國
立
交
通
大
學
分子醫學與生物工程研究所
碩
士
論
文
幽門螺旋桿菌熱休克蛋白 60 N 端之活性
The activities for the N-terminal domain of H.
pylori heat shock protein 60
研 究 生:沈筱薇
指導教授:廖光文 教授
幽門螺旋桿菌熱休克蛋白 60 N 端之活性
The activities for the N-terminal domain of H.
pylori heat shock protein 60
研究生:沈筱薇 Student:Hsiao-Wei Shen 指導教授:廖光文 Advisor:Kuang-Wen Liao 國 立 交 通 大 學 分子醫學與生物工程研究所 碩 士 論 文 A Thesis
Submitted to Institute of Molecular Medicine and Bioengineering National Chiao Tung University
in partial Fulfillment of the Requirements for the Degree of
Master In
Molecular Medicine and Bioengineering June 2010
Hsinchu, Taiwan, Republic of China 中華民國九十九年六月
i 幽門螺旋桿菌熱休克蛋白 60 之 N 端的活性 研究生:沈筱薇 指導教授:廖光文 博士 國立交通大學分子醫學與生物工程研究所 中文摘要 幽門螺旋桿菌的感染與許多上消化道的疾病相關,像是慢性胃 炎、消化性潰瘍、黏膜相關淋巴組織淋巴癌 (MALT lymphoma)以 及胃癌。感染幽門螺旋桿菌患者若無以抗生素加以治療,將轉變為慢 性、持續性感染 ; 而上述疾病的發生則主要起因於該病原菌的慢性 感染。並且該菌所分泌的熱休克蛋白 60 已被證實為一黏附分子可連 結幽門螺旋桿菌以及人類胃上皮細胞,進而造成胃部疾病。 對於幽門螺旋桿菌熱休克蛋白 60 在免疫調節功能上,較多的研 究是指出此蛋白可引發前發炎激素(pro-inflammatory cytokine)例 如:干擾素- (IFN-)、腫瘤壞死因子-(TNF-)、白介素 6、8 (IL-6、8)而引起發炎反應。特別是在氨基酸序列 300 到 435 的位 置可引發大量 IL-8 的表現。在另一方面,近年來也有研究指出不同 物種的熱休克蛋白 60 似乎在人體免疫系統可以抵禦病原菌的慢性感 染。例如人類與日本血吸蟲的熱休克蛋白 60 可引發調節型 T 細胞 (regulatory T cells)的增生達到免疫抑制的效果。然而,幽門螺旋桿 菌熱休克蛋白 60 對於免疫調節的機制依然不清楚。在本篇實驗中,
ii
我 們 利 用 人 類 周 邊 血 液 單 核 球 細 胞 ( human peripheral blood mononuclear cells, PBMCs)作為研究系統,探討幽門螺旋桿菌熱休 克蛋白 60 之 N 端蛋白對其增生所造成之影響。研究結果顯示,幽門 螺旋桿菌熱休克蛋白 60 之 N 端位置於 101-200 和 1-200 之蛋白能夠 降低 PBMCs 之增生以及引發 TGF-之產生。根據上述研究結果,幽 門螺旋桿菌熱休克蛋白 60 可藉由接近 N 端的 101-200 之處引發 TGF-並且達到免疫抑制的效果。
iii
The activities for the N-terminal domain of H. pylori heat shock
protein 60
Student: Hsiao-Wei Shen Advisor: Dr. Kuang-Wen Liao Institute of Molecular Medicine and Bioengineering
National Chiao Tung University Abstract
Helicobacter pylori can lead to variety of upper gastrointestinal
disorders, such as chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue (MALT) lymphoma and gastric cancer. Without treatment, H. pylori would become chronic infection in almost all of those patients. The expression of heat shock protein 60 by H.
pylori (HpHSP60) has been shown as an adhesion molecule that interacts
with host gastric epithelial cells.
For immunoregulation, many literatures showed that HpHSP60 can induce the secretions of pro-inflammatory cytokines, such as IFN TNF-, IL- IL-and cause inflammation. Particularly, the sequence of HpHSP60 from 300 to 435 can induce dramatically IL-8 expression. Interestedly, researchers found that HSP60 in different species seems to be related to the regulation of immune responses in
iv
chronic infection disease. Literatures indicated that HSP60 in human and
S. japonicum can induce regulatory T cell (Treg) expression and suppress
immunity. However, the feature of HpHSP60 for immunoregulation still remains unknown. In this study, the N-terminal domains of HpHSP60 were constructed and measured their activities on immune response. The results showed that the treatment with the sequence 101-200 (HpHSP60101-200) or 1-200 (HpHSP601-200) of HpHSP60 to human
peripheral blood mononuclear cells (PBMCs) decreased the proliferation rate. Furthermore, HpHSP60101-200 and HpHSP601-200 could increase
TGF- secretion from THP-1 cells. Taken together, the sequence of HpHSP60 from 101 to 200 could induce the expression of TGF-and which may have contributions to their immune suppressive activity.
v 致謝 總認為論文最容易寫的部分就是致謝,但是當我要提筆開始寫致 謝時,我才發現好難下筆,原因就是太多人要感謝。 首先一定要感謝實驗室的大佬也就是我的指導教授廖光文博 士,謝謝老師收留當時剛從別的實驗室離開的我,以及教導我做人處 事的道理和做為一個研究人員該有的思維、態度。也感謝鄭添祿教 授、吳彰哲教授、蔡女滿教授抽空參加口試,在口試當中也學習到利 用不同角度和更嚴謹的方式看待科學實驗,學生受益良多。 還有感謝實驗室的伙伴,Hp 組的先鋒辰栖學姐,前輩們啾咪、 何姵,我最愛的小瞳瞳,自我感覺良好且愛拋媚眼的小美哥和感覺很 厲害的小蛙,謝謝你們的陪伴、適時地提供意見和認真討論 data。 當然也很感謝 LPPC 組的 DG,坐在我左邊兩年以及跟我一樣愛好廣 告的于鈴學姐,號稱交大吳彥祖的阿伯(交大無帥哥),正妹們小莉、 小溫,國文造詣很棒的馬馬,我的第二愛人靜敏,坐在我右邊笑點很 低的又又又又,操著一口台語腔的美惠和欣怡,感謝你們兩年的照顧 和度過這充滿喜怒歡樂的實驗室生活。無緣實驗室的朱大哥、辰哥、 周大頭、芳馨、瑤貞,謝謝你們給我 powerful 鼓勵和溫暖擁抱。 最後,謝謝我的家人:爸爸、媽媽、叔、嬸、涵、妮、鼎、庭睿、 友哥等人,謝謝你們的支持和陪伴。
vi
Contents
中文摘要……….i Abstract……… iii Contents……….vi Introduction………...1Materials and methods……….5
Preparation of recombinant HpHSP60………...5
The coupling of HpHSP60 and FITC……….6
The binding of proteins and cells………...7
Cloning and expression and purification of recombinant protein……..7
IL-8 secretion from cells……….8
Peripheral blood mononuclear cells (PBMCs) isolation………9
PBMC proliferation assay………10
Statisticalanalysis……….11
Results………..12
The binding activities of HpHSP60 to cells……….12
The N-terminal sequence of HpHSP60 for cell binding activity……..13
The functions of HpHSP60……….13-15 Discussion………16
vii
Figure and Legend………..20
viii
List of Figure and Legend
Figure and Legend
Figure 1 HpHSP60 could bind to THP-1 cells with different affinities..20 Figure 2 HpHSP60 could bind to AGS cells with different affinities….21 Figure 3 The binding intensities of HpHSP60 for LPS-activated THP-1 cells were dramatically increased than inactive THP-1 cells…………..22 Figure 4 Electrophoresis profile of EGFP-HpHSP60………...23 Figure 5 EGFP-HpHSP60 induced the secretion of pro-inflammatory cytokine IL-8………..24 Figure 6 EGFP interrupted LPS-activated THP-1 cells to bind to HpHSP60………....25 Figure 7 The different domains of HpHSP60……….26 Figure 8 The protein expressions of the different fragments…………..27 Figure 9 The IL-8 secretion of THP-1 cells after treating with HpHSP60 or different fragment proteins……….28 Figure 10 Treatment with HpHSP60 and different fragment proteins to PBMCs decreases the proliferation in the present of anti-CD3 antibody.29 Figure 11 The TGF-secretion of THP-1 cells after treating with HpHSP60 or different fragment proteins……….30
ix
List of Appendix
Appendix 1 The map of pET30-EGFP-HpHSP60………..31
Appendix 2 The map of pET30-HpHSP60 101-200………36
Appendix 3 The map of pET100-HpHSP60 1-200……….40
1
Introduction
Helicobacter pylori (H. pylori) have been shown to play a
pathogenic role in gastric diseases. It is now recognized as a cause of chronic gastritis, peptic ulcer, gastric cancer and MALT lymphoma (Algood and Cover, 2006). H. pylori Heat Shock Protein 60 (HpHSP60) has been first identified as an adhesion molecule that interacts with host gastric epithelial cells and mucin (Huesca et al., 1996). Moreover, HpHSP60 has been indicated that it can cause inflammation by inducing releases of pro-inflammatory cytokines (Matsuura et al., 2009). Recently, HpHSP60 for gastric tumor progression can promote the migration ability of cancer cells, increase in the angiogenic activity of endothelial cells, induction of pro-inflammatory cytokine in both gastric epithelial cells and monocytes. These results suggest that HpHSP60 is able to accelerate tumorigenesis by multiple mechanisms (Lin et al., 2010b).
HSP60s are conserved proteins, even if they from different species are highly similar for their protein sequences (Ellis, 1992). For example, the alignment of amino acid sequence between H. pylori, Pseudomonas
2
to 72% of similarity (Macchia et al., 1993). In addition, they also play the similar role in inducing pro-inflammatory cytokines. The HSP60 of
Mycobacterial tuberculosis was demonstrated to induce secretion of
pro-inflammatory cytokine, IL-6, IL-8and TNF-from human monocytes (Friedland et al., 1993). Chlamydia pneumoniae HSP60 can increase the level of IL-6 in the bronchialveolar lavage fluid to cause acute pulmonary inflammation via TLR4 in mice (Bulut et al., 2009). Bulut Y. and his colleagues have proved the Chlamydia trachomatis HSP60 could promote inflammation through TLR4-mediated NF-B pathway to result in the activation of macrophages and endothelial cells (Bulut et al., 2002). The expressions of IL-6 and TNF-for endothelial cells were also found that they can be induced by E. coli (Galdiero et al., 1997) or human HSP60 (Kol et al., 1999) (Vabulas et al., 2001). Recently,
Helicobacter pylori HSP60 has been shown that it can induce many
expressions of pro-inflammatory cytokines such as TNF-, IL-8, GRO, IFN- (Lin et al., 2009a). This effect could be resulted from the TLR2 or 4 pathway for human monocytes (Takenaka et al., 2004). Moreover, the IL-8 inductive activity of HpHSP60 has been explored by Lin and his colleagues, the effective domain is located between 300 and 435 a.a. (Lin
3
et al., 2005).
In addition to inflammation, HSP60 has been indicated recently that it has the capacity to regulate the host immunity. In 2006, Dr. Zanin-Zhorov, A. showed human HSP60 treatment can enhance the IL-10 and TGF- secretions of Treg via TLR2 to inhibit the expressions of IFN-and TNF- of CD4+CD25- T cells (Zanin-Zhorov et al., 2006). Similarly, Schistosoma japonicaum HSP60 can also increase the numbers of CD4CD25regulatory T cells to raise the expressions of IL-10 and TGF- via TLR2 pathway (Wang et al., 2009). In our published paper, HpHSP60 also show its potential to elevate the secretions of anti-inflammatory cytokine IL-10 and TGF-in human monocytes (Lin
et al., 2009b) Therefore, HpHSP60 may be a dual-function protein for inflammation and immune suppression. However, the effect of HpHSP60 on immunosuppression is still unclear.
Currently, the literatures showed that the numbers of Treg will be elevated in H. pylori-associated gastritis and gastric cancer (Jang, 2010). As our previous results shown, although HpHSP60 can induce strong
4
releases of proinflammatory cytokines, it also increases the secretions of IL-10 and TGF- for THP-1 cells (Lin et al., 2009a) (Lin et al., 2010a). In addition, HpHSP60 would cause the proliferative inhibition by interfering with the cell cycle but not inducing apoptosis in our unpublished results. As well-known, IL-10 and TGF- are important factors for the increase of Treg cell (Toms and Powrie, 2001) and the Treg cell’s activity is associated with the suppression of T-cell proliferation. Thus, we are interesting in which domain is involved in the inhibition of T-cell. Through the identification of this domain, it may not only find out the inhibitory domain for T-cell proliferation but also get the effective sequence for the increase in Treg cell.
5
Materials and methods
Preparation of recombinant HpHSP60
E. coli [BL21(DE3)] (Yeastern Biotech, Taipei, Taiwan) were
transformed with pET-HpHSP60 and grew on LB plate containing kanamycin (30mg/ml) at 37C for 16 hours. Then single colony was picked and inoculated in 100 ml LB medium containing kanamycin (30mg/ml). After 16 hours incubation at 37C, the bacteria in the LB broth were refreshed in 900 ml LB with vigorous shaking. Assayed the OD value until OD600 reaches 0.6~0.8, then protein induction was performed by adding 1.25 ml of IPTG (800mM). After 4 hours incubation, harvested the bacteria by centrifugation at 5000 rpm for 15 min at 4 C. Discarded the supernatant and resuspended the pellet with 30ml binding buffer (20 mM Na2HPO4, 0.5 M NaCl, 40 mM imidazole, pH 7.4). Total
lysates were sonicated for 15 min and then centrifuge at 12,000 rpm for 30 min to collect the supernatant which containing rHpHSP60. HisTrapHP column (General Electric, NY, USA) (1cm) was used to purify rHpHSP60. All the buffers and protein samples needed to be filtered with 0.45µm syringe filter. After protein sample loading into the column, washed the column with 30X volume of binding buffer to
6
remove the unwanted proteins and then eluted the rHpHSP60 with 10X volume of elution buffer (20 mM Na2HPO4, 0.5 M NaCl, 500 mM
imidazole, pH 7.4). Eluted rHpHSP60 were collected and loaded into G-25 column (General Electric, NY, USA) to remove the unnecessary salt and replace the buffer with PBS (Phosphate Buffer Saline, 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KHPO4, pH 7.4). Protein
concentration was quantified by Coomassie Plus reagent (BSA was used as the standard). SDS-PAGE and western blotting with anti-His conjugated HRP (ROCKLAND, PA, USA) were used to confirm the purity of rHpHSP60.
The coupling of HpHSP60 and FITC
First, 2mg HpHSP60 should be in NaHCO3 buffer, so the dialysis of
HpHSP60 was with 1L DDW in 4℃ for 1 hour. After 1 hr, the dialysis bag was placed in 1M NaHCO3 buffer (pH=9) for 2 hours and repeated
this step again. Then the container was changed new NaHCO3 buffer
overnight in cool room. 0.01g FITC (SIGMA, MO, USA) was solved in 10 ml DMSO and the solution was shaken in dark for 90 minutes. In
7
order to separate FITC-HpHSP60 and un-coupling FITC, the solution was loaded into sephadex G-25 column and FITC-HpHSP60 was first through the column. Protein concentration was quantified by Coomassie Plus reagent (BSA was used as the standard).
The binding of proteins and cells
After counting cells number (inactive or LPS-activated THP-1 cells or AGS cells), the cells were washed with staining buffer (0.5% skim milk in 1X PBS) by centrifuging at 4000 rpm for 5 minutes. The cells were re-suspended with staining buffer and treated with protein (FITC-HpHP60 or EGFP-HpHSP60) on ice for 30 minutes. Finishing incubation, proteins were not bound to the cells were washed by staining buffer. Then the pellet was re-suspended by 1X PBS. The intensity of fluorescence was detected by flow cytometry (Franklin Lakes, NJ, USA).
Cloning and expression and purification of recombinant protein
8
interesting DNA (EGFP or HpHSP601-200 or HpHSP60101-200 or
HpHSP601-500 or HpHSP60300-547 or HpHSP601-435) were amplified by
polymerase chain reaction and cloned into vectors (pET30-HpHSP60 or pET100). The proteins were expressed in Escherichia coli (BL21 strain) and purified using HisTrap affinity chromatography followed by sephadex G-25 column. The purity of rEGFP-pET-HpHSP60 was determined by SDS-PAGE, western blot assay (detected by HRP conjugated rabbit polyclonal antibody to polyhistidine) (or detected by mouse anti-HpHSP60 polyclonal antibody and HRP conjugated goat anti-mouse IgG antibody; Jackson ImmunoResearch, PA, USA).
IL-8 secretion from cells
THP-1 cells grown on 24 well plates were stimulated with 1g LPS, 10g HpHSP60, EGFP-HpHSP60, G or 5 g HpHSP60, HpHSP601-200,
HpHSP60101-200, HpHSP60300-547 at 37℃ in THP-1 growth medium
(RPMI 1640 with 4.5g/L glucose, 10mM HEPES, 0.05mM 2-mercaptoethanol and 1mM sodium pyruvate) for 24 hours. Supernatants and cells were separated by centrifugation at 1200rpm for 5
9
minutes. Supernatants were assayed for IL-8 production, and IL-8 was measured by IL-8 ELISA development kit. (R&D system, MN, USA).
Peripheral blood mononuclear cells (PBMCs) isolation
PBMCs were isolated from human whole blood by using Ficoll-PaquePlus. Dilute human whole blood with equal volume of PBS. Added 6 ml Ficoll-PaquePlus into the 15 ml centrifuge tube and loaded 8 ml of the diluted blood sample on Ficoll-Paque Plus reagent carefully. Then centrifuged the tubes at 400g for 40 min at 18C. After centrifuge, removed the plasma layer and collect the PBMC carefully between the plasma layer and Ficoll-PaquePlus solution. Washed the cell with 2X volume of PBS, centrifuged at 1,500 rpm for 15 min. Discarded the supernatant and added 10 ml of ACK lysis buffer (0.15M NH4Cl, 10 mM
KHCO3, 0.1 mM Na2EDTA) to lyse the red blood cells. Centrifuged at
1,500 rpm for 15 min to remove the supernatant and then washed the cell with 10ml PBS again. Counted the cell number and seeded the PBMCs into 96-well culture plate with RPMI1640 culture medium.
10
PBMC proliferation assay
To mimic the action occur when the immune cells encounter antigen, anti-CD3 antibody (kindly provided from Dr. Steve R. Roffler) (OKT3, an antibody that specifically binds to CD3 surface marker and transduces signals continually to activate T lymphocytes) was used to selectively activate T lymphocyte (1µg/ml, 30µl per well of 96-well culture plate, incubate in 37C for two hours). Fresh blood samples from healthy donors were obtained and the PBMCs were isolated by Ficoll-Pague reagent. Then seeded the cells into anti-CD3 mAb pre-coated 96-well plates (2*105/well, triplicate for each treatment) and treated with different
proteins of HpHsp60, HpHSP601-200, HpHSP60101-200, HpHSP60300-547 for
four days incubation. Cell proliferation was verified by MTT assay. In briefly, 96-well was centrifuged at 1,500 rpm for 15 min to remove the supernatant. Added 100 µl MTT solution into each well and incubated the plate at 37C for four hours. Then centrifuged again, removed the supernatant and dissolved the purple crystal with 100 µl DMSO each well. Shook the plate for 10 min then measured the OD value at 595nm. The proliferation index was calculated with following equation: proliferation
11
= (OD value of protein-treated PBMC) (OD value of PBMC treated without HpHsp60)*100%.
Statistical analysis
The results are presented as mean ± SD. Significant is calculated by
12
Results
The binding activities of HpHSP60 to cells
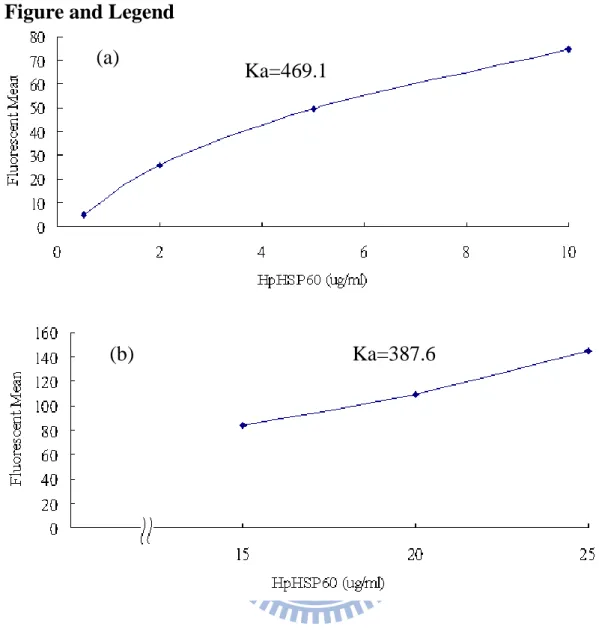
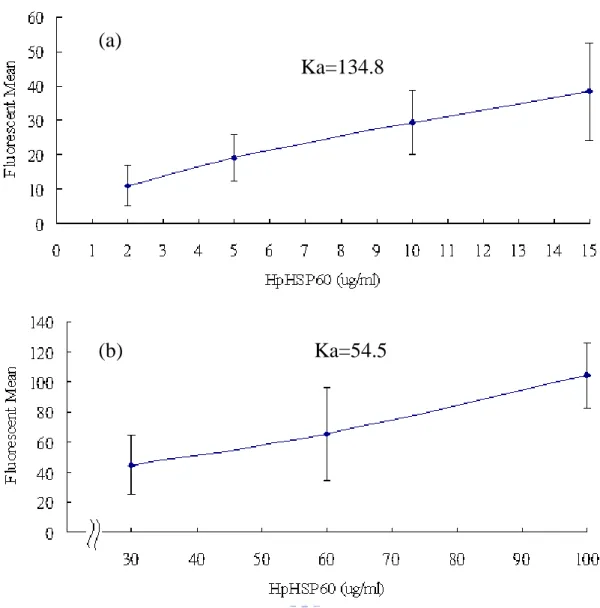
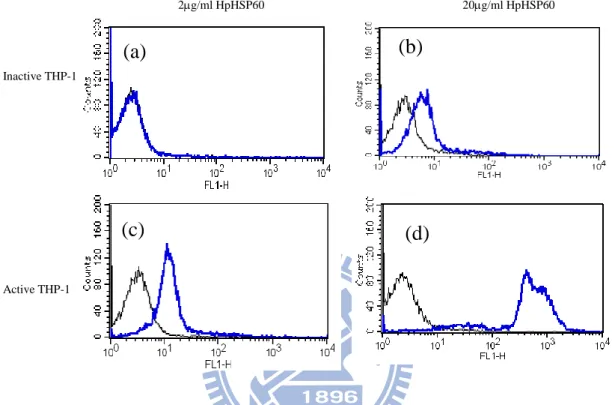
At first, the different dosages of FITC-HpHSP60 were treated with THP-1 cells. As the additional amounts of FITC-HpHSP60 were increased, the fluorescent intensities of cell surface were increase. From 0.5 to 10 g/ml, the Ka value for FITC-HpHSP60 and THP-1 cell was calculated as 469.1 (Fig. 1a). In contrast, the Ka value for FITC-HpHSP60 and THP-1 cell was 387.6 from 15 to 25 g/ml (Fig. 1b). Similarly, FITC-HpHSP60s were treated AGS cells and the Ka values respective were 134.8 for low dosages (from 2 to 15 g/ml, Fig. 2a) and 54.5 for high dosages (from 30 to 150 g/ml, Fig. 2b). In addition, LPS-activated THP-1 cells were also treated with FITC-HpHSP60s (2
g/ml or 20 g/ml) to monitor the surface binding comparing to the inactive THP-1 cells. The results showed the stronger bindings to active THP-1 cells were significantly observed (Fig. 3c and d) than inactive THP-1 cells (Fig. 3a and b). The results seem to indicate that different cells may have different receptors with different affinity to HpHSP60.
13
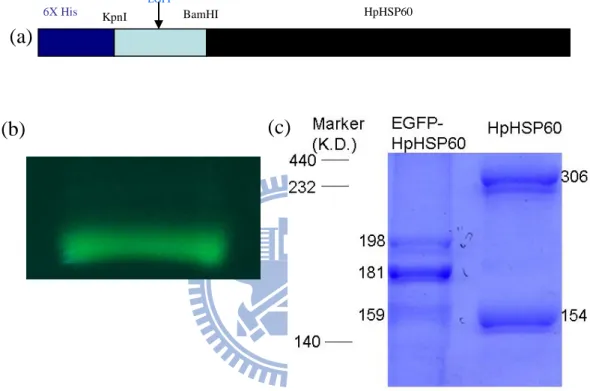
The N-terminal sequence of HpHSP60 for cell binding activity
EGFP-HpHSP60 was constructed by fusing enhance green fluorescence protein (EGFP) to N-terminal of HpHSP60 (Fig. 4a) and such fusion did not abolish the fluorescent activity (Fig. 4b). HpHSP60 proteins were formed as dimmer (154 K.D.) or tetramer (306K.D.) (Fig. 4c) but N-terminal fusion interfered EGFP-HpHSP60 to form tetramer (Fig. 4c). In addition, EGFP-HpHSP60 fusion proteins were also valued their activities to induce proinflammatory cytokines. The results showed these fusion proteins still keep the capabilities to trigger the releases of IL-8 (Fig. 5). However, the N-terminal fusion did not only interfere with the cell binding activities to surface of inactive THP-1 cell but also decrease the surface binding to active THP-1 cells (Fig. 6). Taken together, these results reveal that N-terminal fusion to HpHSP60 did not influence the activity to induce the expression of proinflammatory cytokine but interfere with its binding activity to cell surface.
14
To further investigate the function of N-terminal sequence of
HpHSP60, certain truncated HpHSP60 were constructed. Figure 7 showed the structures of these transgenes. All transgenes were expressed by BL-21 E. coli system but only two recombinant proteins could be significant inducted by IPTG (HpHSP601-200 and HpHSP60101-200), their
molecular weights were about 25 K.D. and 17 K.D. (Fig. 8), respectively. In contrast, HpHSP601-500 (57 K.D.), HpHSP601-435 (50 K.D.) and
HpHSP60300-547 (17 K.D.) were not inducted significantly by IPTG (Fig.
8).
Sequentially, we examine whether these truncated recombinant proteins still remain their activities to induce the releases of proinflammatory cytokines. The results showed that HpHSP60300-547 could
still induce IL-8 expression as intact HpHSP60, but HpHSP601-200 and
HpHSP60101-200 lost this ability, which indicated that the domain to induce
IL-8 release is not located near N-terminal sequence of HpHSP60.
According to our unpublished results that HpHSP60 could inhibit the proliferation of T cells in human PBMC. Therefore, whether N-terminal sequence of HpHSP60 would influence this activity was monitored. Figure10 indicated that HpHSP601-200 and HpHSP60101-200 could inhibit T
15
cells’ proliferation as HpHSP60. However, HpHSP60300-547 could not
inhibit T cells’ proliferation at all. These results indicated the domain respond to suppression of T-cell proliferation is located near amino acid 101 to 200. In addition, the N-terminal domain of HpHSP60 could induce TGF-secretion such as HpHSP601-200 and HpHSP60101-200 but
16
Discussion
In this study, we proposed that the domain located near the N-terminal sequence of HpHSP60 involved in the inhibitory activity to anti-CD3 antibody-induced T-cell proliferation. This domain is not participated in the activities relative to induction of inflammatory cytokines.
As the additional HpHSP60s were increasing, HpHSP60s bound to cell surface by different association constants (Fig. 1 and 2). Thus, we proposed that there are two or more HpHSP60 receptors existed on the cell surface. Although HpHSP60 has been showed that it could bind to TLR2 or TLR4 to induce IL-8 release (Takenaka et al., 2004). However, Dr. Takenaka et al. indicate that TLR2 play an important role in the secretion of HpHSP60-induced IL-8 via the signaling pathway involving NF-B activation but TLR4 play the minor or no effect on this phenomenon. According their experiments, they proposed that receptors other than TLR2 and TLR4 may also recognize HpHSP60 (Takenaka et
al., 2004). In 2007, Zhao et al. also indicate that TLR2 but not TLR4 can
trigger HpHSP60-induced IL-8 expression via the mitogen-activated protein (MAP) kinase pathway associated with activation of NF-B
17
(Zhao et al., 2007). Whereas they also find the IL-8 releases only were partially decreased using siRNA-TLR2. So they suggested there were other receptors for HpHSP60. Thus, HpHSP60 has at least two kinds of receptors on the THP-1 cell, a human monocytic cell is reasonable.
In 2005, Lin, et al. have identified that the activity of HpHSP60 to induce the IL-8 secretion is resulted from the region 300-435 a.a. (Lin
et al., 2005). Subsequently, the sequence of this peptide was compared
with the HSP60 sequences of other species such as C. pneumoniae, M.
leprae, human, M. tuberculosis or E. coli by Vector NTI Suite 9 software.
Their similarities were about 65% and the positions were near 320-460 a.a.. In addition, these HSP60s all can induce proinflammatory cytokine releases (Lin et al., 2009a). Except of inflammation, human HSP60 has been shown it has other activity for immunoregulatory function of T cell by induction of anti-inflammatory cytokine such as IL-10 (Wieten et al., 2007). Vaccination with human HSP6031-50
(KFGADARALMLQGVDLLADA) increases the secretions of IL-10 and TGF-which are immunomodulatory cytokines capable of inhibiting experimental adjuvant arthritis (AA) (Francisco J. Quintana, 2003). IL-10 is an anti-inflammatory cytokine (Quinn et al., 2000) and TGF-can
18
enhance the production of regulatory T cell (Yoshimura et al., 2010). In this study, figure 11 also showed that the sequence of HpHSP60 from 101 to 200 could induce the expression of TGF- but the sequence from 300 to 547 did not. Thus, the information indicates that HSP60s have other functional sequences, except of the domain to result in inflammation. So, HSP60 may engage the receptor which is not responsive to inflammation by these immunoregulatory sequences. For example, CXCL12 can direct the production of Treg cells via CXCR4 (Karin, 2010) and it also controls these processes of cell adhesion, survival and tumor progression via CXCR7 (Mahabaleshwar et al., 2008).
Furthermore, our results showed that the fluorescent intensity of active THP-1 cells bound with EGFP-HpHSP60s was lower than FITC-HpHSP60s (8.8 versus 563.8) (Fig. 3 and 6). The decrease of fluorescence was not resulted from the difference between the fluorescent degree of FITC and EGFP, because the fluorescence of FITC was only higher 3 fold than EGFP. It may be due to N-terminal fusion of EGFP interfere the cell binding activity of HpHSP60, which imply that there is at least one cell binding domain locating near the N-terminal sequence with cells. According to our results, this study showed HpHSP60 can
19
provide the different functions via different receptors. HpHSP60 and HpHSP60300-547 could induce dramatically IL-8 releases but lose of the
sequence from 300-435 a.a. (HpHSP601-200 and HpHSP60101-200) would
abolish this activity (Fig. 9). In contrast, lose of the sequence from 101 to 200 a.a (HpHSP60300-547) would abolish the activity to suppress T cell
proliferation (Fig. 10). Thus, we supported the N-terminal domain near 101-200 a. a. of HpHSP60 could inhibit the proliferation of T cell.
According the literature (Lin et al., 2009a), HpHSP60s were formed as dimmers or tetramers but EGFP-HpHSP60s only were dimmers in native gel (Fig. 4). The information implied that N-terminal sequence of HpHSP60 involved in the polymerization of HpHSP60. Taken together, we proposed that the N-terminal sequence of HpHSP60 have multiple functional domain in which it can trigger TGF- release to cause the proliferative suppression for T-cell and also involved in the formation of structure. This study first explored the immunoregulatory activity of HpHSP60 is came from the domain located the sequence from 101 to 200. This finding may be useful to further study for the chronic infection of H.
20
Figure and Legend
Figure 1 HpHSP60 could bind to THP-1 cells with different affinities.
THP-1 cells (2x105) were treated with FITC-HpHSP60 (0.5 to 25 g/ml) for 30 minutes. Fluorescence means of FITC-HpHSP60 bound on THP-1 cells detected by flow cytometry were plotted. (a) The binding affinity (Ka=469.1) of FITC-HpHSP60s (0.5 to 10 g/ml) bound to THP-1 cells. (b) The binding affinity (Ka=387.6) of FITC-HpHSP60 (15 to 25 g/ml) bound to THP-1 cells. Association constant (Ka) =
ion Concentrat otein Mean ce Fluorescen Pr .
The higher Ka value stands for the better binding affinity. N=1 Ka=469.1
(a)
Ka=387.6 (b)
21
Figure 2 HpHSP60 could bind to AGS cells with different affinities.
AGS cells (2x105) were treated with FITC-HpHSP60 (2 to 150 g/ml) for 30 minutes in 250 l. Fluorescence means of FITC-HpHSP60 bound to AGS cells detected by flow cytometry were plotted. (a) The binding affinity (Ka=134.8) of FITC-HpHSP60 (2 to 15 g/ml) bound to AGS cells. (b) The binding affinity (Ka=54.5) of FITC-HpHSP60 (30 to 150
g/ml) bound to AGS cells. Association constant (Ka) =
ion Concentrat otein Mean ce Fluorescen Pr N=3 Ka=134.8 (a) Ka=54.5 (b)
22
Figure 3 The binding intensities of HpHSP60 for LPS-activated THP-1 cells were dramatically increased than inactive THP-1 cells. (a)
inactive THP-1 cells, (c) active THP-1 cells (1x106) were incubated with 2 mg/ml HpHSP60 in 500 ml. After 30 minutes co-incubation, the fluorescence mean of FITC-HpHSP60 was measured by flow cytometry. In the same way for 20 mg/ml HpHSP60s with (b) inactive THP-1 cells, (d) active THP-1 cells. The fluorescence mean was (a) 3.44, (b) 11.81 for FITC-HpHSP60 binding to inactive cells and (c) 16.59, (d) 563.75 for active cells.
cell alone, FITC-HpHSP60 with cells.
Inactive THP-1 Active THP-1 (b) (d) (a) (c) 2g/ml HpHSP60 20g/ml HpHSP60
23
Figure 4 Electrophoresis profile of EGFP-HpHSP60. (a) Enhance green fluorescence protein (EGFP) was fused at KpnI and BamHI restriction sites on pET30-HpHSP60. (b) The fluorescence of EGFP-HpHSP60. (c) The protein structures were calculated by Rf value.
Therefore, EGFP-HpHSP60 were dimmers and HpHSP60 were dimmers or tetramers. (a) (b) (c) BamHI HpHSP60 6X His EGFP KpnI
24
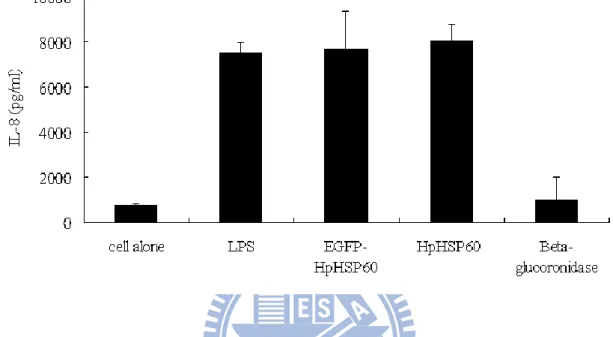
Figure 5 EGFP-HpHSP60 induced the secretion of pro-inflammatory cytokine IL-8. THP-1 cells were treated with 10g EGFP-HpHSP60 or HpHSP60 for 24 hours in 5% CO2 at 37℃ incubator. Positive control:
THP-1 cells were treated with 1g/ml LPS. Negative control: THP-1 cells were treated with 10g β-glucuronidase. After 24 hours, the supernatants were collected and measured for IL-8 expression. EGFP-HpHSP60 triggered THP-1 cells to secrete IL-8 significantly (~8000 pg/ml) as well as HpHSP60 and LPS. N=3
25
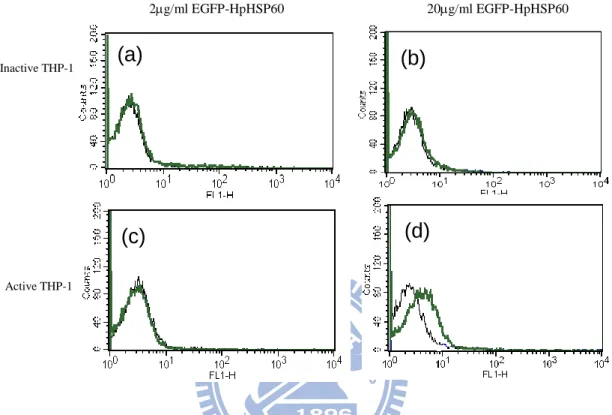
Figure 6 EGFP interrupted LPS-activated THP-1 cells to bind to HpHSP60. (a) inactive THP-1 cells, (c) active THP-1 cells (1x106) were incubated with 2 mg/ml EGFP-HpHSP60 in 500 l. After 30 minutes co-incubation, the fluorescence mean of EGFP-HpHSP60 was measured by flow cytometry.In the same way, 20 mg/ml EGFP-HpHSP60s treated with (b) inactive THP-1 cells or (d) active THP-1 cells. The fluorescence mean was (a) 3.44、(b) 3.64 for EGFP-HpHSP60 binding to inactive cells and (c) 3.46、(d) 8.77 for active cells.
cell alone, EGFP-HpHSP60 with cells. Inactive THP-1 Active THP-1 2g/ml EGFP-HpHSP60 20g/ml EGFP-HpHSP60 (a ) (b) (c ) (d) (a) (c)
26
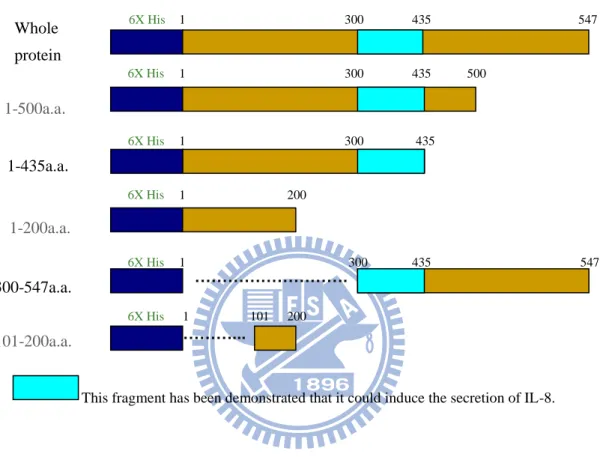
Figure 7 The different domains of HpHSP60. This figure simply
showed that the different sequence of HpHSP60. 1-200a.a. 1-435a.a. 1-500a.a. 300-547a.a. 101-200a.a. 6X His 1 200 6X His 1 435 6X His 1 500 6X His 300 547 6X His 101 200
This fragment has been demonstrated that it could induce the secretion of IL-8.
………. . ………... 300 300 435 435 1 1 6X His 1 300 435 547 Whole protein
27
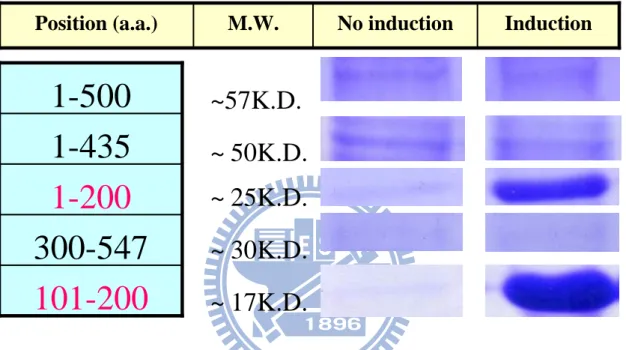
Figure 8 The protein expressions of the different fragments. Different
fragments were constructed by Directional TOPO Expression Kit (invitrogen). Proteins were inducted by IPTG (1mM) in BL21 E.coli system. The induction of HpHSP601-200 and HpHSP60101-200 were
observed.
101-200
300-547
1-200
1-435
1-500
~57K.D.
~
50K.D.
~
25K.D.
~
30K.D.
~
17K.D.
28 0 1000 2000 3000 4000 5000 6000 Cell alone LPS HpHSP60 HpHSP60 1-200 HpHSP60 101-200 HpHSP60 300-547 IL -8 (pg /m l)
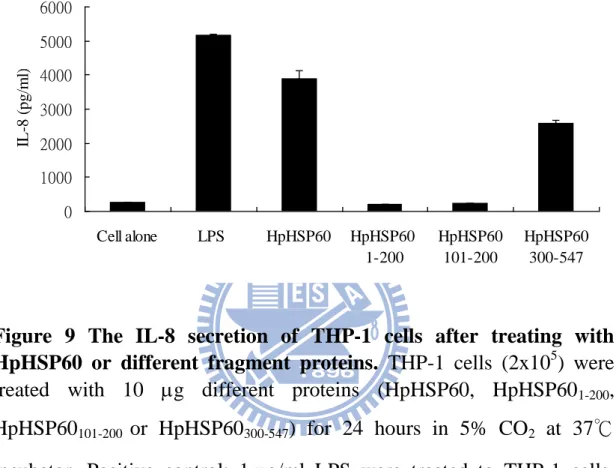
Figure 9 The IL-8 secretion of THP-1 cells after treating with HpHSP60 or different fragment proteins. THP-1 cells (2x105) were treated with 10 g different proteins (HpHSP60, HpHSP601-200,
HpHSP60101-200 or HpHSP60300-547) for 24 hours in 5% CO2 at 37℃
incubator. Positive control: 1g/ml LPS were treated to THP-1 cells. After 24 hours, the supernatants were collected and measured IL-8 expression. Truncated HpHSP601-200, HpHSP60101-200 could not induce
29
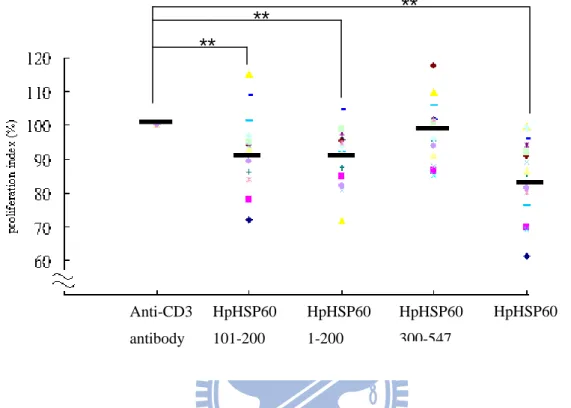
Figure 10 Treatment with HpHSP60 and different fragment proteins to PBMCs decreases the proliferation in the present of anti-CD3 antibody. PBMCs were isolated from fifteen healthy donors and seeded
in 96 well, which was coated with anti-CD3 antibody (1g/ml). Different proteins were co-cultured with PBMCs for four days. After four days incubation, MTT assay was used to detect the proliferation of PBMCs. PBMCs treated with anti-CD3 antibody was identified as 100%. HpHSP60, HpHSP60101-200 and HpHSP601-200 could inhibit PBMCs
proliferation significantly. ** P< 0.01. N=15 Anti-CD3 antibody HpHSP60 101-200 HpHSP60 1-200 HpHSP60 300-547 HpHSP60 ** ** **
30 0 100 200 300 400 500 600 700 800 900 1000 cell alone HpHsp60 101-200 HpHsp60 1-200 HpHsp60 300-547 HpHsp60 T G F-β (p g/ m l)
Figure 11 The TGF-secretion of THP-1 cells after treating with HpHSP60 or different fragment proteins. THP-1 cells (1x105) were treated with 10g different proteins (HpHSP60, HpHSP601-200, HpHSP60 101-200 or HpHSP60300-547) for 24 hours in 5% CO2 at 37℃ incubator.
Negative control: only cells. After 24 hours, the supernatants were collected and measured TGF- expression. HpHSP601-200,
HpHSP60101-200, HpHSP60 could significantly induce TGF- secretion.
*P<0.05, **P<0.01. N=1
*
*
31
Appendix
1. pET30-EGFP-HpHSP60 1.1 Map
1.2 pET30-EGFP-HpHSP60 DNA sequence
1 atccggatat agttcctcct ttcagcaaaa aacccctcaa gacccgttta gaggccccaa 61 ggggttatgc tagttattgc tcagcggtgg cagcagccaa ctcagcttcc tttcgggctt 121 tgttagcagc cggatctcag tggtggtggt ggtggtgctc gagttacatc atgccgccca 181 tgcctcccat accgcccatg ccacccatat caggcattgc tggggttgct ttttcttctt 241 tgatttcatg cacggtggct tctgtggtta aaagcaggct tgaaaccgaa accgcatttt 301 gtaaagcgat cctttctact tttaaggggt caataatgcc ttctttaaac atatccacat 361 acttgccatt gctagcgtta aaaccaaaat gcccttcgtg tttttgcact tcattcacga 421 ccacaccgcc atcataaccg gcattgatag cgatttgagc taatggggct ttaatggcac 481 gcatgatgat ttcatagcct actttttcat catcgtgtaa attcaaatgc actttttgag
541 ccgcgcgaat gagagccgca ccgccgccaa taacaatgcc ctcttcaaca gctgctttag 601 tcgcactcaa tgcgtcatca acccggtctt ttttctcttt catttccact tcactcgcag 661 cgcccacttt aatcacagcc acaccaccag agagtttggc caatctttct tgcaattttt 721 ctttgtcata atcgcttgtc gtgcttgcaa tttgggtttt gatttgcgcg actctgtctt 781 taacatcatg gctatgtcct ttgccatcta cgatcgtggt gttgtctttg tcaatcacaa
32
841 tccttccggc tttgcctaaa aactccactt cagcgttttc taaagtcaag cctaattctt 901 cgctaatgac ttgaccgccg gttaaaacag cgatgtcttt gagcatttct tttcttctgt 961 ccccaaagcc tggagcttta accgctgcga tattcaacac gcctcttaat ttattcacca 1021 ctagagtcgt taaagcttcg ccctcaatgt cttcagcgat gattaaaagc ggtttgccct 1081 ctttcatggt tttttctagt agcgggagaa tgtctttcat gctagagatt tttttatccg 1141 ttaaaaggat gtaagcgtta tccaattgag cggtcatttt ctcagcgttt gttacaaagt 1201 aaggggagag gtagcctcta tcaaattgca tgccctctac gacatctaat tcatcttcaa 1261 tgcccttagc ttcttcaacg gtgatcacgc cgtctttacc cactttttcc atagcgtcag 1321 cgatgagttt cccgatattg tgatcggagt ttgcagaaat ggtcgctact tgggtgattt 1381 cttctttacc acctactttt ttgctcgctt ttttaagctc attaataatg gcttcagcgg 1441 ctttatccat gcctcgtttc acttcaatag ggttagcccc agccgtgata ttcctcaagc 1501 cttctttaaa gatgctataa gcaagcacgg tcgctgtggt cgtgccatcg ccggcagcat 1561 cagcggtttt gctcgctact tctttaacga gttgagcgcc catgttagct accgggcaac 1621 ttaattcaat ctctttagcc acgctcacgc catctttggt gatgcttgga gcgccatagc 1681 ttttttggat caacacattc ctacctcttg gccccatggt tactttgaca gcgtcatgga 1741 gttgtctcac gccttcaaat aaaaggtttc ttgcactatc tgaaaatttg atttcttttg
1801 ccatgaattc ggatcccttg tacagcctgt ccatgccgag agtgatcccg gcggcggtca 1861 cgaactccag caggcacatg tgatcgcgct tctcgttggg gtctttgctc agggcggact 1921 gggtgctcag gtagtggttg tcggcgagca gcacggggcc gtcgccgatg ggggtgttct 1981 gctggtagtg gtcggcgagc tgcacgctgc cgtcctcgat gttgtggcgg atcttgaagt 2041 tcaccttgat gccgttcttc tgcttgtcgg ccatgatata gacgttgtgg ctgttgtagt 2101 tgtactccag cttgtgcccc aggatgttgc cgtcctcctt gaagtcgatg cccttcagct 2161 cgatgcggtt caccagggtg tcgccctcga acttcacctc ggcgcgggtc ttgtagttgc 2221 cgtcgtcctt gaagaagatg gtgcgctcct ggacgtagcc ttcgggcatg gcggacttga 2281 agaagtcgtg ctgcttcatg tggtcggggt agcggctgaa gcactgcacg ccgtaggtca 2341 gggtggtcac gagggtgggc cagggcacgg gcagcttgcc ggtggtgcag
atgaacttca
2401 gggtcagctt gccgtaggtg gcatcgccct cgccctcgcc ggacacgctg aacttgtggc 2461 cgtttacgtc gccgtccagc tcgaccagga tgggcaccac cccggtgaac agctcctcgc 2521 ccttgctcac catggtaccc agatctgggc tgtccatgtg ctggcgttcg aatttagcag 2581 cagcggtttc tttcatacca gaaccgcgtg gcaccagacc agaagaatga tgatgatgat 2641 ggtgcatatg tatatctcct tcttaaagtt aaacaaaatt atttctagag gggaattgtt 2701 atccgctcac aattccccta tagtgagtcg tattaatttc gcgggatcga gatcgatctc 2761 gatcctctac gccggacgca tcgtggccgg catcaccggc gccacaggtg cggttgctgg 2821 cgcctatatc gccgacatca ccgatgggga agatcgggct cgccacttcg ggctcatgag 2881 cgcttgtttc ggcgtgggta tggtggcagg ccccgtggcc gggggactgt tgggcgccat 2941 ctccttgcat gcaccattcc ttgcggcggc ggtgctcaac ggcctcaacc tactactggg 3001 ctgcttccta atgcaggagt cgcataaggg agagcgtcga gatcccggac accatcgaat
33
3061 ggcgcaaaac ctttcgcggt atggcatgat agcgcccgga agagagtcaa ttcagggtgg 3121 tgaatgtgaa accagtaacg ttatacgatg tcgcagagta tgccggtgtc tcttatcaga 3181 ccgtttcccg cgtggtgaac caggccagcc acgtttctgc gaaaacgcgg gaaaaagtgg 3241 aagcggcgat ggcggagctg aattacattc ccaaccgcgt ggcacaacaa
ctggcgggca
3301 aacagtcgtt gctgattggc gttgccacct ccagtctggc cctgcacgcg ccgtcgcaaa 3361 ttgtcgcggc gattaaatct cgcgccgatc aactgggtgc cagcgtggtg gtgtcgatgg 3421 tagaacgaag cggcgtcgaa gcctgtaaag cggcggtgca caatcttctc
gcgcaacgcg
3481 tcagtgggct gatcattaac tatccgctgg atgaccagga tgccattgct gtggaagctg 3541 cctgcactaa tgttccggcg ttatttcttg atgtctctga ccagacaccc atcaacagta 3601 ttattttctc ccatgaagac ggtacgcgac tgggcgtgga gcatctggtc gcattgggtc 3661 accagcaaat cgcgctgtta gcgggcccat taagttctgt ctcggcgcgt ctgcgtctgg 3721 ctggctggca taaatatctc actcgcaatc aaattcagcc gatagcggaa cgggaaggcg 3781 actggagtgc catgtccggt tttcaacaaa ccatgcaaat gctgaatgag ggcatcgttc 3841 ccactgcgat gctggttgcc aacgatcaga tggcgctggg cgcaatgcgc gccattaccg 3901 agtccgggct gcgcgttggt gcggacatct cggtagtggg atacgacgat accgaagaca 3961 gctcatgtta tatcccgccg ttaaccacca tcaaacagga ttttcgcctg ctggggcaaa 4021 ccagcgtgga ccgcttgctg caactctctc agggccaggc ggtgaagggc aatcagctgt 4081 tgcccgtctc actggtgaaa agaaaaacca ccctggcgcc caatacgcaa accgcctctc 4141 cccgcgcgtt ggccgattca ttaatgcagc tggcacgaca ggtttcccga ctggaaagcg 4201 ggcagtgagc gcaacgcaat taatgtaagt tagctcactc attaggcacc gggatctcga 4261 ccgatgccct tgagagcctt caacccagtc agctccttcc ggtgggcgcg gggcatgact 4321 atcgtcgccg cacttatgac tgtcttcttt atcatgcaac tcgtaggaca ggtgccggca 4381 gcgctctggg tcattttcgg cgaggaccgc tttcgctgga gcgcgacgat gatcggcctg 4441 tcgcttgcgg tattcggaat cttgcacgcc ctcgctcaag ccttcgtcac tggtcccgcc 4501 accaaacgtt tcggcgagaa gcaggccatt atcgccggca tggcggcccc
acgggtgcgc
4561 atgatcgtgc tcctgtcgtt gaggacccgg ctaggctggc ggggttgcct tactggttag 4621 cagaatgaat caccgatacg cgagcgaacg tgaagcgact gctgctgcaa aacgtctgcg 4681 acctgagcaa caacatgaat ggtcttcggt ttccgtgttt cgtaaagtct ggaaacgcgg 4741 aagtcagcgc cctgcaccat tatgttccgg atctgcatcg caggatgctg ctggctaccc 4801 tgtggaacac ctacatctgt attaacgaag cgctggcatt gaccctgagt gatttttctc 4861 tggtcccgcc gcatccatac cgccagttgt ttaccctcac aacgttccag taaccgggca 4921 tgttcatcat cagtaacccg tatcgtgagc atcctctctc gtttcatcgg tatcattacc 4981 cccatgaaca gaaatccccc ttacacggag gcatcagtga ccaaacagga
aaaaaccgcc
34
5101 ctggacgcgg atgaacaggc agacatctgt gaatcgcttc acgaccacgc tgatgagctt 5161 taccgcagct gcctcgcgcg tttcggtgat gacggtgaaa acctctgaca catgcagctc 5221 ccggagacgg tcacagcttg tctgtaagcg gatgccggga gcagacaagc
ccgtcagggc
5281 gcgtcagcgg gtgttggcgg gtgtcggggc gcagccatga cccagtcacg tagcgatagc
5341 ggagtgtata ctggcttaac tatgcggcat cagagcagat tgtactgaga gtgcaccata 5401 tatgcggtgt gaaataccgc acagatgcgt aaggagaaaa taccgcatca ggcgctcttc 5461 cgcttcctcg ctcactgact cgctgcgctc ggtcgttcgg ctgcggcgag cggtatcagc 5521 tcactcaaag gcggtaatac ggttatccac agaatcaggg gataacgcag gaaagaacat 5581 gtgagcaaaa ggccagcaaa aggccaggaa ccgtaaaaag gccgcgttgc tggcgttttt 5641 ccataggctc cgcccccctg acgagcatca caaaaatcga cgctcaagtc agaggtggcg 5701 aaacccgaca ggactataaa gataccaggc gtttccccct ggaagctccc tcgtgcgctc 5761 tcctgttccg accctgccgc ttaccggata cctgtccgcc tttctccctt cgggaagcgt 5821 ggcgctttct catagctcac gctgtaggta tctcagttcg gtgtaggtcg ttcgctccaa 5881 gctgggctgt gtgcacgaac cccccgttca gcccgaccgc tgcgccttat ccggtaacta 5941 tcgtcttgag tccaacccgg taagacacga cttatcgcca ctggcagcag ccactggtaa 6001 caggattagc agagcgaggt atgtaggcgg tgctacagag ttcttgaagt ggtggcctaa 6061 ctacggctac actagaagga cagtatttgg tatctgcgct ctgctgaagc cagttacctt 6121 cggaaaaaga gttggtagct cttgatccgg caaacaaacc accgctggta gcggtggttt 6181 ttttgtttgc aagcagcaga ttacgcgcag aaaaaaagga tctcaagaag atcctttgat 6241 cttttctacg gggtctgacg ctcagtggaa cgaaaactca cgttaaggga ttttggtcat 6301 gaacaataaa actgtctgct tacataaaca gtaatacaag gggtgttatg agccatattc 6361 aacgggaaac gtcttgctct aggccgcgat taaattccaa catggatgct gatttatatg 6421 ggtataaatg ggctcgcgat aatgtcgggc aatcaggtgc gacaatctat cgattgtatg 6481 ggaagcccga tgcgccagag ttgtttctga aacatggcaa aggtagcgtt gccaatgatg 6541 ttacagatga gatggtcaga ctaaactggc tgacggaatt tatgcctctt ccgaccatca 6601 agcattttat ccgtactcct gatgatgcat ggttactcac cactgcgatc cccgggaaaa 6661 cagcattcca ggtattagaa gaatatcctg attcaggtga aaatattgtt gatgcgctgg 6721 cagtgttcct gcgccggttg cattcgattc ctgtttgtaa ttgtcctttt aacagcgatc 6781 gcgtatttcg tctcgctcag gcgcaatcac gaatgaataa cggtttggtt gatgcgagtg 6841 attttgatga cgagcgtaat ggctggcctg ttgaacaagt ctggaaagaa atgcataaac 6901 ttttgccatt ctcaccggat tcagtcgtca ctcatggtga tttctcactt gataacctta 6961 tttttgacga ggggaaatta ataggttgta ttgatgttgg acgagtcgga atcgcagacc 7021 gataccagga tcttgccatc ctatggaact gcctcggtga gttttctcct tcattacaga 7081 aacggctttt tcaaaaatat ggtattgata atcctgatat gaataaattg cagtttcatt 7141 tgatgctcga tgagtttttc taagaattaa ttcatgagcg gatacatatt tgaatgtatt 7201 tagaaaaata aacaaatagg ggttccgcgc acatttcccc gaaaagtgcc acctgaaatt
35
7261 gtaaacgtta atattttgtt aaaattcgcg ttaaattttt gttaaatcag ctcatttttt
7321 aaccaatagg ccgaaatcgg caaaatccct tataaatcaa aagaatagac cgagataggg 7381 ttgagtgttg ttccagtttg gaacaagagt ccactattaa agaacgtgga ctccaacgtc 7441 aaagggcgaa aaaccgtcta tcagggcgat ggcccactac gtgaaccatc accctaatca 7501 agttttttgg ggtcgaggtg ccgtaaagca ctaaatcgga accctaaagg gagcccccga 7561 tttagagctt gacggggaaa gccggcgaac gtggcgagaa aggaagggaa
gaaagcgaaa
7621 ggagcgggcg ctagggcgct ggcaagtgta gcggtcacgc tgcgcgtaac caccacaccc
36 2. pET30-HpHSP60 101-200
2.1 Map
2.2 pET30-HpHSP60 101-200 DNA sequence
1 atccggatat agttcctcct ttcagcaaaa aacccctcaa gacccgttta gaggccccaa 61 ggggttatgc tagttattgc tcagcggtgg cagcagccaa ctcagcttcc tttcgggctt 121 tgttagcagc cggatctcag tggtggtggt ggtggtgctc gagggagagg tagcctctat 181 caaattgcat gccctctacg acatctaatt catcttcaat gcccttagct tcttcaacgg 241 tgatcacgcc gtctttaccc actttttcca tagcgtcagc gatgagtttc ccgatattgt 301 gatcggagtt tgcagaaatg gtcgctactt gggtgatttc ttctttacca cctacttttt 361 tgctcgcttt tttaagctca ttaataatgg cttcagcggc tttatccatg cctcgtttca 421 cttcaatagg gttagcccca gccgtgatat tcctcaagcc ttcggatccg atatcagcca 481 tggccttgtc gtcgtcgtcg gtacccagat ctgggctgtc catgtgctgg cgttcgaatt 541 tagcagcagc ggtttctttc ataccagaac cgcgtggcac cagaccagaa gaatgatgat 601 gatgatggtg catatgtata tctccttctt aaagttaaac aaaattattt ctagagggga 661 attgttatcc gctcacaatt cccctatagt gagtcgtatt aatttcgcgg gatcgagatc 721 gatctcgatc ctctacgccg gacgcatcgt ggccggcatc accggcgcca caggtgcggt 781 tgctggcgcc tatatcgccg acatcaccga tggggaagat cgggctcgcc acttcgggct 841 catgagcgct tgtttcggcg tgggtatggt ggcaggcccc gtggccgggg gactgttggg 901 cgccatctcc ttgcatgcac cattccttgc ggcggcggtg ctcaacggcc tcaacctact
37
961 actgggctgc ttcctaatgc aggagtcgca taagggagag cgtcgagatc ccggacacca 1021 tcgaatggcg caaaaccttt cgcggtatgg catgatagcg cccggaagag agtcaattca 1081 gggtggtgaa tgtgaaacca gtaacgttat acgatgtcgc agagtatgcc ggtgtctctt 1141 atcagaccgt ttcccgcgtg gtgaaccagg ccagccacgt ttctgcgaaa acgcgggaaa 1201 aagtggaagc ggcgatggcg gagctgaatt acattcccaa ccgcgtggca
caacaactgg
1261 cgggcaaaca gtcgttgctg attggcgttg ccacctccag tctggccctg cacgcgccgt 1321 cgcaaattgt cgcggcgatt aaatctcgcg ccgatcaact gggtgccagc gtggtggtgt 1381 cgatggtaga acgaagcggc gtcgaagcct gtaaagcggc ggtgcacaat cttctcgcgc 1441 aacgcgtcag tgggctgatc attaactatc cgctggatga ccaggatgcc attgctgtgg 1501 aagctgcctg cactaatgtt ccggcgttat ttcttgatgt ctctgaccag acacccatca 1561 acagtattat tttctcccat gaagacggta cgcgactggg cgtggagcat ctggtcgcat 1621 tgggtcacca gcaaatcgcg ctgttagcgg gcccattaag ttctgtctcg gcgcgtctgc 1681 gtctggctgg ctggcataaa tatctcactc gcaatcaaat tcagccgata gcggaacggg 1741 aaggcgactg gagtgccatg tccggttttc aacaaaccat gcaaatgctg aatgagggca 1801 tcgttcccac tgcgatgctg gttgccaacg atcagatggc gctgggcgca atgcgcgcca 1861 ttaccgagtc cgggctgcgc gttggtgcgg acatctcggt agtgggatac gacgataccg 1921 aagacagctc atgttatatc ccgccgttaa ccaccatcaa acaggatttt cgcctgctgg 1981 ggcaaaccag cgtggaccgc ttgctgcaac tctctcaggg ccaggcggtg
aagggcaatc
2041 agctgttgcc cgtctcactg gtgaaaagaa aaaccaccct ggcgcccaat acgcaaaccg 2101 cctctccccg cgcgttggcc gattcattaa tgcagctggc acgacaggtt tcccgactgg 2161 aaagcgggca gtgagcgcaa cgcaattaat gtaagttagc tcactcatta ggcaccggga 2221 tctcgaccga tgcccttgag agccttcaac ccagtcagct ccttccggtg ggcgcggggc 2281 atgactatcg tcgccgcact tatgactgtc ttctttatca tgcaactcgt aggacaggtg 2341 ccggcagcgc tctgggtcat tttcggcgag gaccgctttc gctggagcgc gacgatgatc 2401 ggcctgtcgc ttgcggtatt cggaatcttg cacgccctcg ctcaagcctt cgtcactggt 2461 cccgccacca aacgtttcgg cgagaagcag gccattatcg ccggcatggc
ggccccacgg
2521 gtgcgcatga tcgtgctcct gtcgttgagg acccggctag gctggcgggg ttgccttact 2581 ggttagcaga atgaatcacc gatacgcgag cgaacgtgaa gcgactgctg ctgcaaaacg 2641 tctgcgacct gagcaacaac atgaatggtc ttcggtttcc gtgtttcgta aagtctggaa 2701 acgcggaagt cagcgccctg caccattatg ttccggatct gcatcgcagg atgctgctgg 2761 ctaccctgtg gaacacctac atctgtatta acgaagcgct ggcattgacc ctgagtgatt 2821 tttctctggt cccgccgcat ccataccgcc agttgtttac cctcacaacg ttccagtaac 2881 cgggcatgtt catcatcagt aacccgtatc gtgagcatcc tctctcgttt catcggtatc 2941 attaccccca tgaacagaaa tcccccttac acggaggcat cagtgaccaa acaggaaaaa 3001 accgccctta acatggcccg ctttatcaga agccagacat taacgcttct ggagaaactc
38
3061 aacgagctgg acgcggatga acaggcagac atctgtgaat cgcttcacga ccacgctgat 3121 gagctttacc gcagctgcct cgcgcgtttc ggtgatgacg gtgaaaacct ctgacacatg 3181 cagctcccgg agacggtcac agcttgtctg taagcggatg ccgggagcag
acaagcccgt
3241 cagggcgcgt cagcgggtgt tggcgggtgt cggggcgcag ccatgaccca gtcacgtagc
3301 gatagcggag tgtatactgg cttaactatg cggcatcaga gcagattgta ctgagagtgc 3361 accatatatg cggtgtgaaa taccgcacag atgcgtaagg agaaaatacc gcatcaggcg 3421 ctcttccgct tcctcgctca ctgactcgct gcgctcggtc gttcggctgc ggcgagcggt 3481 atcagctcac tcaaaggcgg taatacggtt atccacagaa tcaggggata acgcaggaaa 3541 gaacatgtga gcaaaaggcc agcaaaaggc caggaaccgt aaaaaggccg
cgttgctggc
3601 gtttttccat aggctccgcc cccctgacga gcatcacaaa aatcgacgct caagtcagag 3661 gtggcgaaac ccgacaggac tataaagata ccaggcgttt ccccctggaa gctccctcgt 3721 gcgctctcct gttccgaccc tgccgcttac cggatacctg tccgcctttc tcccttcggg 3781 aagcgtggcg ctttctcata gctcacgctg taggtatctc agttcggtgt aggtcgttcg 3841 ctccaagctg ggctgtgtgc acgaaccccc cgttcagccc gaccgctgcg ccttatccgg 3901 taactatcgt cttgagtcca acccggtaag acacgactta tcgccactgg cagcagccac 3961 tggtaacagg attagcagag cgaggtatgt aggcggtgct acagagttct tgaagtggtg 4021 gcctaactac ggctacacta gaaggacagt atttggtatc tgcgctctgc tgaagccagt 4081 taccttcgga aaaagagttg gtagctcttg atccggcaaa caaaccaccg ctggtagcgg 4141 tggttttttt gtttgcaagc agcagattac gcgcagaaaa aaaggatctc aagaagatcc 4201 tttgatcttt tctacggggt ctgacgctca gtggaacgaa aactcacgtt aagggatttt 4261 ggtcatgaac aataaaactg tctgcttaca taaacagtaa tacaaggggt gttatgagcc 4321 atattcaacg ggaaacgtct tgctctaggc cgcgattaaa ttccaacatg gatgctgatt 4381 tatatgggta taaatgggct cgcgataatg tcgggcaatc aggtgcgaca atctatcgat 4441 tgtatgggaa gcccgatgcg ccagagttgt ttctgaaaca tggcaaaggt agcgttgcca 4501 atgatgttac agatgagatg gtcagactaa actggctgac ggaatttatg cctcttccga 4561 ccatcaagca ttttatccgt actcctgatg atgcatggtt actcaccact gcgatccccg 4621 ggaaaacagc attccaggta ttagaagaat atcctgattc aggtgaaaat attgttgatg 4681 cgctggcagt gttcctgcgc cggttgcatt cgattcctgt ttgtaattgt ccttttaaca 4741 gcgatcgcgt atttcgtctc gctcaggcgc aatcacgaat gaataacggt ttggttgatg 4801 cgagtgattt tgatgacgag cgtaatggct ggcctgttga acaagtctgg aaagaaatgc 4861 ataaactttt gccattctca ccggattcag tcgtcactca tggtgatttc tcacttgata 4921 accttatttt tgacgagggg aaattaatag gttgtattga tgttggacga gtcggaatcg 4981 cagaccgata ccaggatctt gccatcctat ggaactgcct cggtgagttt tctccttcat 5041 tacagaaacg gctttttcaa aaatatggta ttgataatcc tgatatgaat aaattgcagt 5101 ttcatttgat gctcgatgag tttttctaag aattaattca tgagcggata catatttgaa
39
5161 tgtatttaga aaaataaaca aataggggtt ccgcgcacat ttccccgaaa agtgccacct 5221 gaaattgtaa acgttaatat tttgttaaaa ttcgcgttaa atttttgtta aatcagctca 5281 ttttttaacc aataggccga aatcggcaaa atcccttata aatcaaaaga atagaccgag 5341 atagggttga gtgttgttcc agtttggaac aagagtccac tattaaagaa cgtggactcc 5401 aacgtcaaag ggcgaaaaac cgtctatcag ggcgatggcc cactacgtga accatcaccc 5461 taatcaagtt ttttggggtc gaggtgccgt aaagcactaa atcggaaccc taaagggagc 5521 ccccgattta gagcttgacg gggaaagccg gcgaacgtgg cgagaaagga
agggaagaaa
5581 gcgaaaggag cgggcgctag ggcgctggca agtgtagcgg tcacgctgcg cgtaaccacc
40 3. pET100-HpHSP60 1-200
3.1 Map
3.2 pET100-HpHSP60 1-200 DNA sequence
1 atggcaaaag aaatcaaatt ttcagatagt gcaagaaacc ttttatttga aggcgtgaga 61 caactccatg acgctgtcaa agtaaccatg gggccaagag gtaggaatgt gttgatccaa 121 aaaagctatg gcgctccaag catcaccaaa gatggcgtga gcgtggctaa agagattgaa 181 ttaagttgcc cggtagctaa catgggcgct caactcgtta aagaagtagc gagcaaaacc 241 gctgatgctg ccggcgatgg cacgaccaca gcgaccgtgc ttgcttatag catctttaaa 301 gaaggcttga ggaatatcac ggctggggct aaccctattg aagtgaaacg aggcatggat 361 aaagccgctg aagccattat taatgagctt aaaaaagcga gcaaaaaagt aggtggtaaa 421 gaagaaatca cccaagtagc gaccatttct gcaaactccg atcacaatat cgggaaactc 481 atcgctgacg ctatggaaaa agtgggtaaa gacggcgtga tcaccgttga agaagctaag 541 ggcattgaag atgaattaga tgtcgtagag ggcatgcaat ttgatagagg ctacctctcc 601 aagggcgagc tcaacgatcc ggctgctaac aaagcccgaa aggaagctga gttggctgct
41
661 gccaccgctg agcaataact agcataaccc cttggggcct ctaaacgggt cttgaggagt 721 tttttgctga aaggaggaac tatatccgga tatcccgcaa gaggcccggc agtaccggca 781 taaccaagcc tatgcctaca gcatccaggg tgacggtgcc gaggatgacg atgagcgcat 841 tgttagattt catacacggt gcctgactgc gttagcaatt taactgtgat aaactaccgc 901 attaaagctt atcgatgata agctgtcaaa catgagaatt aattcttgaa gacgaaaggg 961 cctcgtgata cgcctatttt tataggttaa tgtcatgata ataatggttt cttagacgtc 1021 aggtggcact tttcggggaa atgtgcgcgg aacccctatt tgtttatttt tctaaataca 1081 ttcaaatatg tatccgctca tgagacaata accctgataa atgcttcaat aatattgaaa 1141 aaggaagagt atgagtattc aacatttccg tgtcgccctt attccctttt ttgcggcatt 1201 ttgccttcct gtttttgctc acccagaaac gctggtgaaa gtaaaagatg ctgaagatca 1261 gttgggtgca cgagtgggtt acatcgaact ggatctcaac agcggtaaga tccttgagag 1321 ttttcgcccc gaagaacgtt ttccaatgat gagcactttt aaagttctgc tatgtggcgc 1381 ggtattatcc cgtgttgacg ccgggcaaga gcaactcggt cgccgcatac actattctca 1441 gaatgacttg gttgagtact caccagtcac agaaaagcat cttacggatg gcatgacagt 1501 aagagaatta tgcagtgctg ccataaccat gagtgataac actgcggcca acttacttct 1561 gacaacgatc ggaggaccga aggagctaac cgcttttttg cacaacatgg gggatcatgt 1621 aactcgcctt gatcgttggg aaccggagct gaatgaagcc ataccaaacg acgagcgtga 1681 caccacgatg cctgcagcaa tggcaacaac gttgcgcaaa ctattaactg gcgaactact 1741 tactctagct tcccggcaac aattaataga ctggatggag gcggataaag ttgcaggacc 1801 acttctgcgc tcggcccttc cggctggctg gtttattgct gataaatctg gagccggtga 1861 gcgtgggtct cgcggtatca ttgcagcact ggggccagat ggtaagccct cccgtatcgt 1921 agttatctac acgacgggga gtcaggcaac tatggatgaa cgaaatagac agatcgctga 1981 gataggtgcc tcactgatta agcattggta actgtcagac caagtttact catatatact 2041 ttagattgat ttaaaacttc atttttaatt taaaaggatc taggtgaaga tcctttttga 2101 taatctcatg accaaaatcc cttaacgtga gttttcgttc cactgagcgt cagaccccgt 2161 agaaaagatc aaaggatctt cttgagatcc tttttttctg cgcgtaatct gctgcttgca 2221 aacaaaaaaa ccaccgctac cagcggtggt ttgtttgccg gatcaagagc taccaactct 2281 ttttccgaag gtaactggct tcagcagagc gcagatacca aatactgtcc ttctagtgta 2341 gccgtagtta ggccaccact tcaagaactc tgtagcaccg cctacatacc tcgctctgct 2401 aatcctgtta ccagtggctg ctgccagtgg cgataagtcg tgtcttaccg ggttggactc 2461 aagacgatag ttaccggata aggcgcagcg gtcgggctga acggggggtt
cgtgcacaca
2521 gcccagcttg gagcgaacga cctacaccga actgagatac ctacagcgtg agctatgaga 2581 aagcgccacg cttcccgaag ggagaaaggc ggacaggtat ccggtaagcg
gcagggtcgg
2641 aacaggagag cgcacgaggg agcttccagg gggaaacgcc tggtatcttt atagtcctgt 2701 cgggtttcgc cacctctgac ttgagcgtcg atttttgtga tgctcgtcag gggggcggag 2761 cctatggaaa aacgccagca acgcggcctt tttacggttc ctggcctttt gctggccttt
42
2821 tgctcacatg ttctttcctg cgttatcccc tgattctgtg gataaccgta ttaccgcctt 2881 tgagtgagct gataccgctc gccgcagccg aacgaccgag cgcagcgagt
cagtgagcga
2941 ggaagcggaa gagcgcctga tgcggtattt tctccttacg catctgtgcg gtatttcaca 3001 ccgcaatggt gcactctcag tacaatctgc tctgatgccg catagttaag ccagtataca 3061 ctccgctatc gctacgtgac tgggtcatgg ctgcgccccg acacccgcca acacccgctg 3121 acgcgccctg acgggcttgt ctgctcccgg catccgctta cagacaagct gtgaccgtct 3181 ccgggagctg catgtgtcag aggttttcac cgtcatcacc gaaacgcgcg aggcagctgc 3241 ggtaaagctc atcagcgtgg tcgtgaagcg attcacagat gtctgcctgt tcatccgcgt 3301 ccagctcgtt gagtttctcc agaagcgtta atgtctggct tctgataaag cgggccatgt 3361 taagggcggt tttttcctgt ttggtcactg atgcctccgt gtaaggggga tttctgttca 3421 tgggggtaat gataccgatg aaacgagaga ggatgctcac gatacgggtt actgatgatg 3481 aacatgcccg gttactggaa cgttgtgagg gtaaacaact ggcggtatgg atgcggcggg 3541 accagagaaa aatcactcag ggtcaatgcc agcgcttcgt taatacagat gtaggtgttc 3601 cacagggtag ccagcagcat cctgcgatgc agatccggaa cataatggtg
cagggcgctg
3661 acttccgcgt ttccagactt tacgaaacac ggaaaccgaa gaccattcat gttgttgctc 3721 aggtcgcaga cgttttgcag cagcagtcgc ttcacgttcg ctcgcgtatc ggtgattcat 3781 tctgctaacc agtaaggcaa ccccgccagc ctagccgggt cctcaacgac
aggagcacga
3841 tcatgcgcac ccgtggccag gacccaacgc tgcccgagat gcgccgcgtg cggctgctgg
3901 agatggcgga cgcgatggat atgttctgcc aagggttggt ttgcgcattc acagttctcc 3961 gcaagaattg attggctcca attcttggag tggtgaatcc gttagcgagg tgccgccggc 4021 ttccattcag gtcgaggtgg cccggctcca tgcaccgcga cgcaacgcgg
ggaggcagac
4081 aaggtatagg gcggcgccta caatccatgc caacccgttc catgtgctcg ccgaggcggc 4141 ataaatcgcc gtgacgatca gcggtccaat gatcgaagtt aggctggtaa gagccgcgag 4201 cgatccttga agctgtccct gatggtcgtc atctacctgc ctggacagca tggcctgcaa 4261 cgcgggcatc ccgatgccgc cggaagcgag aagaatcata atggggaagg
ccatccagcc
4321 tcgcgtcgcg aacgccagca agacgtagcc cagcgcgtcg gccgccatgc cggcgataat
4381 ggcctgcttc tcgccgaaac gtttggtggc gggaccagtg acgaaggctt gagcgagggc 4441 gtgcaagatt ccgaataccg caagcgacag gccgatcatc gtcgcgctcc
agcgaaagcg
4501 gtcctcgccg aaaatgaccc agagcgctgc cggcacctgt cctacgagtt gcatgataaa 4561 gaagacagtc ataagtgcgg cgacgatagt catgccccgc gcccaccgga
43 aggagctgac
4621 tgggttgaag gctctcaagg gcatcggtcg agatcccggt gcctaatgag tgagctaact 4681 tacattaatt gcgttgcgct cactgcccgc tttccagtcg ggaaacctgt cgtgccagct 4741 gcattaatga atcggccaac gcgcggggag aggcggtttg cgtattgggc
gccagggtgg
4801 tttttctttt caccagtgag acgggcaaca gctgattgcc cttcaccgcc tggccctgag 4861 agagttgcag caagcggtcc acgctggttt gccccagcag gcgaaaatcc tgtttgatgg 4921 tggttaacgg cgggatataa catgagctgt cttcggtatc gtcgtatccc actaccgaga 4981 tatccgcacc aacgcgcagc ccggactcgg taatggcgcg cattgcgccc agcgccatct 5041 gatcgttggc aaccagcatc gcagtgggaa cgatgccctc attcagcatt tgcatggttt 5101 gttgaaaacc ggacatggca ctccagtcgc cttcccgttc cgctatcggc tgaatttgat 5161 tgcgagtgag atatttatgc cagccagcca gacgcagacg cgccgagaca gaacttaatg 5221 ggcccgctaa cagcgcgatt tgctggtgac ccaatgcgac cagatgctcc acgcccagtc 5281 gcgtaccgtc ttcatgggag aaaataatac tgttgatggg tgtctggtca gagacatcaa 5341 gaaataacgc cggaacatta gtgcaggcag cttccacagc aatggcatcc tggtcatcca 5401 gcggatagtt aatgatcagc ccactgacgc gttgcgcgag aagattgtgc accgccgctt 5461 tacaggcttc gacgccgctt cgttctacca tcgacaccac cacgctggca cccagttgat 5521 cggcgcgaga tttaatcgcc gcgacaattt gcgacggcgc gtgcagggcc
agactggagg
5581 tggcaacgcc aatcagcaac gactgtttgc ccgccagttg ttgtgccacg cggttgggaa 5641 tgtaattcag ctccgccatc gccgcttcca ctttttcccg cgttttcgca gaaacgtggc 5701 tggcctggtt caccacgcgg gaaacggtct gataagagac accggcatac tctgcgacat 5761 cgtataacgt tactggtttc acattcacca ccctgaattg actctcttcc gggcgctatc 5821 atgccatacc gcgaaaggtt ttgcgccatt cgatggtgtc cgggatctcg acgctctccc 5881 ttatgcgact cctgcattag gaagcagccc agtagtaggt tgaggccgtt gagcaccgcc 5941 gccgcaagga atggtgcatg caaggagatg gcgcccaaca gtcccccggc
cacggggcct
6001 gccaccatac ccacgccgaa acaagcgctc atgagcccga agtggcgagc ccgatcttcc
6061 ccatcggtga tgtcggcgat ataggcgcca gcaaccgcac ctgtggcgcc ggtgatgccg 6121 gccacgatgc gtccggcgta gaggatcgag atctcgatcc cgcgaaatta atacgactca 6181 ctatagggga attgtgagcg gataacaatt cccctctaga aataattttg tttaacttta 6241 agaaggagat atacatatgc ggggttctca tcatcatcat catcatggta tggctagcat 6301 gactggtgga cagcaaatgg gtcgggatct gtacgacgat gacgataagg atcatccctt 6361 cacc
44 4. pET100-HpHSP60 300-547
4.1 Map
4.2 pET100-HpHSP60 300-547 DNA sequence
1 attagcgaag aattaggctt gactttagaa aacgctgaag tggagttttt aggcaaagcc 61 ggaaggattg tgattgacaa agacaacacc acgatcgtag atggcaaagg acatagccat 121 gatgttaaag acagagtcgc gcaaatcaaa acccaaattg caagcacgac aagcgattat 181 gacaaagaaa aattgcaaga aagattggcc aaactctctg gtggtgtggc tgtgattaaa 241 gtgggcgctg cgagtgaagt ggaaatgaaa gagaaaaaag accgggttga tgacgcattg 301 agtgcgacta aagcagctgt tgaagagggc attgttattg gcggcggtgc ggctctcatt 361 cgcgcggctc aaaaagtgca tttgaattta cacgatgatg aaaaagtagg ctatgaaatc 421 atcatgcgtg ccattaaagc cccattagct caaatcgcta tcaatgccgg ttatgatggc 481 ggtgtggtcg tgaatgaagt gcaaaaacac gaagggcatt ttggttttaa cgctagcaat 541 ggcaagtatg tggatatgtt taaagaaggc attattgacc ccttaaaagt agaaaggatc 601 gctttacaaa atgcggtttc ggtttcaagc ctgcttttaa ccacagaagc caccgtgcat 661 gaaatcaaag aagaaaaagc aaccccagca atgcctgata tgggtggcat gggcggtatg
45
721 ggaggcatgg gcggcatgat gtaaaagggc gagctcaacg atccggctgc taacaaagcc 781 cgaaaggaag ctgagttggc tgctgccacc gctgagcaat aactagcata accccttggg 841 gcctctaaac gggtcttgag gagttttttg ctgaaaggag gaactatatc cggatatccc 901 gcaagaggcc cggcagtacc ggcataacca agcctatgcc tacagcatcc agggtgacgg 961 tgccgaggat gacgatgagc gcattgttag atttcataca cggtgcctga ctgcgttagc 1021 aatttaactg tgataaacta ccgcattaaa gcttatcgat gataagctgt caaacatgag 1081 aattaattct tgaagacgaa agggcctcgt gatacgccta tttttatagg ttaatgtcat 1141 gataataatg gtttcttaga cgtcaggtgg cacttttcgg ggaaatgtgc gcggaacccc 1201 tatttgttta tttttctaaa tacattcaaa tatgtatccg ctcatgagac aataaccctg 1261 ataaatgctt caataatatt gaaaaaggaa gagtatgagt attcaacatt tccgtgtcgc 1321 ccttattccc ttttttgcgg cattttgcct tcctgttttt gctcacccag aaacgctggt 1381 gaaagtaaaa gatgctgaag atcagttggg tgcacgagtg ggttacatcg aactggatct 1441 caacagcggt aagatccttg agagttttcg ccccgaagaa cgttttccaa tgatgagcac 1501 ttttaaagtt ctgctatgtg gcgcggtatt atcccgtgtt gacgccgggc aagagcaact 1561 cggtcgccgc atacactatt ctcagaatga cttggttgag tactcaccag tcacagaaaa 1621 gcatcttacg gatggcatga cagtaagaga attatgcagt gctgccataa ccatgagtga 1681 taacactgcg gccaacttac ttctgacaac gatcggagga ccgaaggagc taaccgcttt 1741 tttgcacaac atgggggatc atgtaactcg ccttgatcgt tgggaaccgg agctgaatga 1801 agccatacca aacgacgagc gtgacaccac gatgcctgca gcaatggcaa
caacgttgcg
1861 caaactatta actggcgaac tacttactct agcttcccgg caacaattaa tagactggat 1921 ggaggcggat aaagttgcag gaccacttct gcgctcggcc cttccggctg gctggtttat 1981 tgctgataaa tctggagccg gtgagcgtgg gtctcgcggt atcattgcag cactggggcc 2041 agatggtaag ccctcccgta tcgtagttat ctacacgacg gggagtcagg caactatgga 2101 tgaacgaaat agacagatcg ctgagatagg tgcctcactg attaagcatt ggtaactgtc 2161 agaccaagtt tactcatata tactttagat tgatttaaaa cttcattttt aatttaaaag 2221 gatctaggtg aagatccttt ttgataatct catgaccaaa atcccttaac gtgagttttc 2281 gttccactga gcgtcagacc ccgtagaaaa gatcaaagga tcttcttgag atcctttttt 2341 tctgcgcgta atctgctgct tgcaaacaaa aaaaccaccg ctaccagcgg tggtttgttt 2401 gccggatcaa gagctaccaa ctctttttcc gaaggtaact ggcttcagca gagcgcagat 2461 accaaatact gtccttctag tgtagccgta gttaggccac cacttcaaga actctgtagc 2521 accgcctaca tacctcgctc tgctaatcct gttaccagtg gctgctgcca gtggcgataa 2581 gtcgtgtctt accgggttgg actcaagacg atagttaccg gataaggcgc agcggtcggg 2641 ctgaacgggg ggttcgtgca cacagcccag cttggagcga acgacctaca
ccgaactgag
2701 atacctacag cgtgagctat gagaaagcgc cacgcttccc gaagggagaa aggcggacag
46 cagggggaaa
2821 cgcctggtat ctttatagtc ctgtcgggtt tcgccacctc tgacttgagc gtcgattttt 2881 gtgatgctcg tcaggggggc ggagcctatg gaaaaacgcc agcaacgcgg cctttttacg 2941 gttcctggcc ttttgctggc cttttgctca catgttcttt cctgcgttat cccctgattc
3001 tgtggataac cgtattaccg cctttgagtg agctgatacc gctcgccgca gccgaacgac 3061 cgagcgcagc gagtcagtga gcgaggaagc ggaagagcgc ctgatgcggt attttctcct 3121 tacgcatctg tgcggtattt cacaccgcaa tggtgcactc tcagtacaat ctgctctgat 3181 gccgcatagt taagccagta tacactccgc tatcgctacg tgactgggtc atggctgcgc 3241 cccgacaccc gccaacaccc gctgacgcgc cctgacgggc ttgtctgctc ccggcatccg 3301 cttacagaca agctgtgacc gtctccggga gctgcatgtg tcagaggttt tcaccgtcat 3361 caccgaaacg cgcgaggcag ctgcggtaaa gctcatcagc gtggtcgtga
agcgattcac
3421 agatgtctgc ctgttcatcc gcgtccagct cgttgagttt ctccagaagc gttaatgtct 3481 ggcttctgat aaagcgggcc atgttaaggg cggttttttc ctgtttggtc actgatgcct 3541 ccgtgtaagg gggatttctg ttcatggggg taatgatacc gatgaaacga gagaggatgc 3601 tcacgatacg ggttactgat gatgaacatg cccggttact ggaacgttgt gagggtaaac 3661 aactggcggt atggatgcgg cgggaccaga gaaaaatcac tcagggtcaa
tgccagcgct
3721 tcgttaatac agatgtaggt gttccacagg gtagccagca gcatcctgcg atgcagatcc 3781 ggaacataat ggtgcagggc gctgacttcc gcgtttccag actttacgaa acacggaaac 3841 cgaagaccat tcatgttgtt gctcaggtcg cagacgtttt gcagcagcag tcgcttcacg 3901 ttcgctcgcg tatcggtgat tcattctgct aaccagtaag gcaaccccgc cagcctagcc 3961 gggtcctcaa cgacaggagc acgatcatgc gcacccgtgg ccaggaccca
acgctgcccg
4021 agatgcgccg cgtgcggctg ctggagatgg cggacgcgat ggatatgttc tgccaagggt 4081 tggtttgcgc attcacagtt ctccgcaaga attgattggc tccaattctt ggagtggtga 4141 atccgttagc gaggtgccgc cggcttccat tcaggtcgag gtggcccggc tccatgcacc 4201 gcgacgcaac gcggggaggc agacaaggta tagggcggcg cctacaatcc
atgccaaccc
4261 gttccatgtg ctcgccgagg cggcataaat cgccgtgacg atcagcggtc caatgatcga 4321 agttaggctg gtaagagccg cgagcgatcc ttgaagctgt ccctgatggt cgtcatctac 4381 ctgcctggac agcatggcct gcaacgcggg catcccgatg ccgccggaag
cgagaagaat
4441 cataatgggg aaggccatcc agcctcgcgt cgcgaacgcc agcaagacgt agcccagcgc
4501 gtcggccgcc atgccggcga taatggcctg cttctcgccg aaacgtttgg tggcgggacc 4561 agtgacgaag gcttgagcga gggcgtgcaa gattccgaat accgcaagcg
47
4621 catcgtcgcg ctccagcgaa agcggtcctc gccgaaaatg acccagagcg ctgccggcac
4681 ctgtcctacg agttgcatga taaagaagac agtcataagt gcggcgacga tagtcatgcc 4741 ccgcgcccac cggaaggagc tgactgggtt gaaggctctc aagggcatcg
gtcgagatcc
4801 cggtgcctaa tgagtgagct aacttacatt aattgcgttg cgctcactgc ccgctttcca 4861 gtcgggaaac ctgtcgtgcc agctgcatta atgaatcggc caacgcgcgg
ggagaggcgg
4921 tttgcgtatt gggcgccagg gtggtttttc ttttcaccag tgagacgggc aacagctgat 4981 tgcccttcac cgcctggccc tgagagagtt gcagcaagcg gtccacgctg gtttgcccca 5041 gcaggcgaaa atcctgtttg atggtggtta acggcgggat ataacatgag ctgtcttcgg 5101 tatcgtcgta tcccactacc gagatatccg caccaacgcg cagcccggac tcggtaatgg 5161 cgcgcattgc gcccagcgcc atctgatcgt tggcaaccag catcgcagtg ggaacgatgc 5221 cctcattcag catttgcatg gtttgttgaa aaccggacat ggcactccag tcgccttccc 5281 gttccgctat cggctgaatt tgattgcgag tgagatattt atgccagcca gccagacgca 5341 gacgcgccga gacagaactt aatgggcccg ctaacagcgc gatttgctgg tgacccaatg 5401 cgaccagatg ctccacgccc agtcgcgtac cgtcttcatg ggagaaaata atactgttga 5461 tgggtgtctg gtcagagaca tcaagaaata acgccggaac attagtgcag gcagcttcca 5521 cagcaatggc atcctggtca tccagcggat agttaatgat cagcccactg acgcgttgcg 5581 cgagaagatt gtgcaccgcc gctttacagg cttcgacgcc gcttcgttct accatcgaca 5641 ccaccacgct ggcacccagt tgatcggcgc gagatttaat cgccgcgaca atttgcgacg 5701 gcgcgtgcag ggccagactg gaggtggcaa cgccaatcag caacgactgt
ttgcccgcca
5761 gttgttgtgc cacgcggttg ggaatgtaat tcagctccgc catcgccgct tccacttttt 5821 cccgcgtttt cgcagaaacg tggctggcct ggttcaccac gcgggaaacg gtctgataag 5881 agacaccggc atactctgcg acatcgtata acgttactgg tttcacattc accaccctga 5941 attgactctc ttccgggcgc tatcatgcca taccgcgaaa ggttttgcgc cattcgatgg 6001 tgtccgggat ctcgacgctc tcccttatgc gactcctgca ttaggaagca gcccagtagt 6061 aggttgaggc cgttgagcac cgccgccgca aggaatggtg catgcaagga
gatggcgccc
6121 aacagtcccc cggccacggg gcctgccacc atacccacgc cgaaacaagc gctcatgagc
6181 ccgaagtggc gagcccgatc ttccccatcg gtgatgtcgg cgatataggc gccagcaacc 6241 gcacctgtgg cgccggtgat gccggccacg atgcgtccgg cgtagaggat
cgagatctcg
6301 atcccgcgaa attaatacga ctcactatag gggaattgtg agcggataac aattcccctc 6361 tagaaataat tttgtttaac tttaagaagg agatatacat atgcggggtt ctcatcatca 6421 tcatcatcat ggtatggcta gcatgactgg tggacagcaa atgggtcggg atctgtacga
48 6481 cgatgacgat aaggatcatc ccttcacc