349
Ann. N.Y. Acad. Sci. 1042: 349–356 (2005). © 2005 New York Academy of Sciences. doi: 10.1196/annals.1338.032
Protection by Resveratrol, a Component of
Red Wine
HUEI-MEI HUANG,a YU-CHIH LIANG,b TZU-HURNG CHENG,c CHENG-HEIEN CHEN,c AND SHU-HUI JUANd
aGraduate Institute of Cell and Molecular Biology, Center for Stem Cells Research at Wan-Fang Hospital, Taipei Medical University, Taipei, Taiwan
bBiomedical Technology, Taipei Medical University, Taipei, Taiwan
cDepartment of Medicine, Taipei Medical University–Wan Fang Hospital, Taipei, Taiwan dGraduate Institute of Medical Sciences and Department of Physiology,
School of Medicine, Taipei Medical University, Taipei, Taiwan
ABSTRACT: Resveratrol-mediated heme oxgenase-1 (HO-1) induction has been shown to occur in primary neuronal cultures and has been implicated as having potential neuroprotective action. Further, antioxidant properties of resveratrol have been reported to protect against coronary heart disease. We attempted to examine the HO-1 inducing potency of resveratrol and the regulatory mecha-nism of its induction in rat aortic smooth muscle cells (RASMC). We showed that resveratrol-induced HO-1 expression was concentration- and time-depen-dent. The level of HO-1 expression and its promoter activity mediated by res-veratrol was attenuated by nuclear factor-kappa B (NF-B) inhibitors, but not by mitogen-activated protein kinase (MAPK) inhibitors. Deletion of NF-B binding sites in the promoter region strongly reduced luciferase activity. Col-lectively, we suggest that resveratrol-mediated HO-1 expression occurs, at least in part, through the NF-B pathway.
KEYWORDS: rat aortic smooth muscle cell; heme oxygenase-1; resveratrol; nuclear factor-kappa B
INTRODUCTION
Resveratrol, a polyphenolic compound, is abundant in red wine, grape skin, and seeds. It is present in the cis and trans isoforms, of which the latter is the biologically active one. Epidemiologic studies have reported that a low incidence of cardiovas-cular disease is associated with moderate red wine consumption in southern France—the so-called French paradox.1 Additionally, in vivo and in vitro studies have revealed that resveratrol has significant antioxidant properties and is able to protect against coronary heart disease.2
Address for correspondence: Shu-Hui Juan, Ph.D., Graduate Institute of Medical Sciences and Department of Physiology, Taipei Medical University, 250 Wu-Hsing Street, Taipei 110, Taiwan. Voice: +886-2-27361661 ext. 3717; fax: +886-2-27390214.
Heme oxygenase (HO) is a rate-limiting catalyst in the heme degradation that produces biliverdin, free iron, and carbon monoxide. Three HO isozymes have been identified to have distinct genes.3 Among them, HO-1, a stress-response protein, can be induced by various oxidative-inducing agents, including heme, heavy metals, UV radiation, cytokines, and endotoxin.4 The potential use of HO-1 on therapeutic tar-gets in various diseases has been explored. For instance, adenovirus-mediated gene transfer of HO-1 in animal models can protect against hyperoxia-induced lung injury5 and reperfusion-induced injury of a transplanted liver.6 We recently reported that adenovirus-mediated HO-1 gene transfer inhibits the development of athero-sclerosis in apolipoprotein E–deficient mice.7 Its notable effect prompts us to search for an alternative medicine to serve as a potent HO-1 inducer.
Activation of the transcription of HO-1 or other genes is mediated by a cascade of signal transduction pathways, mainly by modulating the activities of transcription factors. MAPKs contribute to the major pathway for regulating numerous cellular processes such as cell proliferation, differentiation, stress response, and cell death. Nevertheless, conflicting results exist in the literature: stress-related stimuli (sodium arsenite, cobalt chloride, hemin, and cadmium) might be regulated through extracel-lular signal-regulated kinase 1/2 (ERK1/2) or c-Jun N-terminal kinase (JNK or p38MAPK) in the transcription of HO-1 gene expression in different tissues or spe-cies. Furthermore, the redox-sensitive transcription factors of AP-1, Nrf2, and NF-κB have been shown to be involved in heavy metals’ or curcumin’s (a major compo-nent of the food spice turmeric) induction of HO-1 in tumor and human renal prox-imal tubule cells.8,9
Our study attempted to reveal resveratrol’s HO-1 inducing effect in preventing cardiovascular diseases, and to explore its inducing potency and molecular mecha-nism in rat aortic smooth muscle cells (RASMC).
MATERIALS AND METHODS
Resveratrol and curcumin were purchased from Sigma Chemical Co. U0126 and SB202190 were obtained from Tocris Cookson (Bristol, UK). [γ-32P]dATP (6000 Ci/mmol) was purchased from Perkin-Elmer Life Science (North Point, Hong Kong). Dulbecco’s modified Eagle’s medium (DMEM) medium, fetal bovine serum (FBS), and tissue culture reagents were obtained from Invitrogen (Carlsbad, CA). Protein assay agents were purchased from Bio-Rad (Hercules, CA).
Culture of Rat Aortic Smooth Muscle Cells
RASMC were isolated from thoracic aorta of male Sprague-Dawley rats (200– 250 g) by explant outgrowth10 and subculture in DMEM supplemented with 10% FBS. The purity and identity of cells were examined by immunostaining using spe-cific antibody against smooth muscle cell α-actin. Cells from passage 5 to 12 were used for experiments.
Constructs of Plasmid Variants and Luciferase Activity Assay
The pGL3/hHO-1 reporter plasmid, which contains a 3293-bp fragment, −3106 to +186 relative to the transcription start of the human HO-1 gene, was amplified
from the human BAC clone CTA-286B1011 using the primers 5′-AGAGAACAGT-TAGAAAAGAAAG-3′ (sense) and 5′-TACGGGCACAGGCAGGATCAGAA-3′ (antisense). The PCR products were inserted to the pCR2.1-TOPO cloning vector (Invitrogen) and cut with KpnI/XbaI, such that the resulting PCR products contained the KpnI/XbaI site, and this was ligated into the unique KpnI/NheI site present with-in the pGL3 plasmid (Promega). Therefore, we obtawith-ined a pGL3/hHO-1 reporter construct containing about a 3.3-kbp region of human HO-1 promoter driving luciferase gene expression. The luciferase reporter gene constructs pHO-2920 (Bsi-HKAI/BglII), pHO-2739 (SspI/BglII), pHO-2520 (HindIII/BglII), pHO-1890 (BstEII/ BglII), and pHO-1041 (BciVI/BglII) were generated by deletion of the −3293/−2920 fragment of HO-2920, −3293/−2520 fragment of HO-2520, etc., by the restriction enzymes indicated in parentheses. The Klenow enzyme was used to create a blunt end at the 5′-sequence, and BglII was used to generate a 3′-cohesive end. These frag-ments were ligated into the SmaI and BglII sites of pGL3. The identities of the se-quences were confirmed using an ABI PRISM 377 DNA Analysis System (Perkin-Elmer).
For the reporter activity assay, cells were seeded in six-well plates at a density of 1 × 105 cells/well. In brief, RASMC were transiently transfected with 3 µg of plas-mid DNA containing 1 µg of the Renilla luciferase construct, phRL-TK (Promega) to control transfection efficiency, and 2 µg of the appropriate HO-1 promoter firefly luciferase (FL) construct. The next day, cells were transfected with pGL3/hHO-1 and phRL-TK (Promega) as internal control plasmid using the Lipofet-AMINE 2000ΤΜ (Invitrogen). After transfection (12 h), the medium was replaced with com-plete medium and continually incubated for another 36 h. Transfected cells were then treated with drugs for 12 h, and cell lysates were collected. Luciferase activities were recorded in a TD-20/20 luminometer (Turner Designs) using the dual luciferase assay kit (Promega) according to the manufacturer’s instructions. Luciferase activi-ties of reported plasmids were normalized to luciferase activiactivi-ties of the internal con-trol plasmid.
Western Blots
Western blot analysis was carried out using the following antibodies: HO-1 and α-tubulin. To prepare whole-cell lysates, cells were washed twice with ice-cold PBS, resuspended in ice-cold RIPA buffer containing 1 mM Na3VO4, 1 mM PMSF, 1 µg/ mL leupeptin, and 1 µg/mL aprotinin, incubated on ice for 30 min, and vortexed ev-ery 10 min followed by centrifugation at 12,000 rpm for 30 min at 4°C. Whole-cell lysates (80 µg) were electrophoresed on 10% SDS-polyacrylamide gels and then transblotted onto Hybond-P membranes Pharmacia). Membranes were blocked in PBS containing 0.1% Tween-20 and 5% skim milk at room temperature for 30 min. Blots were incubated, in blocking buffer, with the indicated antibodies (with dilu-tions used according to the manufacturer’s instrucdilu-tions) for 1 h at room temperature. After three washes with PBS containing 0.1% Tween-20, blots were incubated with peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG)/anti-mouse IgG (1:5000) for 1 h at room temperature, followed by another washing. Expression of protein was detected using an enhanced chemiluminescence system.
Electrophoretic Mobility Shift Assay
To prepare nuclear protein extracts, RASMC, in 10-cm2 dishes, were washed twice with ice-cold PBS and scraped off into 1 mL PBS after having been treated with resveratrol for 8 h in various concentrations as were indicated. After centrifu-gation of the cell suspension at 500 × g for 3 min, the supernatant was removed and cell pellets were subjected to NE-PERΤΜ nuclear extraction reagents (Pierce) with the addition of 0.5 mg/mL benzamidine, 2 µg/mL aprotinin, 2 µg/mL leupeptin, and 2 mM phenylmethylsulfonyl fluoride. The subsequent procedures for the nuclear protein extraction followed the manufacturer’s instructions. The fraction containing the nuclear protein was used for the assay or was stored at −70°C until use. The se-quence of the oligonucleotides used was AGTTGAGGGGACTTTCCAGGC for NF-κB binding (Promega). The oligonucleotides were end-labeled with [γ-32P] dATP. Extracted nuclear proteins (10 µg) were incubated with 0.1 ng of 32P-labeled DNA for 15 min at room temperature in 25 µL of binding buffer containing 1 µg of poly (dI-dC). For competition with unlabeled oligonucleotides, a 100-fold molar excess of unlabeled oligonucleotides relative to the radiolabeled probe was added to the binding assay. Mixtures were electrophoresed on 5% nondenaturing polyacrylamide gels. Gels were dried and imaged by means of autoradiography.
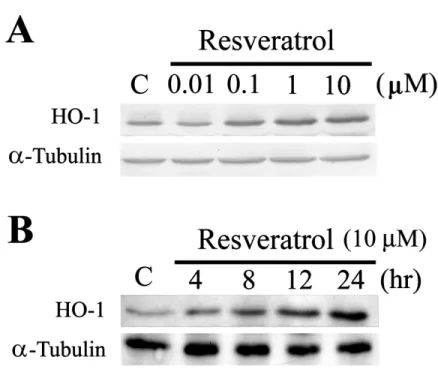
FIGURE 1. Western blot analysis of resveratrol-mediated HO-1 expression in various
concentrations (A) and time points (B). Equal loading was confirmed by incubating with an anti-tubulin antibody. Results of three experiments are shown.
Statistical Analysis
Values are expressed as the mean ± SD. The significance of the difference from the control group was analyzed by Student’s t-test. A value of P < 0.05 was consid-ered statistically significant.
RESULTS AND DISCUSSION
Cells were treated with various concentrations of resveratrol. After 48 h in cul-ture, cell lysates were prepared, and the protein levels of HO-1 in treated cells were assessed by Western blot analysis. A 32-kDa protein band corresponding to HO-1 was highly expressed in resveratrol-treated cells in a concentration-dependent man-ner (FIG. 1A). Time-course experiments demonstrated that the expression of HO-1
was observed at 4 h after resveratrol treatment and sustained ≥ 24 h after treatment (FIG. 1B). Given that resveratrol induces HO-1, it is tempting to postulate that the
antioxidant and anti-inflammatory properties of resveratrol may be, at least in part, related to HO-1 induction. Resveratrol-mediated HO-1 induction is regulated at both the transcriptional and translational levels, because actinomycin D (a transcriptional inhibitor) and cycloheximide (a translational inhibitor) elminated HO-1 mRNA and protein expression (data not shown). The mechanism of translation regulation of HO-1 induction by resveratrol remains to be established.
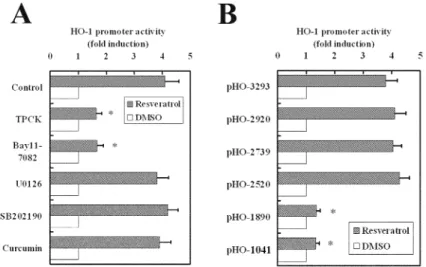
FIGURE 2. Resveratrol-mediated HO-1 promoter activity in relation to NF-κB and
MAPK inhibitors (A) and deletion mutations in the HO-1 promoter region (B). RASMC were transiently transfected with pGL3/hHO-1 variants and phRL-TK as an internal control plasmid for 48 h, followed by treatment of inhibitors (10 µM) for 1 h prior to incubation with resveratrol (5 µM) for another 12 h. Luciferase activities of the reported plasmid were normalized to those of the internal control plasmid and are presented as the mean ± SD of three independent experiments. ∗P < 0.01 indicates a significant difference from the control.
To reveal the molecular mechanism of resveratrol-mediated HO-1 induction, MAPK and NF-κB inhibitors were employed. The results showed that the level of resveratrol-induced HO-1 expression was attenuated by TPCK (a protease inhibitor that blocks activation of NF-κB) and BAY 11-7082 (an inhibitor of IκB phosphory-lation), but not by inhibitors of Erk1/2, JNK, and p38MAPK (data not shown).
Another approach consisting of deletion mutations was used to confirm that the importance of the NF-κB binding site is attributable to resveratrol-mediated HO-1 induction. A luciferase reporter vector containing −3293 bp of the human HO-1 pro-moter region was obtained. This construct was transiently transfected into RASMC for 48 h followed by treatment with MAPK and NF-κB inhibitors (10 µM) for 1 h prior to the addition of resveratrol (5 µM). After incubation for another 12 h, cells were harvested and analyzed for luciferase activity. Consistently, NF-κB inhibitors abolished the HO-1 promoter activity induced by resveratrol, but not by MAPK in-hibitors (FIG. 2A). NF-κB consensus binding elements are located at −2641 to −2632
and −2451 to −2442 bp in the HO-1 promoter region. A series of deletion mutations on HO-1 promoter were carried out, and their activities were eliminated when the consensus binding sites for NF-κB were removed (FIG. 2B).
The most significant finding in this study is the demonstration of the involvement of the NF-κB pathway in resveratrol-mediated HO-1 gene induction. The
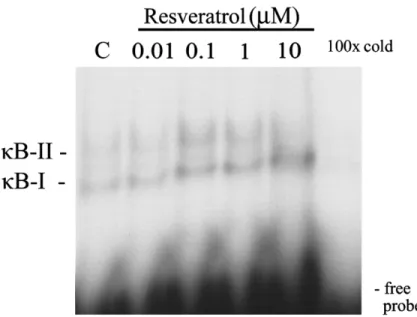
electro-FIGURE 3. The electrophoretic mobility shift assay (EMSA) of the NF-κB binding
ac-tivity by resveratrol. Binding acac-tivity of NF-κB in RASMC exposed to resveratrol. Cells were cultured and treated with increasing concentrations of resveratrol as indicated. Nuclear proteins were assayed for NF-κB binding activity by EMSA. “100× cold” denotes a 100-fold molar excess of unlabeled oligonucleotides relative to the 32P-labeled probe; this was added
to the binding assay for competition with the unlabeled oligonucleotides. The mobility of specific NF-κB complexes is indicated. Representative results of three separate experiments are shown.
phoretic mobility shift assay revealed that resveratol—within a range of 0.1 to 10µM—indeed increased NF-κB binding activity (FIG. 3). Evidence in support of
this pathway was provided by two approaches. The NF-κB inhibitors, TPCK and BAY 11-7082, completely prevented resveratrol-induced HO-1 expression and the activity of the HO-1 promoter, which indicated involvement of the NF-κB pathway. In addition, serial mutants confirmed that NF-κB binding elements are responsible for resveratrol-mediated HO-1 induction. Furthermore, our study revealed that the transactivation of NF-κB by resveratrol within a range of 0.1 to 10 µM occurs by in-creasing the extent of IκBα phosphorylation. As a result, resveratrol facilitated the translocation of NF-κB into nucleus and then modulated HO-1 gene expression (data not shown).
NF-κB is generally considered a redox-sensitive and proinflammatory transcription factor. Interestingly, the results of our study are in accord with a recent study showing that curcumin-induced HO-1 upregulation occurs through the NF-κB pathway.9 Both resveratrol and curcumin are natural polyphenolic compounds possessing antitumor and anti-inflammatory properties. Thus, the structural and functional similarities of resveratrol and curcumin also exert similar molecular actions on HO-1 induction. It is intriguing that resveratrol, on the one hand, induces HO-1 via a pro-oxidative and proinflammatory transcription factor (NF-κB) but, on the other hand, possesses anti-oxidant and anti-inflammatory properties, further emphasizing the functional impor-tance of HO-1 as an adaptive response to oxidative stress and inflammation.
In conclusion, results from our study indicate that the activation of NF-κB is at-tributable to resveratrol-mediated HO-1 induction. Recently, numerous in vitro and
in vivo studies have shown that the induction of HO-1 is an important cellular
pro-tective mechanism against oxidative injury.4 Therefore, resveratrol might be a poten-tial dietary component for protecting cells and tissues against oxidative injuries. Further studies using resveratrol will clarify the possibility of developing this new “drug” for the prevention or treatment of cardiovascular disease.
ACKNOWLEDGMENTS
This study was supported by grants from the National Science Council, Taiwan (NSC92-2320-B-038-015; NSC93-2320-B-038-022).
REFERENCES
1. RENAUD, S. & M. DE LORGERIL. 1992. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339: 1523–1526.
2. BORS, W. & M. SARAN. 1987. Radical scavenging by flavonoid antioxidants. Free Radic. Res. Commun. 2: 289–294.
3. MAINES, M.D. 1997. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37: 517–554.
4. PLATT, J.L. & K.A. NATH. 1998. Heme oxygenase: protective gene or Trojan horse. Nat. Med. 4: 1364–1365.
5. OTTERBEIN, L.E. et al. 1999. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J. Clin. Invest.
103: 1047–1054.
6. AMERSI, F. et al. 1999. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J. Clin. Invest. 104: 1631–1639.
7. JUAN, S.H. et al. 2001. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation
104: 1519–1525.
8. ELBIRT, K.K. et al. 1998. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells. Role of mitogen-activated protein kinases. J. Biol. Chem. 273: 8922–8931.
9. HILL-KAPTURCZAK, N. et al. 2001. Mechanism of heme oxygenase-1 gene induction by curcumin in human renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 281: F851–859.
10. FISHER-DZOGA, K. et al. 1973. Ultrastructural and immunohistochemical studies of pri-mary cultures of aortic medial cells. Exp. Mol. Pathol. 18: 162–176.
11. KIM, U.J. et al. 1996. Construction and characterization of a human bacterial artificial chromosome library. Genomics 34: 213–218.