行政院國家科學委員會專題研究計畫 成果報告
台灣地區 B 型肝炎病毒基因型 B/C 雜交病毒株之盛行率和臨
床重要性
計畫類別: 個別型計畫 計畫編號: NSC92-2314-B-002-135- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學醫學院臨床醫學研究所 計畫主持人: 高嘉宏 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 10 月 11 日
Prevalence and Clinical Significance of HBV Genotype B/C
Recombinant in Taiwan
Jia-Horng Kao
Hepatitis Research Center, Graduate Institute of Clinical Medicine, Division of Gastroenterology, Department of Internal Medicine, Department of Medical Research, National Taiwan University College of Medicine and National Taiwan University Hospital, Taipei, TAIWAN
Grant support: The study was supported by grants from the National Science
Council, Executive Yuan, Taiwan
Correspondence:
Jia-Horng Kao, M.D., Ph.D. Hepatitis Research Center
National Taiwan University Hospital 7 Chung-Shan South Road, Taipei 100 TAIWAN
Tel.: 886-2-23123456 ext. 7307 Fax: 886-2-23317624
Abstract
Hepatitis B virus (HBV) infection is a major health problem in Taiwan. Currently, 4 subtypes and 8 genotypes of HBV are identified worldwide, and most of them have distinct geographic distributions. The impact of HBV genotypes on the clinical outcome of chronic HBV infection in Taiwan has been partially clarified. Our recent data showed that subtypes adw and adr or genotypes B and C are the predominant HBV strains in Taiwan. In addition, all adr strains are genotype C whereas 81% and 12% of the adw strains are genotype B and genotype C, respectively. Clinically, genotype C is associated with more severe liver disease including cirrhosis and hepatocellular carcinoma (HCC) whereas genotype B is associated the development of HCC in young non-cirrhotic patients. Serologically, genotype C tends to have a higher frequency of hepatitis B e antigen (HBeAg) positivity and a higher serum HBV DNA level than genotype B. Virologically, genotype C bears a higher frequency of core promoter mutation than genotype B. Although superinfection of HBV on top of hepatitis B carriers indeed occurs in Taiwan, it is rarely associated with acute exacerbations. As to the response to antiviral treatments, genotype C is associated with a lower response rate to interferon therapy as compared to genotypes B. Although pathogenic and therapeutic differences do exist among HBV genotypes in Taiwan, the relationship between HBV genotypes and clinical phenotypes of patients with chronic hepatitis B as well as the molecular virological mechanisms contributing these clinical differences, especially hepatocarcinogenesis, deserve further exploration. In addition, although the recombination between genotypes B and C has been reported, its clinical relevance remains largely unknown. In this study, we therefore investigated the prevalence of HBV genotype B/C
recombinant and its association with clinical phenotype of chronic hepatitis B patients as well as progression of liver disease in Taiwan. Our results showed that none of our HBV/B carriers were infected with HBV/Bj, and HBV/Ba accounted for all of the HBV/B strains in Taiwan, irrespective of the severity of liver disease. Therefore the discrepancy in genotype predominance between HCC patients in Japan and Taiwan, particularly at a younger age, remains to be elucidated.
Introduction
Hepatitis B virus (HBV) infection is a global health problem. About 2 billion people in the world have been infected by HBV, 350 million of whom are chronic carriers of the virus [1]. The infection can cause acute and chronic liver disease including cirrhosis and hepatocellular carcinoma (HCC) [1, 2]. The natural history can be divided into three phases based on virus-host interactions [2, 3]. In the first immune tolerance phase, patients are hepatitis B e antigen (HBeAg) positive and have high serum HBV DNA levels, but have no symptoms, normal serum aminotransferase levels and minimal histological activities. During the second immune clearance phase, a proportion of previously symptomless HBV carriers start to have bouts of symptoms and signs suggestive of acute hepatitis, i.e., the so-called “acute exacerbations”, when previous immune tolerance no longer exists. In the third low-replicative or integration phase, serum hepatitis B surface antigen (HBsAg) persists, but HBeAg is no longer detectable and patients are usually asymptomatic and liver disease is inactive. It is known that early seroconversion from HBeAg to anti-HBe in the natural course of chronic HBV infection usually indicates a favorable outcome, because it is usually associated with the cessation of virus replication and non-progressive liver disease [2, 3]. In contrast, late seroconversion of HBeAg after multiple bouts of reactivation and remission may accelerate the progression of chronic hepatitis to liver cirrhosis and thus have a poor clinical outcome [4-6]. Thus the final outcome of chronic HBV infection depends on the frequency and severity of acute exacerbation in the second immune clearance phase, i.e. the more frequent and severe acute exacerbation in this phase, the more chance to develop liver cirrhosis and even HCC in later life.
Having only 3,200 base pairs in its genome, HBV is the smallest known DNA virus [1]. The partially double-stranded circular HBV DNA consists of four overlapping genes encoding the viral envelope (pre-S and S), nucleocapsid (precore and core), polymerase with error prone reverse transcriptase activity, and X protein. Because of the spontaneous error rate of viral reverse transcriptase, HBV genome evolves with an estimated rate of nucleotide substitution at 1.4-3.2x10-5/site/year [7]. After a long-time evolution, currently 4 major HBV serological subtypes (adw, ayw, adr and ayr) or nine minor subtypes are identified by the antigenic determinants of HBsAg and 8 HBV genotypes (A to H) are defined by divergence in the entire HBV genomic sequence > 8% [8-10].The interrelation of subtypes to genotypes has been clarified [8]. In general, genomes encoding adw are found in genotypes A, B, C, F, and G, while the genomes encoding both adr and ayr occurs in genotype C alongside with adw. Most of the HBV genotypes have distinct geographic distributions. In brief, genotypes B and C are prevalent in Asia, whereas genotypes A and D prevail in Western countries and India. Genotype E is restricted to Africa, and genotype F in Central and South America. Genotype G has been recently identified in France and North America [8, 9].
Taiwan is an area endemic for HBV infection. Previous epidemiological survey indicated the carrier rate of HBsAg in the general population of Taiwan was as high as 15% to 20% [11], which is one of the highest in the world. Since chronic HBV infection will result in chronic hepatitis, cirrhosis, and HCC in the carriers, the liver cancer is therefore very common in Taiwan [12]. Actually, HCC has ranked first for cancer mortality in men and second in women since the early 1980s. In addition, chronic liver diseases rank sixth
among causes of death. In about half of the Taiwanese chronic HBsAg carriers, the infection is attributed to perinatal transmission of the virus from mothers to infants [13]. Fortunately, a mass immunization program against hepatitis B has been launched in Taiwan since 1 July 1984 [14], and the efficacy of universal immunization has been shown, with dramatic reductions of the prevalence of HBsAg carriage in children and adolescents [15, 16]. In addition, hepatitis B vaccination can protect children against HCC and fulminant hepatitis [17-20]. Nevertheless, there are still many carriers (~2.4 million) left in our population. And thus, the focus of hepatitis B research in the new century will be the search of factors, host or virus, that determine the clinical outcomes of patients with chronic HBV infection and the development of more effective therapies that can be applied to all hepatitis B carriers.
The clinical relevance of HBV genotypes in Taiwan remains unknown until very recently. Our previous data showed that subtypes adw and adr or genotypes B and C are the predominant HBV strains in Taiwan [21]. In addition, all adr strains are genotype C whereas 81% and 12% of the adw strains are genotype B and genotype C, respectively. Clinically, genotype C is associated with more severe liver disease including cirrhosis and HCC whereas genotype B is associated the development of HCC in young non-cirrhotic patients [22]. Serologically, genotype C tends to have a higher frequency of HBeAg positivity and a higher serum HBV DNA level than genotype B [23]. Virologically, genotype C bears a higher frequency of core promoter mutation than genotype B [24]. Although superinfection of HBV on top of hepatitis B carriers indeed occurs in Taiwan, it is rarely associated with acute exacerbations [25]. As to the response to antiviral treatments, genotype
C is associated with a lower response rate to interferon therapy as compared to genotypes B [24]. In addition, genotype B seems to have a better virological response to lamivudine as compared to genotype C but both genotypes have a similar risk in developing lamivudine resistance [26].
One of our major findings that HBV genotype C is associated with a poor response to IFN therapy and the development of HCC has been confirmed by subsequent studies from Japan and China [27-30]. Wai et al. studied 73 patients received IFN and 34 received no treatment (controls). Antiviral response was achieved in 39% and 17% of IFN-alpha-treated patients (P = 0.03) and in 10% and 8% of untreated controls (P = 0.88) with HBV genotype B and C, respectively [29]. Furhter multivariate analysis identified HBV genotype B, elevated pretreatment ALT levels, and low pretreatment HBV-DNA levels but not IFN treatment as independent factors associated with antiviral response. In the meantime, Orito et al. performed a large-scale survey of the geographic distribution of HBV genotypes in Japan and investigated the clinical characteristics among the patients with different genotypes [28]. They found that genotypes C and B are predominant in Japan, and there are significant differences in the geographic distribution. Compared with genotype C patients, genotype B patients were older, had a lower rate of positive HBeAg and a lower serum HBV DNA level. The number of patients with liver cirrhosis or HCC increased with age in the patients with genotype C, indicating genotype C is also closely associated with the development of HCC in Japan. However, none of their HCC patients younger than 35 years had genotype B. Similarly, Ding et al. have shown genotype C is associated with the development of HCC, while genotype B has a relatively good prognosis in China [27]. In summary, their data indicated genotype B is rarely
associated with the development of HCC in Japan and China. On the contrary, more than 50% of HBV-related HCC patients in Taiwan are infected with HBV genotype B [22]. Accordingly, The genotype B strains in Taiwan are somewhat different from those in Japan and China, and are proposed to divide into three subtypes based on the rate of liver disease progression [31]. The first is the slowly progressive subtype that is associated with a tendency for early disappearance of HBeAg during a carrier's lifetime or in the course of chronic hepatitis and subsequently leads to the low death rate from HCC as has been observed in Okinawa of Japan [32]. The second is the rapidly progressive subtype that is associated with the development of HCC in young hepatitis B carriers before their fourth decade even in the absence of cirrhosis, suggesting that an oncogenic potential of certain particular HBV strains may exist as is in the case of HCC in woodchucks. Chronic HBV infection in woodchucks never results in cirrhosis but contains viral DNA with preferential integration sites that activate myc family genes [33]. The third is the intermediately progressive subtype that runs a typical natural course of chronic HBV infection with the development of HCC usually in their sixth decade, as does genotype C. However, further large studies are awaited to prove or disprove these speculations. Of particular note is that Sumi et al. recently indicated that, although the patients with genotype B experience earlier HBe seroconversion, slower progression of liver fibrosis, and slower development of HCC, the life-long risk of progression to advanced fibrosis and development of HCC may not differ among genotypes B- and C-related chronic liver disease [30].
Similar situation has been observed in other HBV genotypes. Sanchez-Tapias et al. studied 258 Spanish patients with chronic hepatitis B
infected with different HBV genotypes, mostly genotype A, D and F [34]. They found that concomitant sustained biochemical remission and clearance of HBV DNA occurred at a higher rate in genotype A- than in genotype D- (log-rank, 14.2; P = 0.002) or genotype F-infected patients (log-rank, 4.2; P = 0.03). The rate of HBsAg clearance was higher in genotype A than in genotype D hepatitis (log-rank, 4.6; P = 0.03). Sustained remission and clearance of HBsAg were associated with infection with genotype A by Cox regression analysis. Seroconversion to anti-HBe was unrelated to HBV genotype, but the rate of sustained remission after seroconversion was higher in genotype A than in genotype D hepatitis both in patients who seroconverted to anti-HBe during follow-up (log-rank, 4.5; P = 0.03) and in patients with positive anti-HBe at baseline (log-rank, 6.66; P = 0.009). Death related to liver disease was more frequent in genotype F than in genotype A (P = 0.02) or genotype D (P = 0.002) hepatitis. Thus the long-term outcome of chronic hepatitis B was also is different in patients infected with HBV genotype A, D, or F.
The molecular virological mechanisms related to the different clinical outcomes among HBV genotypes remains largely unknown. Previous studies have indicated that several HBV variants display alteration of epitopes important in the host immune recognition, enhanced virulence with increased replication of HBV, resistance to antiviral therapies or facilitated cell attachment/penetration [35]. Concerning the genomic variability of human viruses, homologous recombination as a new source of variation has been documented in HIV [36], HBV [37], and HDV [38]. Recombination during reverse transcription of the pregenomic RNA, a process taking place simultaneously with encapsidation, appears unlikely because it would require two pregenomic RNA molecules to be encapsidated, and such
phenomena have not been observed in studies of HBV particle sedimentation rates. It seems more likely that recombination would take place in the nucleus where co-existing covalently closed circular DNA copies derived from different genotypes could exchange genomic segments. Regardless of mechanism, recombination requires co-infection with different genotypes and this has just been documented in our recent study [25]. One case of recombination between different HBV genotypes has also been reported [39], and a recent study by using phylogenetic analysis to examine the evolutionary relationships among 99 complete HBV sequences revealed that 9 viral genomes of them cluster with different genotypes depending on genome region analyzed [40]. This discordance suggests that recombination events could occur during HBV history. Thus co-infection may be more frequent than previously thought, but it is rarely detected because infection with a second strain is suppressed or merely cannot be established due to the very high virus load of the first strain. In such cases, co-infection may become visible only when the first strain disappears. Of 9 mosaic viral genomes as reported by Morozov et al. [40], 6 representing B/C hybrids were isolated in East Asia and three A/D hybrids in Italy. At least some recombinant strains appear to be fully viable and possess high evolutionary potential. As a result, B/C recombinants are overspreading through the East Asia region. They have been found among the isolates from Japan, China and Indonesia. Accordingly, recombination is a significant and relatively frequent event in the evolution of HBV genome. The putative breakpoints between genomes of different genotypes have also been mapped [40]. Eight out of 9 intertype genomes reported contained the breakpoints, which lie in the vicinity of DR1 and encapsidation signal of the HBV pregenome. Hino et al. found that the
fragment of HBV DNA covering nucleotides 1855–1915 is indispensable for enhancement of in vitro recombination [41]. Pineau et al. reported that the region encompassing nucleotides 1600–2000 reaches a recombination site density almost five-fold higher than the remaining part of the genome [42].
Recently, Sugauchi et al. compared the entire nucleotide sequences of 70 HBV isolates of genotype B (HBV/B) phylogenetically, and 2 subgroups of HBV/B could be identified based on sequence divergence in the precore region plus the core gene, one with the recombination with genotype C and the other without it [43]. Due to the frequency of the distribution of HBV genotype B without the recombination (91%) and the fact that it was exclusive to Japan, they provisionally classified HBV genotype B into the Bj (j for Japan) subgroup, and HBV genotype B with the recombination was classified into the Ba (a for Asia) subgroup. They therefore suggested that the virological differences between HBV genotype Bj and Ba may be reflected in the severity of clinical disease in the patients infected with HBV genotype B, which seems to be under strong geographic influences in Asia. However, the frequency and mechanism of such recombination as well as the possible implications of recombination in the natural history of HBV and clinically important properties such as whether recombinant viruses differ in their pathogenicity need further studies.
Taking advantage of common HBV genotype B and C infection in Taiwan, we therefore investigated the prevalence of HBV genotype B/C recombinant and its association with clinical phenotype of chronic hepatitis B patients as well as progression of liver disease.
Materials and Methods
Patients
Serum samples were obtained from 150 chronic HBV/B carriers undergoing long-term follow-up at the gastroenterological clinics of the National Taiwan University Hospital. These included 50 inactive carriers of hepatitis B surface antigen (HBsAg) who had persistently normal serum alanine aminotransferase levels for at least 3 years in their periodic biochemical examinations (every 3–6 months), 50 histologically verified HBsAg-positive patients with chronic hepatitis, and 50 HBsAg-positive HCC patients. Diagnosis of HCC was based on biochemical, radiological, and histological findings.
Methods
The recombination between different HBV genotypes can be detected when different genes, or different intergenic regions from a single genome, generate incongruent phylogenetic trees [39, 43]. Thus the conserved surface gene and the most frequent recombinant region encompassing nucleotides 1600–2000 among HBV genotypes B and C are chosen for sequence determination and subsequent phylogenetic tree analysis in the present study [42, 43].
Genotyping of HBV
HBV genotypes will be determined by using the polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) of the surface gene of HBV as previously described [44].
Amplification and sequencing of surface gene and core/precore/core promoter gene encompassing nucleotides 1600–2000
containing 2 l of the cDNA sample, 1x PCR buffer (10 mM tris -HCl pH 9.0, 50mM KCl, 1.5 mM MgCl2, 0.01% gelatin and 0.1% Triton X-100), 10 mM of each dNTP, 100 ng of each outer primer (outer sense: S1s 5'- AGAACATCGCATCAGGACTC -3', positions 159 - 178; outer antisense: S2a 5’- CATAGGTATCTTGCGAAAGC -3’, positions 642 - 623) and 1 unit of Taq DNA polymerase is amplified in a thermal cycler (Perkin-Elmer Cetus, Norwalk, CT) for 30 cycles. After the first amplification, 1 l of the PCR products is reamplified for another 30 cycles with 100 ng of each inner primer (inner sense: S3s 5'- AGGACCCCTGCTCGTGTTAC -3', positions 181 - 200; inner antisense: S4a 5'- AGATGATGGGATGGGAATAC -3', positions 619 – 600).
2. Core/precore/core promoter gene: For the first stage PCR, a 25 l of reaction mixture containing 2 l of the cDNA sample, 1x PCR buffer (10 mM tris-HCl pH 9.0, 50mM KCl, 1.5 mM MgCl2, 0.01% gelatin and 0.1% Triton X-100), 10 mM of each dNTP, 100 ng of each outer primer (outer sense: P1s 5'-CAGACGGTCTGGAGCAAACC-3', positions 1302-1321; outer antisense: P2a 5’-ATACTAACATTGACATTCCC-3’, positions 2455 - 2436) and 1 unit of Taq DNA polymerase is amplified in a thermal cycler (Perkin-Elmer Cetus, Norwalk, CT) for 30 cycles. After the first amplification, 1 l of the PCR products is reamplified for another 30 cycles with 100 ng of each inner primer (inner sense: P3s 5'-CTCATCTGCCGGACCGTGTG-3', positions 1562–1581; inner antisense: P4a 5'-CAATGCTCAGGAGACTCTAAGGC-3', positions 2043–2021).
For both PCR reactions, each cycle entails denaturation at 95 oC for 60 s, primer annealing at 55 oC for 30 s and extension at 72 oC for 60 s with a final extension step at 72°C for 7 min. Nucleotide sequences of amplified products
will be directly determined by using fluorescence labelled primers with a 377 Sequencer (Applied Biosystems, Foster City, CA). Sequencing conditions are specified in the protocol for the Taq DyeDeoxy Terminator Cycle Sequencing Kit (Applied Biosystems). The inner primer pairs are used as sequencing primers for both directions of the amplicons.
Phylogenetic analysis
A phylogenetic tree will be constructed by using the program of unweighted pair-group method with arithmetic mean (UPGMA) method (PHYLIP [Phylogeny Inference Package], version 3.5c; J. Felstein, University of Washington, Seattle, USA) based on the nucleotide sequences of surface gene or core/precore/core promoter region encompassing nucleotides 1600–2000. The prototype genotypes B and C sequences will be used as references.
Statistical Analysis
Data will be analyzed by Fisher's exact test, Chi-square test with Yates’ correction where appropriate. Logistic regression analysis was used to assess the likelihood of HBV genotype B/C recombinant in different clinical stages of chronic HBV infection.
Results and Discussion
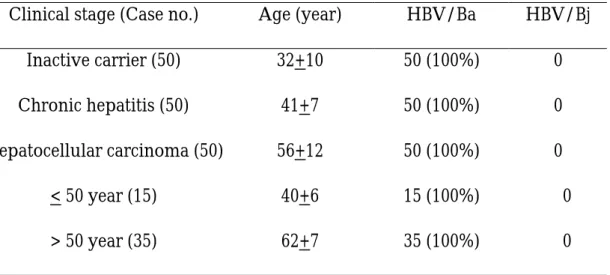
In agreement with a previous report based on a limited number of cases
from Taiwan [43], none of our HBV/B carriers were infected with HBV/Bj.
Our results showed that HBV/Ba accounts for all of the HBV/B strains in
indicated that the prevalence of HBV/Ba was comparable between younger
(< 50 years) and older (> 50 years) HCC patients. Taken together, the clinical
and virological differences between genotype Ba and Bj as indicated by
Sugauchi et al. [43] cannot explain the variable clinical outcomes of patients
with HBV/B infection, at least in Taiwan.
In conclusion, the discrepancy in genotype predominance between HCC
patients in Japan and Taiwan, particularly at a younger age, remains to be
elucidated. The possibility of certain HBV/B strains having higher oncogenic
References
1. Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infectious Dis 2002;2:395-403.
2. Chen DS. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science 262:369-370;1993.
3. Chu CM. Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol 15 (suppl):E25-30;2000.
4. Liaw YF, Tai DI, Chu CM, Pao CC, Chen TJ. Acute exacerbation in chronic type B hepatitis: comparison between HBeAg and antibody-positive patients. Hepatology 7:20-23;1987.
5. Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol 10:29-34;1990.
6. Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 120:1009-1022;2001.
7. Okamoto H, Imai M, Kametani M, Nakamura T, Mayumi M. Genomic heterogeneity of hepatitis B virus in a 54 year old woman who contracted the infection through materno-fetal transmission. Japan J Exp Med 57:231-236;1987.
8. Magnius LO, Norder H. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology 38:24-34;1995.
9. Stuyver L, De Gendt S, Van Geyt C, Zoulim F, Fried M, Schinazi RF, Rossau R. A new genotype of hepatitis B virus: complete genome and
phylogenetic relatedness. J Gen Virol 81:67-74;2000.
10. Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol 69:2575-2583;1988.
11. Chen DS, Sung JL. Hepatitis B virus infection and chronic liver diseases in Taiwan. Acta Hepatogastroenterol 25:423-430;1978.
12. Beasley RP. Hepatitis B virus, the major etiology of hepatocellular carcinoma. Cancer 61:1942-1956;1988.
13. Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 292:771-774;1975.
14. Chen DS, Hsu NH, Sung JL, Hsu TC, Hsu ST, Kuo YT, Lo KJ, Shih YT. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen carrier mothers. JAMA 257:2597-2603;1987.
15. Hsu HM, Lu CF, Lee SC, Lin SR, Chen DS. Seroepidemiologic survey for hepatitis B virus infection in Taiwan: the effect of hepatitis B mass immunization. J Infect Dis 179:367-370;1999.
16. Ni YH, Chang MH, Huang LM, Chen HL, Hsu HY, Chiu TY, Tsai KS, Chen DS. Hepatitis B Virus Infection in Children and Adolescents in a Hyperendemic Area: 15 Years after Mass Hepatitis B Vaccination. Ann Intern Med 135:796-800;2001.
17. Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 336:1855-1859;1997.
18. Chang MH, Shau WY, Chen CJ, Wu TC, Kong MS, Liang DC, Hsu HM, Chen HL, Hsu HY, Chen DS. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA 284:3040-3042;2000.
19. Kao JH, Chen DS. Recent updates in hepatitis vaccination and the prevention of hepatocellular carcinoma. Int J Cancer (in press).
20. Kao JH, Hsu HM, Shau WY, Chang MH, Chen DS. Universal hepatitis B vaccination and the decreased mortality from fulminant hepatitis in infants in Taiwan. J Pediatr 139:349-352;2001.
21. Liu CJ, Kao JH, Chen PJ, Lai MY, Chen DS. Molecular epidemiology of hepatitis B viral serotypes and genotypes in Taiwan. J Biomed Sci (in press).
22. Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 118:554-559;2000.
23. Kao JH, Chen PJ, Lai MY, Chen DS. Clinical and virological aspects of hepatitis B genotypes B and C-infected blood donors. J Clin Microbiol (in press).
24. Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol 33:998-1002;2000.
25. Kao JH, Chen PJ, Lai MY, Chen DS. Acute exacerbations of chronic hepatitis B are rarely associated with superinfection of hepatitis B virus. Hepatology 34:817-823;2001.
26. Kao JH, Liu CJ, Chen DS. Hepatitis B viral genotypes and lamivudine resistance. J Hepatol (in press).
27. Ding X, Mizokami M, Yao G, Xu B, Orito E, Ueda R, Nakanishi M. Hepatitis B virus genotype distribution among chronic hepatitis B virus carriers in Shanghai, China. Intervirology 44:43-47;2001.
28. Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, Okita K, Okanoue T, Iino S, Tanaka E, Suzuki K, Watanabe H, Hige S, Mizokami M. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology 34:590-594;2001.
29. Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology 2002;36:1425-30.
30. Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology 2003;37:19-26. 31. Kao JH, Chen DS. Hepatitis B virus genotypes and hepatocellular
carcinoma in Japan-Reply. Gastroenterology 120:1564-1565;2001.
32. Sakugawa H. Correlation between hepatitis B virus infection and chronic liver disease in Okinawa (in Japanese). Kansenshogaku Zasshi 66:14-21;1992.
33. Roggendorf M, Tolle TK. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology 38:100-112;1995.
34. Sanchez-Tapias JM, Costa J, Mas A, Bruguera M, Rodes J. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology. 2002;123:1848-56.
35. Gunther S, Fischer L, Pult I, Sterneck M, Will H. Naturally occurring variants of hepatitis B virus. Adv. Virus Res 52:125-137;1999.Hunt CM,
McGill JM, Allen MI, Condreay LD. Clinical relevance of hepatitis B viral mutations. Hepatology 31:1037-1044;2000.
36. Malim MH, Emerman M. HIV-1 sequence variation: drift, shift, and attenuation. Cell. 2001;104:469-472.
37. Georgi-Geisberger, P., Berns, H., Loncarevic, I. F., Yu, Z. Y., Tang, Z. Y., Zentgraf, H. & Schroder, C. H. (1992). Mutations on free and integrated hepatitis B virus DNA in a hepatocellular carcinoma: footprints of homologous recombination.Oncology 49, 386-395.
38. Wu JC, Chiang TY, Shiue WK, Wang SY, Sheen IJ, Huang YH, Syu WJ. Recombination of hepatitis D virus RNA sequences and its implications. Mol Biol Evol 1999;16:1622-1632.
39. Bollyky, P. L., Rambaut, A., Harvey, P. H. & Holmes, E. C. Recombination between sequences of hepatitis B virus from different genotypes.Journal of Molecular Evolution 1996;42, 97-102.
40. Morozov V, Pisareva M, Groudinin M. Homologous recombination between different genotypes of hepatitis B virus. Gene 2000;260:55-65. 41. Hino O, Tabata S, Hotta Y. Evidence for increased in vitro recombination
with insertion of human hepatitis B virus DNA. Proc Natl Acad Sci U S A 1991;88:9248-9252.
42. Pineau P, Marchio A, Mattei MG, Kim WH, Youn JK, Tiollais P, Dejean A. Extensive analysis of duplicated-inverted hepatitis B virus integrations in human hepatocellular carcinoma. J Gen Virol 1998;79:591-600.
43. Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Ueda R, Miyakawa Y, Mizokami M. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J Virol. 2002;76:5985-92.
44. Mizokami M, Nakano T, Orito E, Tanaka Y, Sakugawa H, Mukaide M, Robertson BH. Hepatitis B virus genotype assignment using restriction fragment length polymorphism patterns. FEBS Lett 1999;450:66-71.
Table 1. Prevalence of HBV genotype Ba and Bj in different clinical stages
of patients with chronic HBV infection in Taiwan
Clinical stage (Case no.) Age (year) HBV/Ba HBV/Bj
Inactive carrier (50) 32+10 50 (100%) 0
Chronic hepatitis (50) 41+7 50 (100%) 0
Hepatocellular carcinoma (50) 56+12 50 (100%) 0
< 50 year (15) 40+6 15 (100%) 0
> 50 year (35) 62+7 35 (100%) 0
HBV/Ba (a for Asia), HBV genotype B with recombination with genotype C;