 13, 730-737.
Nakao, K., Hamasaki, K., Ichikawa, T., Arima, K., Eguchi, K., and Ishii, N. (2006).
Survivin downregulation by siRNA sensitizes human hepatoma cells to
TRAIL-induced apoptosis. Oncol Rep 16, 389-392.
Nandi, D., Tahiliani, P., Kumar, A., and Chandu, D. (2006). The
ubiquitin-proteasome system. J Biosci 31, 137-155.
O'Connor, D.S., Grossman, D., Plescia, J., Li, F., Zhang, H., Villa, A., Tognin, S.,
Marchisio, P.C., and Altieri, D.C. (2000a). Regulation of apoptosis at cell
division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci U S A 97,
13103-13107.
O'Connor, D.S., Schechner, J.S., Adida, C., Mesri, M., Rothermel, A.L., Li, F., Nath,
A.K., Pober, J.S., and Altieri, D.C. (2000b). Control of apoptosis during
angiogenesis by survivin expression in endothelial cells. Am J Pathol 156,
393-398.
Cell Death Differ 6, 303-313.
Pidgeon, G.P., Kandouz, M., Meram, A., and Honn, K.V. (2002). Mechanisms
controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase
inhibition in prostate cancer cells. Cancer Res 62, 2721-2727.
Psahoulia, F.H., Drosopoulos, K.G., Doubravska, L., Andera, L., and Pintzas, A.
(2007). Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by
inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther 6,
2591-2599.
Ramanathan, R.K., Clark, J.W., Kemeny, N.E., Lenz, H.J., Gococo, K.O., Haller,
D.G., Mitchell, E.P., and Kardinal, C.G. (2003). Safety and toxicity analysis of
oxaliplatin combined with fluorouracil or as a single agent in patients with
previously treated advanced colorectal cancer. J Clin Oncol 21, 2904-2911.
Rathkopf, D., Dickson, M.A., Feldman, D.R., Carvajal, R.D., Shah, M.A., Wu, N.,
Lefkowitz, R., Gonen, M., Cane, L.M., Dials, H.J., et al. (2009). Phase I study of
flavopiridol with oxaliplatin and fluorouracil/leucovorin in advanced solid
tumors. Clin Cancer Res 15, 7405-7411.
Riedl, S.J., and Shi, Y. (2004). Molecular mechanisms of caspase regulation during
apoptosis. Nat Rev Mol Cell Biol 5, 897-907.
and Tsushida, T. (2007). Baicalein, a flavonoid extracted from a methanolic
extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro
via induction of apoptosis. Pharmazie 62, 149-153.
Sakahira, H., Enari, M., and Nagata, S. (1998). Cleavage of CAD inhibitor in CAD
activation and DNA degradation during apoptosis. Nature 391, 96-99.
Salvesen, G.S., and Duckett, C.S. (2002). IAP proteins: blocking the road to death's
door. Nat Rev Mol Cell Biol 3, 401-410.
Salz, W., Eisenberg, D., Plescia, J., Garlick, D.S., Weiss, R.M., Wu, X.R., Sun, T.T.,
and Altieri, D.C. (2005). A survivin gene signature predicts aggressive tumor
behavior. Cancer Res 65, 3531-3534.
Satoh, K., Kaneko, K., Hirota, M., Masamune, A., Satoh, A., and Shimosegawa, T.
(2001). Tumor necrosis factor-related apoptosis-inducing ligand and its receptor
expression and the pathway of apoptosis in human pancreatic cancer. Pancreas
23, 251-258.
Savill, J., and Fadok, V. (2000). Corpse clearance defines the meaning of cell death.
Nature 407, 784-788.
Scaffidi, C., Fulda, S., Srinivasan, A., Friesen, C., Li, F., Tomaselli, K.J., Debatin,
K.M., Krammer, P.H., and Peter, M.E. (1998). Two CD95 (APO-1/Fas)
Scalbert, A., and Williamson, G. (2000). Dietary intake and bioavailability of
polyphenols. J Nutr 130, 2073S-2085S.
Schlette, E.J., Medeiros, L.J., Goy, A., Lai, R., and Rassidakis, G.Z. (2004). Survivin
expression predicts poorer prognosis in anaplastic large-cell lymphoma. J Clin
Oncol 22, 1682-1688.
Schwartz, G.K., and Shah, M.A. (2005). Targeting the cell cycle: a new approach to
cancer therapy. J Clin Oncol 23, 9408-9421.
Shapiro, G.I. (2006). Cyclin-dependent kinase pathways as targets for cancer
treatment. J Clin Oncol 24, 1770-1783.
Shariat, S.F., Ashfaq, R., Karakiewicz, P.I., Saeedi, O., Sagalowsky, A.I., and Lotan,
Y. (2007). Survivin expression is associated with bladder cancer presence, stage,
progression, and mortality. Cancer 109, 1106-1113.
Shariat, S.F., Karakiewicz, P.I., Godoy, G., Karam, J.A., Ashfaq, R., Fradet, Y.,
Isbarn, H., Montorsi, F., Jeldres, C., Bastian, P.J., et al. (2009). Survivin as a
prognostic marker for urothelial carcinoma of the bladder: a multicenter external
validation study. Clin Cancer Res 15, 7012-7019.
Shi, Y. (2002). Mechanisms of caspase activation and inhibition during apoptosis.
Mol Cell 9, 459-470.
Y.K., and Oh, B.H. (2001). An anti-apoptotic protein human survivin is a direct
inhibitor of caspase-3 and -7. Biochemistry 40, 1117-1123.
Siegelin, M.D., Reuss, D.E., Habel, A., Herold-Mende, C., and von Deimling, A.
(2008). The flavonoid kaempferol sensitizes human glioma cells to
TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol Cancer
Ther 7, 3566-3574.
Siegelin, M.D., Reuss, D.E., Habel, A., Rami, A., and von Deimling, A. (2009).
Quercetin promotes degradation of survivin and thereby enhances
death-receptor-mediated apoptosis in glioma cells. Neuro Oncol 11, 122-131.
Spencer, J.P., Rice-Evans, C., and Williams, R.J. (2003). Modulation of pro-survival
Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo
metabolites underlie their action on neuronal viability. J Biol Chem 278,
34783-34793.
Srinivasula, S.M., Datta, P., Fan, X.J., Fernandes-Alnemri, T., Huang, Z., and
Alnemri, E.S. (2000). Molecular determinants of the caspase-promoting activity
of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem 275,
36152-36157.
Suh, J., and Rabson, A.B. (2004). NF-kappaB activation in human prostate cancer:
Tamm, I., Wang, Y., Sausville, E., Scudiero, D.A., Vigna, N., Oltersdorf, T., and
Reed, J.C. (1998). IAP-family protein survivin inhibits caspase activity and
apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer
Res 58, 5315-5320.
Tanaka, K., Iwamoto, S., Gon, G., Nohara, T., Iwamoto, M., and Tanigawa, N.
(2000). Expression of survivin and its relationship to loss of apoptosis in breast
carcinomas. Clin Cancer Res 6, 127-134.
Tong, W.G., Ding, X.Z., Witt, R.C., and Adrian, T.E. (2002). Lipoxygenase
inhibitors attenuate growth of human pancreatic cancer xenografts and induce
apoptosis through the mitochondrial pathway. Mol Cancer Ther 1, 929-935.
Tran, J., Master, Z., Yu, J.L., Rak, J., Dumont, D.J., and Kerbel, R.S. (2002). A role
for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc
Natl Acad Sci U S A 99, 4349-4354.
Tran, J., Rak, J., Sheehan, C., Saibil, S.D., LaCasse, E., Korneluk, R.G., and Kerbel,
R.S. (1999). Marked induction of the IAP family antiapoptotic proteins survivin
and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res
Commun 264, 781-788.
Tu, S.P., Jiang, X.H., Lin, M.C., Cui, J.T., Yang, Y., Lum, C.T., Zou, B., Zhu, Y.B.,
inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res 63,
7724-7732.
Vagnarelli, P., and Earnshaw, W.C. (2004). Chromosomal passengers: the
four-dimensional regulation of mitotic events. Chromosoma 113, 211-222.
Verdecia, M.A., Huang, H., Dutil, E., Kaiser, D.A., Hunter, T., and Noel, J.P. (2000).
Structure of the human anti-apoptotic protein survivin reveals a dimeric
arrangement. Nat Struct Biol 7, 602-608.
Walczak, H., and Krammer, P.H. (2000). The CD95 (APO-1/Fas) and the TRAIL
(APO-2L) apoptosis systems. Exp Cell Res 256, 58-66.
Wall, N.R., O'Connor, D.S., Plescia, J., Pommier, Y., and Altieri, D.C. (2003).
Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances
tumor cell apoptosis. Cancer Res 63, 230-235.
Wang, C.Z., Li, X.L., Wang, Q.F., Mehendale, S.R., and Yuan, C.S. (2009). Selective
fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7
human breast cancer cells. Phytomedicine 17, 63-68.
Wang, I.K., Lin-Shiau, S.Y., and Lin, J.K. (1999). Induction of apoptosis by apigenin
and related flavonoids through cytochrome c release and activation of caspase-9
and caspase-3 in leukaemia HL-60 cells. Eur J Cancer 35, 1517-1525.
Saio, M., Moriwaki, H., and Takami, T. (2005). Expression of survivin and of
antigen detected by a novel monoclonal antibody, T332, is associated with
outcome of diffuse large B-cell lymphoma and its subtypes. Pathol Int 55,
324-330.
Wheatley, S.P., Carvalho, A., Vagnarelli, P., and Earnshaw, W.C. (2001). INCENP is
required for proper targeting of Survivin to the centromeres and the anaphase
spindle during mitosis. Curr Biol 11, 886-890.
Yang, G.Y., Liao, J., Kim, K., Yurkow, E.J., and Yang, C.S. (1998). Inhibition of
growth and induction of apoptosis in human cancer cell lines by tea polyphenols.
Carcinogenesis 19, 611-616.
Yin, F., Giuliano, A.E., Law, R.E., and Van Herle, A.J. (2001). Apigenin inhibits
growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK
MAP kinase activation in breast carcinoma cells. Anticancer Res 21, 413-420.
Zhang, T., Otevrel, T., Gao, Z., Ehrlich, S.M., Fields, J.Z., and Boman, B.M. (2001).
Evidence that APC regulates survivin expression: a possible mechanism
contributing to the stem cell origin of colon cancer. Cancer Res 61, 8664-8667.
Zhao, J., Tenev, T., Martins, L.M., Downward, J., and Lemoine, N.R. (2000). The
ubiquitin-proteasome pathway regulates survivin degradation in a cell