Biological Energy from the Igneous Rock Enhances Cell
Growth and Enzyme Activity
Yuh-Ling Lin,
1Hsien-Shou Kuo,
1Chien-Tsu Chen
1and Sheng-Chu Kuo
2 1DEPARTMENT OF BIOCHEMISTRY, TAIPEI MEDICAL COLLEGE, TAIPEI, TAIWAN; AND2DEPARTMENT OF PHARMACEUTICALCHEMISTRY, CHINA MEDICAL COLLEGE, TAICHUNG, TAIWAN
ABSTRACT. Some effects from natural resources might be ignored and unused by humans. Environmental hormesis could be a phenomena necessary to bio-organism existence on earth. Since 1919, radiation and some heavy metal hormesis from the environment were proved in various reports. In this study, igneous rock with very low radioactivity and high ferrous activity was measured by multichannel analyzer and inductively coupled plasma analyzer. The water treated by igneous rock, both directly soaked or indirectly in contact, induced increased activities of glucose oxidase, catalase, peroxidase, and superoxide dismutase. It also increased cell growth of SC-M1, HCT-15, Raji, and fibroblast cell lines. The water after treatment of igneous rock had no change in pH values, but displayed decreased conductivity values. We assume that the igneous rock could transfer energy to water to change the molecular structure or conformation of water cluster, or by radiation hormesis effect could then induce increased enzyme activity and cell growth. It is also possible that the energy from rock may combine radiation hormesis with other transferable biological energy forms to change water cluster conformation. NUCL MED BIOL27;6:611– 616, 2000. © 2000 Elsevier Science Inc. All rights reserved.
KEY WORDS. Igneous rock, Hormesis, Scavenger enzyme, Cell growth, Water conformation, Conductivity
INTRODUCTION
There are many reports in the scientific literature dating as early as 1919 (8) demonstrating that a low level of ionizing radiation or low concentrations of some virulent substances can stimulate rather than depress biological function and may, in some instances, produce net benefits including stimulation of metabolic detoxifica-tion and repair network (7, 10, 15, 24 –26, 28, 32, 36). In the field of ionizing radiation research, the concept of hormesis is often taken to imply some element of physiologic benefit from low-linear energy transfer radiation in the range of 0.2–50 cGy total absorbed dose (24). These reports support the existence of “hormesis,” defined as a stimulatory or beneficial effect. According the hypothesis of the hormesis phenomenon, the determining event in the stimulatory effects of low-dose radiation is the excitation of receptor molecules incorporated in condensed, ordered membrane structure (22, 23). The act of excitation in these structures may form solitons and plasmons, capable of changing the receptor conformation as specific receptors do (3, 4). The excited receptors would increase the activity of corresponding enzymes and become more sensitive to the action of specific effectors (23). However, some reports showed that the enzymatic scavengers of free radicals increased within cells of any biological system after low-dose radiation exposure (10, 36). This overproduction of scavengers provides a transient increase in cellular defenses against free radicals that would be protective during a subsequent exposure to ionizing radiation. In addition, there are many factors that could influence the enzyme activity including conductivity, water structure (except pH), temperature, cofactor, and inhibitor (11, 37).
In this report, we found that the natural material of igneous rock (IGR) also has similar effects, both in direct and indirect methods. It is interesting that energy or wave seems to be transferred from IGR to the water by low radiation or/and another mechanism. The transferring wave or energy might change the water structure in some way, then affect cell growth and enzyme activity. The transferring energy could be too tiny to be quantified. We believe that the transferring energy would be very tiny, but large enough to enhance enzyme activity and cell growth and decrease conductivity. The element components and radionuclides of the IGR were also measured by inductively coupled plasma (ICP) and by multichannel analyzer (MCA), respectively.
MATERIALS AND METHODS
The cell lines used were: SC-M1, human adenocarcinoma; Raji, human lymphoma; HCT-15, human colon adenocarinoma; HeLa, human cervical carcinoma; Hep G2, human hepatoma; SF, human foreskin fibroblast; NHEK, normal human epidermal keratinocyte; and PC12, pheochromocytoma of rat adrenal gland.
Materials
The IGR was a kind gift from Mr. Wako Kimio in Yakohama, Japan. The source of IGR is the mountain of Omachi in Nagano, Japan. The IGR was ground to sandlike particles by grindstone before use. The density of IGR was 3.33 g/cm3. Culture mediums used were:
RPMI 1640 medium, minimum essential media (MEM) medium, Dulbecco’s modified Eagle’s Media (DMEM) medium, fetal bovine serum (FBS), MEM nonessential amino acid, L-glutamine, sodium pyruvate, penicillin-streptomycin, and fungizone, purchased from Gibico Laboratories (Grand Island, NY, USA); keratinocyte growth medium (KGM kit), purchased from Clontics Laboratories (Walk-ersville, MD, USA); catalase, peroxidase, glucose oxidase,
superox-Address correspondence to: Dr. Yuh-Ling Lin, Taipei Medical College, Department of Biochemistry, 250 Wu-Hsing St., Taipei, Taiwan; e-mail: yllin@tmc.edu.tw.
Received 31 December 1999. Accepted 7 April 2000.
ide dismutase (SOD), xanthine oxidase, tetra-methylbenzidine (TMB), thymol, o-dianisidine, and 4-aminoantipyrine, purchased from Sigma Chemical Co. (St. Louis, MO, USA). All others chemical reagents were purchased from Merck (Darmstadt, Germany).
Human Cancer and Normal Cell Lines Cultured
SC-M1 and HCT-15 were cultured in RPMI 1640 medium with 10% FBS, 2 mM L-glutamine, 25 mM Hepes, Hep G2, and SF. Raji and PC12 were cultured in DMEM medium with 10% FBS, 2 mM L-glutamine, and 10 mM sodium pyruvate. HeLa was cultured in MEM medium with 10% FBS. All mediums contained antibacterial reagents with 100 U/mL penicillin, 100g/mL streptomycin, and 0.1% fungizone. Normal human epidermal keratinocyte (NHEK) were grown in keratinocyte growth medium kits. All cells were incubated in a humidified atmosphere of 5% CO2 at 37°C. Cell
cultures were subcultured once or twice weekly using trypsin-ethylene-diamine-tetraacetic acid (EDTA) to detach the cell from the culture flask. Numbers of cells were counted after trypsinization by a Neubauer hemocytometer (VWR, Scientific Corp. Philadel-phia, PA, USA). All the cultured mediums were prepared from direct method (direct IGR water) and the IGR was sterilized by autoclave firstly.
Measurement of Cell Growth of Various Cell Lines
The same number of various cell lines, detached by trypsin-EDTA, were seeded individually in six-well cultured plates. These various cell lines were cultured in mediums prepared from direct IGR water or untreated control water at 37°C, 5% CO2in an incubator for afew days. In 80% cell density of controls, cells were harvested after trypsinization, then the optical density (O.D.) was measured at 620 nm wavelength. The increasing percentage of cell number over control was according to the value of [(O.D. of treated sample⫼ O.D. of control)⫺ 1] ⫻ 100%.
Water Treated by IGR Included Direct and Indirect
Methods
The treatment with IGR water was divided into two methods to treat the mini-Q H2O. The direct method was that 0.1% (wt/vol)
IGR sand was immersed overnight in mini-Q H2O directly before
use. The direct IGR water was that the supernatant of IGR sand was immersed in mini-Q H2O. The IGR water from indirect method
was from a tightly enclosed eppendrof vial containing 0.1 g of IGR sand inside, immersed in 100 mL of mini-Q H2O overnight before
use; the IGR did not have direct contact with mini-Q H2O. The
indirect IGR water was from the mini-Q H2O after the eppendrof
vial was removed. The direct and indirect IGR water were the sources of cell culture medium and enzyme reaction buffer prepara-tion, respectively.
The Element Components and Radionuclide of IGR
Measured by ICP and MCA
The element components of IGR were extracted by HNO3 and
hydrofluoric acid (HF) mixed solvent under pressure pump at 170 °C for 4 h. The extracted solution was detected by ICP analyzer (Perkin Elmar; Stimultaneous ICP-AES Allied Analytical System JARREL-ASH and ICP-Mass Spectrometer, Model ICAP 9000, OPT-DV 3000 and ICP-MS model Elam 5000). The IGR
radionu-clides were measured by MCA of high purity germanium (HPGe) detector (Canberra Model 1510, MCA-series 35 PLUS, and soft-ware SAMPO 90).
Measurement of Conductivity, pH, and Half-Peak Width
(
) of IGR Water
Both direct and indirect IGR water in 50 mL plastic tubes were measured for conductivity and pH value by conductivity meter (Horiba; model DS-15) and pH meter (Horiba, model F-23) at room temperature, respectively. The half-peak width of direct and indirect IGR water were prepared with 70% D2O (Acrose; Geel,
Belgium) and 30% IGR water. The mixed water was measured for the half-peak width of water in 4.633 ppm peak by Brucker 500 nuclear magnetic resonance (NMR).
Measurement of Catalase and Peroxidase Activity in IGR
Water
Catalase activity in IGR water was assayed according the method of Aebi (1). Briefly, the substrate solution was prepared from direct and indirect IGR water with thymol (0.66 mM), 4-aminoantipy (1 mM), and Tris-EDTA buffer, respectively. Ten microliters of peroxidase (100 U/mL) and 50L of H2O2(10 mM) were added to
890L of substrate solution. In the meantime, 50 L of catalase (500 U/mL) was added to the reacting mixture solution, then the O.D. was immediately measured by spectrophotometer (Beckman DU-70) at 505 nm wavelength. The catalase activity monitored the O.D. increase at the fifth minute. The peroxidase activity was measured according the method of Bos et al. (5). In brief, the substrate solution tetramethylbenzidine (TMB, 0.1 mg/mL) was dissolved in IGR water with 0.1 M of sodium-acetate buffer (pH 6.0). The O.D. was measured at 450 nm wavelength when 0.01% H2O2 and peroxidase were integrated to substrate solution. The
peroxidase activity monitored the O.D. slope decrease.
Measurement of Xanthine Oxidase Activity
Xanthine oxidase activity was assayed according the method of Watts et al. (35). In brief, the substrate solution hypoxanthine (2.5g/mL) was prepared in 0.05 M of phosphate buffer, pH 7.5, from IGR water. The xanthine oxidase (0.15 u/mL) was added to substrate solution, and the O.D. was measured at 290 nm wave-length by spectrophotometer. The xanthine oxidase activity mon-itored the O.D. slope increase.
Measurement of Glucose Oxidase Activity
Glucose oxidase activity was measured by the method of Kleppe (20). Briefly, 1.8%-D-glucose solution was prepared in 0.1 M of phosphate buffer, pH 5.6, with 0.01% o-dianisidine from IGR water. Glucose oxidase (5 U/mL) was added after the enzyme peroxidase (1 g/mL) integrated the substrate solution. Then the O.D. was measured at 460 nm wavelength. The glucose oxidase activity monitored the O.D. slope increase.
Measurement of SOD Activity
SOD activity was measured according to the method of Kim et al. (19). Briefly, 42g/mL of auto-oxidative substrate pyrogallol was dissolved in Tris-HCl buffer, pH 8.0, with IGR water. The O.D. was
measured by spectrophotometer at 420 nm wavelength after the enzyme SOD (0.25 U/mL) integrated the substrate solution. The SOD activity measured the O.D. slope increase.
RESULTS
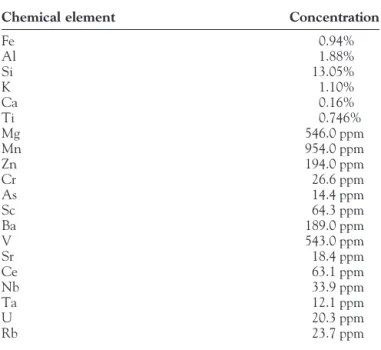
The component elements of IGR were measured by ICP. The major chemical elements in IGR were about 20 elements (Table 1). Fe and Si comprised almost 23% of the total elements of the IGR. Most rocks in earth contain some radionuclide. The radionuclides in this IGR were Pb212, Pb214, and TL208, and the radio intensities
were about three to six times higher than that in blank (Table 2). The -emission of IGR sand was detected by the -counter (Beckman L7), and was almost the same as background (data not shown). The IGR sand was also exposed with X-film (Kodak Bio-Max film) at⫺70°C; some black spots were observed after 6 weeks of exposure (data not shown). From the results, it can be seen that the radioactivity of IGR was very low.
There were no significant differences in the pH values between treated and untreated mini-Q H2O (Table 3). However, no factor
seems to influence the pH value other than conductivity after IGR treatment. The conductivity values in treated IGR water were much lower than untreated, and the indirect IGR water showed the lowest value (Table 3). For the half-peak width () of H2O measured by
NMR, the lowest value was direct IGR water, and there were no
significant differences between indirect IGR water and untreated water. It is possible that the direct IGR water could contain some salt or metal ion dissolved from IGR to water to make the half-peak width decrease.
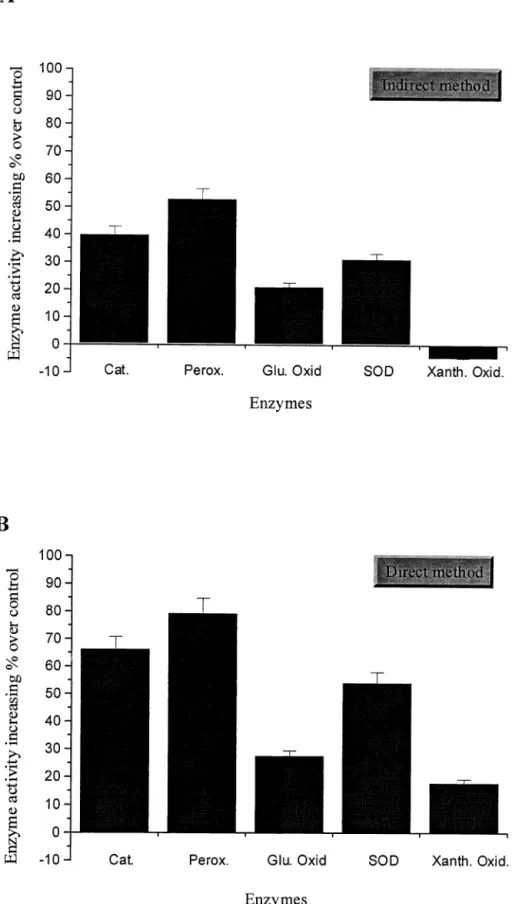
In biological effects, cell growth and enzyme activity were enhanced by the existence of IGR water. Different cells showed different growth effects after culture in direct IGR water. The digestive system cells, colon adenocarcinoma HCT-15 and gastric adenocarcinoma SC-M1 cells, had greater cell number increase than the others. The cell numbers of HCT-15 and SC-M1 cells were increased 87% and 50% over control, respectively (Fig. 1). The cell numbers of lymphocyte Raji and foreskin fibroblast SF cells were within 25% of control (Fig. 1). However, the other cells, including cervical carcinoma HeLa, liver carcinoma Hep G2, normal keratinocyte NHEK, and adrenal gland pheochromocytoma PC12, showed no significant effects in cell number increase (Fig. 1). In the enzyme assays, the enzyme activities were obviously potent with IGR water, in both the direct and the indirect methods. The three antioxidant enzymes catalase, peroxidase, and SOD showed more potent enzyme activity than the other two enzymes, glucose oxidase and xanthine oxidase. The most potent enzyme of the three antioxidants was peroxidase, whose activity increased 53% with indirect IGR water and 82% with direct IGR water compared to untreated water (control). The second most potent enzyme was catalase, whose activity increased 40% with indirect IGR water and 67% with direct IGR water compared to control water. The SOD was third; the potent activity increased 32% with indirect IGR water and 56% with direct IGR water. The glucose oxidase increased 20% and 25%, respectively. The least potent enzyme was xanthine oxidase, which decreased 3% with indirect IGR water and increased 18% with direct IGR water (Figs. 2A and 2B). It is interesting to note that different cells with different growth effects and different enzymes with different activity effects, under the cell-cultured medium or enzyme reaction buffer, were prepared from IGR, especially with direct IGR water.
DISCUSSION
Thus far, the phenomena of radiation hormesis has been observed by many scientists. The concept of hormesis is accepted by most researchers. The radiation hormesis effect includes many aspects, such as growth stimulation and accelerated development (7, 29), fecundity increase (30), tumor-induction decrease (14, 31), radio-resistance and radio-resistance to infection (6, 13), and faster wound healing (27). The phenomena of low-dose radiation hormesis could induce not only beneficial effects but also damage. Since bio-organisms live within the humidity system, it is worth noting that most or part of the radiation hormesis effects could be mediated by the change in water conformation, showing benefits in biological systems.
TABLE 1. The Chemical Element Concentrations of IGR Measured by ICP
Chemical element Concentration
Fe 0.94% Al 1.88% Si 13.05% K 1.10% Ca 0.16% Ti 0.746% Mg 546.0 ppm Mn 954.0 ppm Zn 194.0 ppm Cr 26.6 ppm As 14.4 ppm Sc 64.3 ppm Ba 189.0 ppm V 543.0 ppm Sr 18.4 ppm Ce 63.1 ppm Nb 33.9 ppm Ta 12.1 ppm U 20.3 ppm Rb 23.7 ppm
TABLE 2. The Radionuclide Elements and Activities of IGR Measured by MCA
Radionuclide
Intensity (gps)
Blank IGR sample
Th-Pb212 8.76 39.2⫾ 6.7
Pb214 2.26 7.7⫾ 1.7
Ra-Pb214 2.26 14.3⫾ 2.3
T1208 6.92 16.5⫾ 1.9
TABLE 3. pH, Conductivity Values, and Half-Peak Width of IGR Water Treated (Direct or Indirect Method) and Untreated (Control) Treatment pH (unit) Conductivity (s/cm) Half-peak width () (Hz) Control 5.66⫾ 0.16 3.79⫾ 0.5 18.36⫾ 0.16 Indirect 5.61⫾ 0.05 1.84⫾ 0.2 17.58⫾ 0.81 Direct 5.71⫾ 0.11 2.54⫾ 0.2 12.18⫾ 1.2
The mysterious water molecule is present in all macromolecular biological systems and is regarded as an essential component of both their structures and functions, but its actual role in the life process is relatively poorly understood. The water molecules near the surface of a protein crystal show evidence of some order, so-called bound water, but further from the surface, the unbound water molecules show no evidence of order (16, 21). Both proteins and nucleic acids are greatly hydrated in the crystalline state, where between 20 and 50% of the crystal volume can be occupied by water molecules. The water is more disordered than ordered (16).
The importance of water in living processes derives not only from its ability to form hydrogen bonds with other water molecules but also from its capacity to interact with various types of biological molecules (12, 33, 34). The hydrogen bond is important, but not very well understood in the role in biological structure and function. However, it is now regarded as an essential component of the structure and function of biological molecules (17, 18). There are two types of hydrogen bonds; one is strong hydrogen bond and the other is moderate hydrogen bond. Strong hydrogen bonds are rare in biological structures since they are too rigid and not easily broken (16). Water has considerable flexibility in its hydrogen bonding, and it can cover irregular surfaces and occupy channels or pockets in irregularly shaped macromolecules (2). Water molecules are proposed to be of two states; one is free water with small molecules (dense water) and the other is cluster water with larger conforma-tions (icy water). Water has two types existing at same time (called mixture model), but the ratio of dense/icy molecules will be influenced by water surface energy of contacting surfaces (16, 33). Water has a high dielectric constant associated with the distor-tion or breaking of hydrogen bonds. The fact that hydrogen bonding facilitates, or restricts, proton transfer is considered by some to be the most important chemical property of the hydrogen bond (16, 38). It also has the anomalous conductivity associated with the transfer of H⫹ and OH⫺ ions through the hydrogen-bonded structure. The facility of hydrogen bonds to transmit H⫹and OH⫺ ions in water or an aqueous media provides a catalysis mechanism for many reactions. In the field of molecular biology, proton transfer has come to be recognized as a significant component of enzyme catalysis and transmission of ion through membranes (9). However,
we observed the same kind of biological energy from IGR in this study, which is an interesting phenomenon and reported here for first time.
The radioactivity of IGR was very low, less than background from our results (Table 2). We assume that the low radiation or some kind of energy from IGR could be mediated by changing the water cluster to induce the decrease of conductivity and increase enzyme activity and cell growth. Pure water (mini-Q H2O) has a low
conductivity based on low concentrations of H⫹and OH⫺ions. Our study results show that the indirect IGR water had the lowest conductivity compared with direct IGR water and control water. We propose that the indirect IGR water had a higher ratio of dense/icy molecules, and it was easier with the dense molecule to surround the H⫹and OH⫺ions then decrease the conductivity, but there was no effect on pH values (Table 3). In the direct IGR water, this was also possible with more dense molecules, but there were some metals and ions dissolved from IGR that caused the conduc-tivity to increase slightly. It is also possible that the much more dense molecules in IGR water could more easily help the nutrient and ion transmission through membrane to enhance cell growth. However, the conductivity change in IGR water might influence the osmolarity of cell membrane to enhance cell growth and enzyme activity. It is also possible that these metals and ions released from IGR could provide some cofactors or various organic molecules that enhance the cell growth and enzyme activity not only in direct IGR water, but also in indirect contact IGR water. The cells of SC-M1, HCT-15, Raji, and fibroblast could be more sensitive to IGR effects (Fig. 1) and to the enzyme catalysis especially, by using of small molecule substrates of antioxidant scavenger enzymes (Fig. 2).
The total radioactivity of IGR in this study was much lower than the general dosage used in radiation hormesis. Based on the low radioactivity of IGR and the fact that it did not irradiate to enzyme or cell directly, the effect of radiation hormesis from IGR could a factor in inducing cell growth and enzyme activities. In our experimental design, the rock was soaked in water directly or contacted indirectly with water, then both the waters treated by IGR were used to study the enzyme activity and cell growth, respectively. Therefore, we assumed the IGR study could be not only another example of radiation hormesis, but also could concern FIG. 1. The increasing cell number percentage of various cell lines in-duced by direct IGR water. The vari-ous cell lines were seeded in six-well cultured plates and cultured in culture mediums prepared from either direct IGR water or untreated water as de-scribed in Materials and Methods. The percentage of increasing cell number was calculated as the treated sample over control. All data are meanⴞ SD.
another biological energy from IGR that induces decreased conduc-tivity, some kinds of cell growth, and increased enzyme activities.
This observation is very interesting, and we explained this energy transfer with a changing hypothesis of water cluster conformation.
Undoubtedly we need more evidence to prove this phenomena that we observed in this report. However, we hope that the results from this study will provide new ways of thinking about natural resources in the earth.
FIG. 2. Measurement of increasing enzyme activity percentage over control in various enzymes induced by direct IGR water (A) and indirect IGR water (B). The assays of various enzyme activities are as described in Materials and Methods. The percent-age of increasing cell number was calculated as the treated sample over control. All data are meanⴞ SD.
We thank Mr. Wako Kimio for providing the IGR. Help was provided for the ICP and MCA measurements of IGR by Mr. M. F. Chang of the Institute of Atomic Science and Dr. F. Y. Chou of the Center of Isotopic Science, respectively, at Chin-Haw University, Hsin-Chu, Taiwan.
References
1. Aebi H. (1984) Catalase in vitro. Methods Enzymol. 105, 121–126. 2. Baker E. L. and Hubbard R. E. (1984) Hydrogen bonding in globular
protein. Prog. Biophys. Molec. Biol. 44, 97–179.
3. Balanovski E. and Beaconsfield P. (1982) The role of non-linear field and soliton formation and propagation in DNA function. Phys. Lett. 93, 52–54. 4. Bednar I. (1985) Electronic excitations in condensed biological matter.
Int. J. Radiat. Biol. 48, 147–166.
5. Bos E. S., van der Doelen A. A., van Rooy N. and Schuurs A. H. (1981) 3,3⬘,5,5⬘-tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J. Immunoassay 2, 187–204.
6. Bryant P. E. and Paker J. (1979) Changes in sensitivity of HeLa cells after split doses of X-rays. Int. J. Radiat. Biol. Med. 35, 189 –192. 7. Congdon C. C. (1987) A review of certain low-level ionizing radiation
studies in mice and guinea pigs. Health Phys. 52, 593–597.
8. Davey W. P. (1919) Prolongation of life of pribolium confusum apparently due to small doses of x-rays. J. Exp. Zool. 28, 447– 458. 9. Eckert M. and Zundel G. (1988) Energy surface and proton
polarizabil-ity of hydrogen-bonded chains: An ab-inito treatment with respect to charge conduction in biological systems. J. Phys. Chem. 92, 7016 –7023. 10. Feinendgen L. E., Bond V. P., Booz J. and Muhlensiepen H. (1988) Biochemical and cellular mechanisms of low dose effects. Int. J. Radiat.
Biol. 53, 23–27.
11. Galinski E. A. (1993) Compatible solutes of halophilic eubacteria: Molecular principles, water-solute interaction, stress protection.
Expe-rientia 49, 487– 496.
12. Gerstein M. and Levitt M. (1998) Simulating of water and molecules of life. Sci. Am. 279, 74 –79.
13. Gidali J., Bojtor I. and Feher I. (1979) Kinetic basis for compensated hematopoiesis during continuous irradiation with low doses. Radiat. Res.
77, 285–291.
14. Hellstrom I. and Hellstrom K. E. (1979) Antitumor effect of whole-body X-irradiation: Possible role of X-ray sensitive T-suppressor cell popula-tion. Transplant Proc. 11, 1073–1076.
15. Hendry J. H. (1988) Survival of cells in mammalian tissues after low dose of irradiation: A short review. Int. J. Radiat. Biol. 53, 89 –94. 16. Jeffery G. A. (1997) An Introduction to Hydrogen Bonding. Oxford
University Press, Oxford.
17. Jeffrey G. A. (1994) The role of hydrogen bond and water in biological processes. J. Mol. Struc. 322, 21–25.
18. Jeffrey G. A. and Saenger W. (1991) Hydrogen Bonding in Biological
Structures. Springer-Verlag, Berlin, New York, Heidelberg.
19. Kim S. J., Han D., Moon K. D. and Rhee J. S. (1995) Measurement of
superoxide dismutase-like activity of natural antioxidants. Biosci.
Bio-technol. Biochem. 59, 822– 823.
20. Kleppe K. (1966) The effect of hydrogen peroxide on glucose oxidase from Aspergillus niger. Biochem. 5, 139 –143.
21. Kossiakoff A. A., Sintchak M. D., Shpung I. J. and Presta L. G. (1992) Analysis of solvent structure in protein using neutron deuterium water solvent maps pattern of primary and secondary hydration of trypsin.
Proteins 12, 223–236.
22. Kuzin A. M. (1991) The problem of low-level radiation and hormesis in radiobiology. J. Radiobiol. 31, 16 –21.
23. Kuzin A. M., Ruda V. P. and Mozgovoi E. G. (1991) The role of receptors in radiation hormesis. Radiat. Environ. Biophys. 30, 259 –266. 24. Leukey T. D. (1982) Physiological benefits from low level of radiation
exposure. Health Phys. 43, 771–785.
25. Moghissi A. A. and Ray D. L. (1988) Radiation hormesis and radiation cancer risk. Health Phys. 54, 473.
26. Okamoto K. (1987) Critical values of linear energy transfer, dose rates, and doses for radiation hormesis. Health Phys. 52, 671– 674.
27. Omarov M. A. (1973) Effect of multiple total body radiation on healing of bone fractures. Med. Radio. 18, 64 – 66.
28. Pollycove M. (1995) The issue of the decade: Hormesis. Eur. J. Nucl.
Med. 22, 399 – 401.
29. Semagin V. N. (1973) Influence of prolonged irradiation in low doses on the rat embryo brain. Radiobiol. 15, 583–585.
30. Tanaka S. and Saka A. (1979) Stimulation of allogenic lymphocytes by skin epidermal cells in rat. Transplant 27, 194 –199.
31. Tuschi H., Altmann H., Kovac R., Topalogu A., Egg D. and Gunther R. (1980) Effects of low dose radiation on repair processes in human lymphocytes. Radiat. Res. 81, 1–9.
32. Van Wyngaaarden K. E. and Pauwels E. K. (1995) Hormesis: Are low doses of ionizing radiation harmful or beneficial? Eur. J. Nucl. Med. 22, 481–186.
33. Vogler E. A. (1998) Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 74, 69 –117.
34. Watterson J. G. (1987) A role for water in cell structure. Biochem. J.
248, 615– 618.
35. Watts R. W. E., Watts J. and Seegmiller J. E. (1965) Xanthine oxidase activity in human tissues and its inhibition by allopurinol (4-hydroxy-pyrazolo(3,4-d) pyriimidine. J. Lab. Clin. Med. 66, 688 – 693. 36. Wolff S., Azal V. and Wiencke J. K. (1988) Human lymphocytes
exposed to low doses of ionizing radiations become refractory to high doses as well to high chemical mutagens that induce double strand breaks in DNA. Int. J. Radiat. Biol. 53, 39 – 48.
37. Ye U. J. and Ladik J. (1996) Influence of conformational flexibility of their active sites on electronic conductivity of enzymes. Physiol. Chem.
Physics Med. NMR 28, 123–128.
38. Zundel G. (1992) Proton polarizability and proton transfer processes in hydrogen bonds and cation polarizabilities of other cation bonds—Their importance to understanding molecular processes in electrochemistry and biology. Trends. In. Phys. Chem. 3, 129 –156.