國立交通大學
分子醫學與生物工程研究所
碩 士 論 文
水汽的存在與介電層材料的親水性對有機薄膜電晶
體感測氨氣的影響
Moisture Existence and Hydrophilic Material of Dielectric Layer
Affect Ammonia Sensing of Organic Thin Film Transistor
研究生:王欣怡
指導教授:楊裕雄 教授
冉曉雯 教授
水汽的存在與介電層材料的親水性對有機薄膜電晶體感測氨氣的
影響
Moisture Existence and Hydrophilic Material of Dielectric Layer
Affect Ammonia Sensing of Organic Thin Film Transistor
研究生:王欣怡 Student:Hsin-I Wang
指導教授:楊裕雄 教授 Advisor:Prof. Yuh-Shyong Yang
冉曉雯 教授 Prof. Hsiao-Wen Zan
國 立 交 通 大 學
分 子 醫 學 與 生 物 工 程 研 究 所
碩 士 論 文
A Thesis
Submitted to Institute of Molecular Medicine and Bioengineering College of Biological Science and Technology
National Chiao Tung University in partial Fulfillment of the Requirements
for the Degree of Master
in
Molecular Medicine and Bioengineering August 2010
Hsinchu, Taiwan, Republic of China
水汽的存在與介電層材料的親水性對有機薄膜電晶體感測氨氣的
影響
研究生:王欣怡 指導教授:楊裕雄 教授
冉曉雯 教授
國立交通大學生物科技學院 分子醫學與生物工程研究所 碩士班
摘 要 肝病為盛行於台灣的疾病,可堪稱為國民病。多數人罹患慢性肝炎後,約有5%的 機會會轉變為肝硬化,為一種嚴重且不可逆的病變,目前沒有理想的治療藥物。一般透 過侵入式方法監控肝病患者的血氨濃度,當超過血氨的正常值約每百毫升5~50毫克,患 者依個人耐受性不同即會產生肝昏迷現象,故使氨氣成為肝病患者的監控指標之ㄧ。肝 硬化患者多為居家照護,因此發展非侵入式的氨氣感測器監測患者的血氨濃度可幫助監 控患者的病程,並提供肝昏迷一個預警機制。有研究報告指出肝硬化病者之呼氨濃度為 0.745 ppm高於正常未患病者0.278 ppm。 有機薄膜電晶體 (OTFTs) 可被大面積製造、具低成本、可拋棄式的優點,適合用 來開發非侵入式醫療診斷感測產品或環境監測上之應用。於前期實驗中,已開發出對氨 氣具有高靈敏感測之元件 (可偵測最低氨氣濃度0.5ppm)。我們推測當水分子存在時,氨 氣極易與之結合形成銨離子 (NH4+)。而銨離子為主要影響有機薄膜電晶體的感測能 力。在本研究中旨在探討水分子濃度的提升影響銨離子的形成且增加元件電性的變化 量。其次,我們改變元件介電層的材料:PMMA及PVP,利用材料的親水特性吸引水分 子靠近,能形成更多銨離子,進而與元件作用,提升氨氣感測的靈敏度。Moisture Existence and Hydrophilic Material of Dielectric Layer
Affect Ammonia Sensing of Organic Thin Film Transistor
Student:Hsin-I Wnag Advisor:Prof. Yuh-Shyong Yang Prof. Hsiao-Wen Zan
Institute of Molecular Medicine and Bioengineering College of Biological Science and Technology
National Chiao Tung University
ABSTRACT
Liver disease is common in Taiwan. Most people infected with hepatitis B or C develop chronic liver disease; less cases of liver disease are caused by alcoholism and fatty liver diseases. Cirrhosis is a consequence of chronic liver disease characterized by replacement of liver tissue by fibrous scar tissue. Cirrhosis is generally irreversible and no has ideal medication for therapy. When the liver is dysfunctional, nitrogen compounds cannot be metabolized to ammonium (NH4+); however, ammonia (NH3) is an important indicator for chronic liver disease. Reports
have shown that breath ammonia levels are significantly higher in cirrhotic patients (0.745 ppm) than in healthy subjects (0.278 ppm). Organic thin film transistor (OTFT) is a promising non-invasive, inexpensive, portable, and disposable diagnostic device because of its low-cost fabrication process and high-sensitivity to gas molecules. In our previous study, the OTFT sensor was sensitive to ammonia gas of 0.5 ppm (parts per million), and did not respond to carbon dioxide, ethanol, formaldehyde or methane. Ammonia is highly soluble in water and binds with water molecules to form ammonium (NH4+). We propose that ammonium is a major
factor affecting the sensing ability of OTFT. In this study, we raised water vapor content to form more ammonium ions and enhance the change of electrical characteristics. Second, we chose two materials, PMMA and PVP as a dielectric layer of OTFT. Based on the fact that PVP is more hydrophilic, we discovered that raising water vapor content enhances the ammonia sensing ability of OTFT.
誌 謝
回首過往,滿懷夢想與期望進入理想中的學府就讀,而今好多日子過去了。就學期 間,受到許多人的教導與照顧,在此逐一感謝。 首先感謝我的指導教授 楊裕雄 老師耐心寬容,不勞心煩的教導,並提供優渥的資 源,使我完成碩士論文研究。也謝謝LEPE 實驗室的每位學長、姊及同學們,在我求學 期間的鼓勵、共同奮鬥與相互扶持。特別是淵仁學長,在論文題目與實驗上的大力協助。 再謝謝我的共同指導教授 冉曉雯 老師,在實驗上熱心的與我討論並給予許多建 議,讓我在渾沌之際仍有一盞明燈指引方向。謝謝低溫薄膜元件實驗室的庭毓及洪正不 厭其煩的日夜幫我製作元件材料。 再謝謝 陳皇銘 老師對於我實驗上提供資源的協助,經常性的撥冗與我討論。謝謝 液晶顯示科技暨光電材料實驗室的柏叡、維康、瑞然及每一位同學對我如此親切。 最後感謝我的家人,不論何時總是給我最大的支持。CONTENTS
Abstract (Chinese) ……….…… i Abstract (English) ……….…… ii Acknowledgement ……….……… iv Contents ……….…… v Tables ……….…… vii figures ……….…… viiiAbbreviations and symbols ……….…… x
Chapter I. Natural Product: Ammonia I.1 Sources of Ammonia ….. 1
I.1.1 Atmospheric Sources ….. 1
I.1.2 Human Metabolic ….. 1
I.2 The Importance of Ammonia Sensing (Ammonia in Diseases) ..… 2
I.2.1 Ammonia in Liver Diseases (Liver Cirrhosis) ….. 3
I.3 The Medical Applications for Gas Sensors in Human Body Care ..… 4
I.3.1 Ammonia Sensors and Their Comparisons ..… 4
Chapter II. Theoretical Background of Organic Thin Film Transistor II.1 Introduction ….. 12
II.2 Organic Thin Film Transistor as An Ammonia sensors ….. 12
II.3 Electrical Characteristics of Organic Thin Film Transistor ….. 13
II.3.1 Threshold Voltage ….. 13
II.3.2 Mobility ….. 14
II.3.3 On/Off Current Ratio ….. 14
II.3.4 Subthreshold Swing ….. 15
II.4 Organic Thin Film Transistor Fabrication ….. 15
II.5 Mechanisms of Organic Thin Film Transistors for Ammonia Sensing ….. 16
II.5.1 Electron doping ….. 16
II.5.2 Charge trapping ….. 16
II.5.3 Dielectric layer interaction ….. 17
Chapter III. Materials and Methods
III.1 Chemicals ….. 25
III.2 Facilities ….. 25
III.3 Sensing System ….. 25
III.4 The Measurement of Electrical Characteristics of Organic Thin Film Transistor
….. 26
III.4.1 Id-Vg curve ….. 26
III.4.2 Id-Time measurement ….. 26
III.4.3 I/I0 normalization ….. 26
III.5 Experiment Design ….. 27
Chapter IV. Results and Discussions
IV.1 Under Nitrogen Gas Environment as An <5% Relative Humidity ….. 35 IV.2 Under Air Containing 50% Relative Humidity ….. 36 IV.3 Under >90% Relative Humidity of Water Vapor Environment ….. 37
IV.4 Ammonia in Nitrogen Gas ... 38
IV.5 Ammonia in Air ….. 39
Chapter V. Conclusions ….. 51
Tables
Table I.1 Diseases associated with unusual breath odors. ….. 10
Table I 2 Current ammonia sensors. ….. 11
Figures
Figure I.1 Nitrogen cycle. ….. 7
Figure I.2 Depiction of a general transamination reaction. ….. 8 Figure I.3 Ammonia production from catalyzed by glutamate dehydrogenase. ….. 8 Figure I.4 Overview of catabolism of amino groups in liver. ….. 9 Figure II.1 Chemical structure of pentacene. ….. 19
Figure II.2 Fabrication of OTFT sensors. ….. 20
Figure II.3 Surface morphology of pentacene based PMMA and PVP sensor. 21 Figure II.4 Schematic diagram of mechanisms of ammonia sensing. …..
22 Figure II.5 Kinds odors dependent OTFT responses. ….. 23 Figure II.6 Concentration dependent ammonia sensing response by time
measurement of OTFT-1200. ….. 24
Figure III.1 Sensing-system. ….. 28
Figure III.2 PC-540 Four-channel MFC readout power supply. ….. 28
Figure III.3 Mass flow controller 5850E. ….. 29
Figure III.4 Gas chamber. ….. 29
Figure III.5 PC-615 vacuum gauge controller. ….. 30 Figure III.6 Model 2636A Dual-Channel System Source Meter Instrument (Low
Current). ….. 30
Figure III.7 4200 semiconductor characterization system. ….. 31 Figure III.8 The electric characteristics of PMMA and PVP OTFTs. 32 Figure III.9 The schematic of water molecule content affect ammonia sensing
ability. 33
Figure III.10 Chemical structure and contact angle of PMMA and PVP ….. 34 Figure IV.1 Responses of ammonia after introduced nitrogen gas. ….. 41 Figure IV.2 Responses of ammonia after introduced air, content water vapor
(relative humidity 50±5%). ….. 42
Figure IV.4 Responses of ammonia after introduced water vapor, content water
vapor (relative humidity >90%). …... 44
Figure IV.5 Ammonia in nitrogen gas of PMMA with long time treatment. ….. 45 Figure IV.6 R Ammonia in nitrogen gas of PVP with long time treatment. ….. 46 Figure IV.7 Ammonia changing rate in 200s and 500s of PMMA and PVP in
nitrogen gas. ….. 47
Figure IV.8 Ammonia in air of PMMA with long time treatment. ….. 48 Figure IV.9 Ammonia in air of PVP with long time treatment. ….. 49 Figure IV.10 Ammonia changing rate in 200s and 500s of PMMA and PVP in air. 50
Symbols
Vth :threshold voltage Id :drain current VG :gate voltage
VD :drain bias voltage
W :width of active layer L :length of active layer COX :oxide capacitance μ :mobility S :subthreshold swing k :Boltzmann’s constant T :absolute temperature Ci :capacitance density
Abbreviations
ppm :parts per million
OTFT :organic thin film transistor
MOSFET :metal-oxide-semiconductor field effect transistor PMMA :poly (methyl methacrylate)
PVP :poly-4-vinylphenol
PGMEA :propylene glycol 1-monomethyl ether 2-acetate PMF :poly melamine-co-formaldehyde
Chapter I. Natural Product: Ammonia
I.1. Sources of Ammonia I.1.1. Atmospheric Sources
Ammonia is a natural gas presenting in the atmosphere. There are two pathways for atmospheric nitrogen to enter the ecosystem. The first pathway is the direct deposition of ammonium and nitrate salts by addition to the soil or in rain water. The second pathway is bacterial nitrogen fixation. Some species of bacteria can bind nitrogen and metabolized to nitrogen compounds and ammonium. They release an excess of ammonia into the environment.
A third source of ammonia is combustion from chemical plants and motor vehicles. There are numerous smaller sources of ammonia, produced because of the existence of ammonium ions that are transformed to gaseous ammonia by alkaline rainwater.
A larger source in the overall nitrogen cycle is ammonification, a series of metabolic activities decomposing organic nitrogen. This is performed by bacteria and fungi. The released ammonium ions and gaseous ammonia is again converted to nitrite and nitrate by bacteria. The nitrogen cycle is illustrated in [Figure I.1] [1].
I.1.2. Human Metabolic
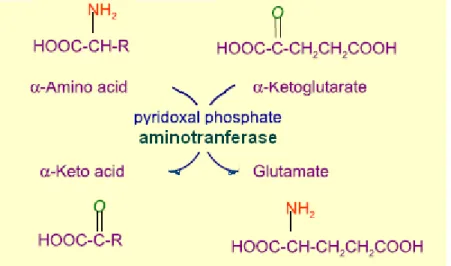
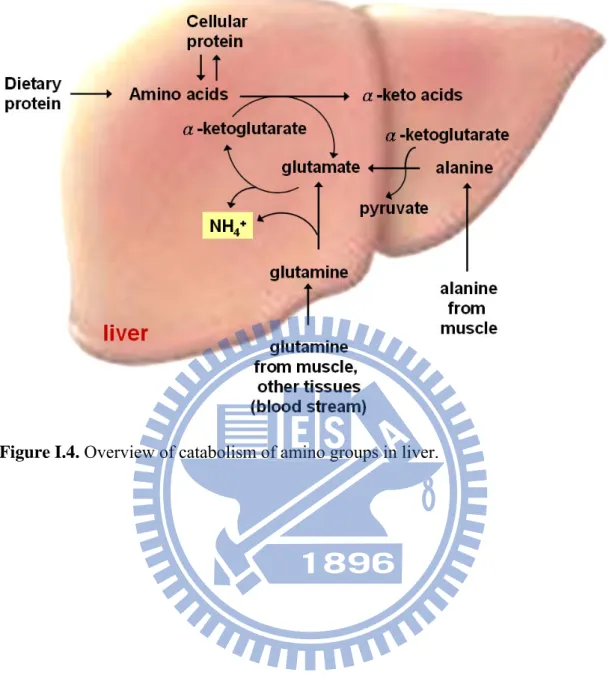
Ammonia is produced by catalyzing of alanin, glutamine and glutamate from dietary amino acids, amine, nucleic acids or tissue protein. The first step in the catabolism is that the most L-amino acids once reached the liver, α-amino groups is
aminotranferases and cofactor, pyridoxal phosphate [Figure I.2]. In hepatocytes, glutamate is transported from the cytosol into mitochondria, where it undergoes oxidative deamination catalyzed by L-glutamate dehydrogenase. Amino groups are
gave up to form ammonium (NH4+) [Figure I.3 and Figure I.4]. Then, in the form of
urea and ammonium salts in urine excrete outside the body. It is considered no accumulation in the body. Some ammonia is removed from the body through sweat glands.
Ammonia is quite toxic to animal tissue. The level of ammonia presenting in blood is regulated. In many tissues, including the brain, some processes such as nucleotide degradation generate free ammonia. Most of the free ammonia is converted to nontoxic compounds before exported into the blood and transported to the liver and kidney. Glutamate can be combined with free ammonia to glutamine, or be transferred amino group to pyruvate forming alanine for passing into the blood and travelling to the liver [2].
I.2. The Importance of Ammonia Sensing (ammonia in diseases)
High concentrations of ammonia form a threat to the human health. However, even below the limit, ammonia is irritating to the respiratory system, skin and eyes. The long term allowed concentration that people may work in is therefore set to be 20 ppm. Immediate and severe irritation of the nose and throat occurs at 500 ppm. Exposure to high ammonia concentrations, 1000 ppm or more, can cause pulmonary oedema; accumulation of fluid in the lungs. It can take up to 24 h before the symptoms develop: difficulty with breathing and tightness in the chest. Short term exposure to such high
ammonia concentrations can lead to fatal or severe long term respiratory system and lung disorders. Extremely high concentrations, 5000–10,000 ppm, are suggested lethal within 5–10 min [1].
Ammonia is toxic to human body. The molecular basis for the toxicity is not entirely understood. The terminal stages of ammonia intoxication in human are characterized by onset of a comatose state accompanied by cerebral edema (an increase in the brain’s water content) and increased cranial pressure [2].
I.2.1. Ammonia in Liver Diseases (Liver Cirrhosis)
Cirrhosis is a consequence of chronic liver disease characterized by replacement of liver tissue by fibrous scar tissue as well as regenerative nodules (lumps that occur as a result of a process in which damaged tissue is regenerated),leading to progressive loss of liver function. It is most commonly caused by hepatitis B (about 70~80%) and C (10%), and less caused by alcoholism and fatty liver disease. Cirrhosis is generally irreversible once it occurs. Treatment generally focuses on preventing progression and complication. So far there is no ideal drug therapy. In advanced stages of cirrhosis the only option is liver transplantation. When liver is dysfunctional, nitrogen compounds can not be metabolized to ammonium salts or urea but ammonia. Because of that, ammonia becomes an important indicator for chronic liver disease. In previous clinical observation, Shimamoto et. al. (2000) reported the cirrhotic patients with the venous blood ammonia level was 59 μg/dL [3]; DuBois et. al. (2005) reported the arterial blood ammonia was 155 μg/dL [4]. Both reports showed the breath ammonia level was above 0.5 ppm. In contrast, the blood ammonia and the
breath ammonia of healthy people were 30 μg/dL and 0.28 ppm.
I.3. The Medical Applications for Gas Sensors in Human Body care
Analyzing the chemical compositions of human breath helps people to examine their health conditions. It is a convenient method for a non-invasive diagnosis of disease and has been used for centuries [5]. More than 200 organic or inorganic gaseous molecules [6] were examined in human breath. These gaseous species were produced through normal physiological processes or pathological conditions such as gastric ulcer, liver disease, cancer, or renal failure. As shown in Table I.1.
I.3.1. Ammonia Sensors and Their Comparisons
In current clinical examination, blood ammonia level is the major parameter to perform ammonia concentration. Blood ammonia levels are determined by using an enzyme-based assay in which the enzyme, glutamate dehydrogenase converts 2-oxoglutarate and ammonium to glutamate and water. In this reaction, the UV absorbance of the NADPH cofactor is monitored to represent the blood ammonia level [7-9].
There are numerous ammonia gaseous sensors have been reported in reviewed articles. Based on different principles, they are illustrated below:
Metal-oxide gas sensors
Metal-oxide gas sensors are manufactured in large quantities for ammonia sensing. Different materials have been applied to sensors are zinc oxide [10], iridium oxide [11], indium oxide [12], Pt-, and SiO2-doped SnO2 [13], Au and
MoO3 modified WO3 [14], etc. The analyte interacts with adsorbed oxygen b
oxidation at the surface. The removal of oxygen modulates the height of barriers, causing the conductance changed. These sensors have higher detection limit, 1ppm, but poor selectivity. The operation temperature is more than 400℃.
Catalytic metal sensors
Catalytic sensors are studied the reactivity of catalytic metals to specific gases. They are altered by a change in charge carriers of concentration variant. The detection limit could be 1 ppm and the accuracy limited.
Conducting polymer gas sensors
The sensing mechanism of conducting polymer gas sensors is ammonia can reversibly reduced the oxidized form of polymers. The reduction causes a change in the conductivity of the material, making it for resistomertric or amperometric detection [15, 16]. Two materials were reported to apply in this type of sensors. Polypyrrole was reported its reaction with ammonia is irreversible. Polyaniline was proved to be a much more stable conducting polymer material [17]. Both two materials of polymer were described lower detection limit of 1 ppm.
Optical gas sensors
There are two main optical principles for the detection. One is based on change in color when ammonia reacts with reagent. The best known is the Nessler reaction [18], for determining total ammonia concentration in water. There is not much literature about quantitative measurements with this reaction, because of the reagent is toxic. The Berthelot reaction was now much studied for
measuring ammonia concentration. A combination of ammonia, phenol and hypochlorite results in a blue color [19]. The detection limit is about 90 ppb of ammonia in water. The other principle is optical absorption. Spectroscopic systems with detection limit of 1 ppb have been reported [20]. Although the high sensitivity of ammonia detection, there are some disadvantages, equipment is very expensive and for accurate analysis the system should be very large.
Figure I.2. Depiction of a general transamination reaction. In many aminotransferase reactions, α-ketoglutarate is the amino group acceptor. All aminotransferases have pyridoxal phosphate as cofactor.it is rapidly reversible.
Table I.1. Diseases associated with unusual breath odors. Breath component as a
disease marker
Diseases References
Acetone Diabetes; Lung Cancer Ebeler et al., 1997 Grote et al., 1997 Ammonia Uremia; Liver Cirrhosis;
Renal Failure
Manolis, 1983 Davies et al., 1997 Butyric acid Liver Cirrhosis Manolis, 1983 Ethanethiol Liver Cirrhosis Manolis, 1983 Hydrogen sufide Periodontal Disease Manolis, 1983 Carbon dioxide H. Pylori infection William, 2007
Table I.2. Current ammonia sensors [1].
Principles Detection limit;
Response time; Temperature range
Remarks
Metal-oxide 1 ppm, ~5 min, 400℃ Low selectivity Catalytic metal 1 ppm, ~1 min, up to 600℃ Low accuracy Conducting polymer 1 ppm, ~5 min, up to 150℃ Irreversible reaction Optical gas sensors 1 ppb, ~5 min, RT Selectivity, sensitivity,
large and expensive OTFT 0.5 ppm, ~3 min, RT Sensitivity, cheap and
Chapter II. Theoretical Background of Organic Thin Film
Transistor
II.1. Introduction
The concept of using organic materials as semiconductors layer in transistors are realized at least since 1980s [21, 22]. They are interested in the fabrication of low-cost, large-area, flexible displays and low-end electronics. Low power-consumption, small size and portable device system are also important issues. Organic thin film transistors (OTFTs) are based on the conjugated polymers, oligoacenes, or fused aromatics. Similar to inorganic semiconductors, the organic material ones can function either as p-type or n-type. P-type semiconductors are the most widely studied in organic semiconductors, the major carriers are holes while n-type are electrons. Among the p-type material, pentacene (C14H22), an aromatic
compound of five benzene [Figure II.1], is common used as an active layer of its high mobility (>1 cm2/v-sec) with proper dielectric properties since 1996 [23, 24]. It purity leads to diffuse for the charge transporting with less interaction with lattice; the impurity in the material tends to chemically combine with the organic semiconductor which leads irregularity in the band gap [25].
II.2. Organic Thin Film Transistor as An Ammonia Sensors
OTFT sensors offer a great deal for applications in chemical and biological sensing, for instance, medical diagnosis, food monitoring, and detection of chemical or biological warfare agents. Some OTFT sensors were developed as chemical and biological sensors [Table II.1][26]. OTFT is promising to be a non-invasive,
inexpensive, portable and disposable diagnostic device because of its low cost fabrication process and high sensitivity to gas molecules [27]. The molecular active channel in OTFT sensors enable the devices to exhibit rapid response in both gaseous [26] and aqueous [28] sensing environment. It has been proposed that the gas molecules penetrate organic active layer through grain boundaries and diffuse into channel region to react with carriers [29, 30]. As a result, the sensitivity is high and is strongly dependent on the morphology and the grain boundary density of the organic film [31]. OTFT sensors provide multiple sensing parameters such as field-effect mobility, turn-on conductivity, turn-off conductivity, threshold voltage and subthreshold swing [32].
II.3. Electrical Characteristics of Organic Thin Film Transistor
There are several of electrical characteristics be preformed in transistor, such as threshold voltage, mobility, on/off current ratio and subthreshold swing. The methods of extraction are characterized, respectively.
II.3.1. Threshold Voltage
For bulk MOSFET (metal–oxide–semiconductor field effect transistor), the gate voltage at which the electron density at the interface is the same as the hole density in the neutral bulk material is called the threshold voltage [33]. Threshold voltage is related to the operation voltage and the power consumptions of an OTFT. We extracted the threshold voltage from the slope of the curve of the square-root of drain current versus the gate voltage in the saturation region.
II.3.2. Mobility
Generally, mobility can be extracted from the transconductance maximum Gm in
the linear region:
D OX t cons V G D m V L WC V I G D tan D OX m V WC L G
ID: Drain current L: Length of active layer
VG: Gate voltage COX: Oxide capacitance
μ: Mobility VD: Drain bias voltage
W: Width of active layer
II.3.3. On/Off current ratio
The ratio of the current in the on state to that in the off state of the device is called the on/off ratio. The “off” state of the transistor occurs when no bias is applied between the gate and source electrodes. Usually, devices operate in the accumulation mode, applying a bias to the gate induces a mobile carrier channel, which charge can move in response to the applied source–drain voltage (Vd). This is the “on” state of the transistor [34]. Devices with high on/off current ratio represent large turn-on current and small off current. It determines the gray-level switching of the displays. High on/off current ratio means there are enough
turn-on current to drive the pixel and sufficiently low off current to keep in low power consumption.
II.3.4. Subthreshold swing
Subthreshold swing is also an important characteristic for device application. It is a measurement of how rapidly the device switches from the off state to the on state in the region of exponential current increase.
V cons t D G D I V S tan log , when VG<VT for p-type.
Moreover, the subthreshold swing also represents the interface quality and the defect density [23], the maximum interface state trap-density, at the pentacene/dielectric interface can be estimated from the value of the subthreshold swing. q C q kT e S N i SS 1 / ) log( k: Boltzmann’s constant S: Subthreshold swing
Ci: capacitance density T: absolute temperature
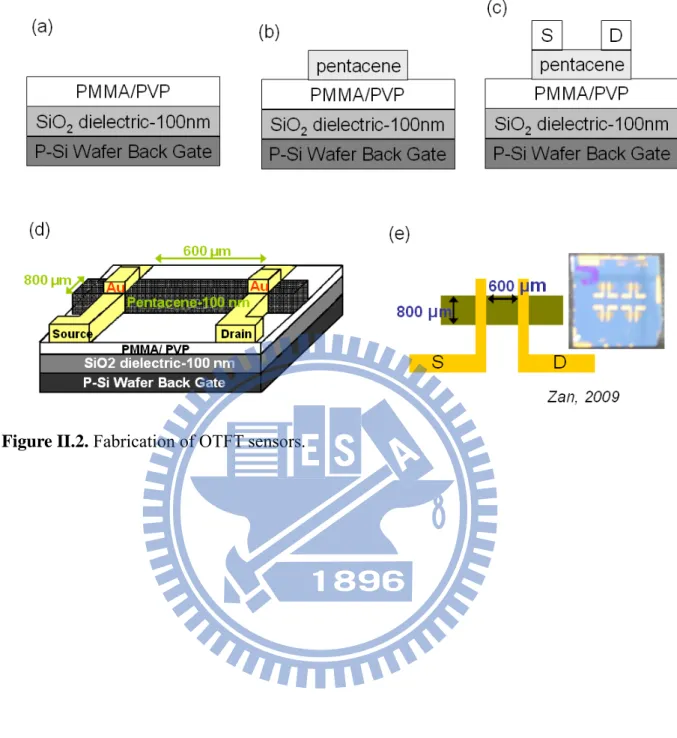
II.4. Organic Thin Film Transistor Fabrication
(OTFT devices were supplied from Zan’s laboratory in NCTU.)
OTFTs in this study were fabricated on silicon substrates as shown in Figure II.2. A highly doped p-type silicon wafer with a 100-nm-thick SiO2 was used as the gate electrode and gate
insulator, respectively. Poly(methyl methacrylate) [(PMMA) with a molecular weight of 95000] and cross-linked PVP (poly-4-vinylphenol) were used as the buffer layer to modify an SiO2 dielectric surface. Cross-linked PVP was derived from PVP dissolving in propylene
glycol 1-monomethyl ether 2-acetate (PGMEA) with cross-linking agent, (PMF) poly melamine-co-formaldehyde (PGMEA). Poly(methyl methacrylate)/SiO2 or cross-linked
PVP/SiO2 dielectrics were transferred into a vacuum chamber for the deposition of
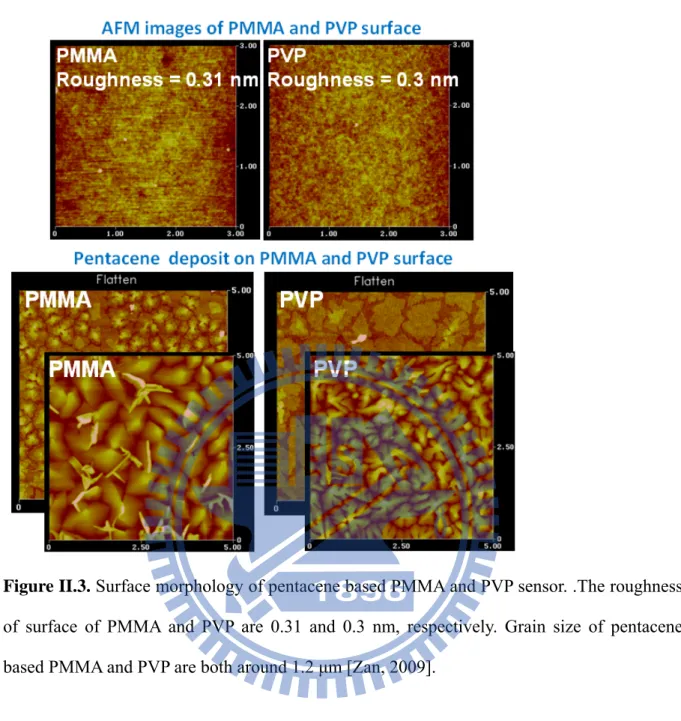
100-nm-thick pentacene film. The pentacene (Aldrich, 99.9 % pure without further purification) was evaporated through a shadow mask to form the active layer. After the formation of a 100-nm-thick pentacene, 100-nm-thick gold was deposited through the shadow mask to form source/drain contacts. The roughness of surface and grain size of pentacene-based PMMA and the PVP sensor were measured by AFM [Figure II.3].
II.5. Mechanisms of Organic Thin Film Transistors for Ammonia Sensing
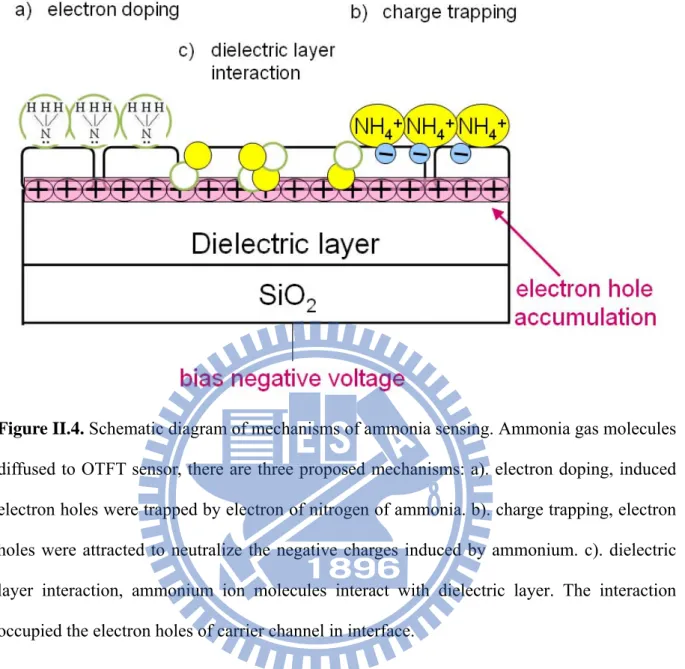
We propose possible mechanisms of OTFTs for ammonia sensing, which include electron doping, charge trapping, and dielectric layer interaction [Figure II.4].
II.5.1. Electron doping
We considered ammonia a polar molecule that acts like an electron donor. When the OTFT sensor was exposed to ammonia gas, the electron holes induced by gate bias negative voltage were trapped to the lone pair of electrons of the nitrogen of ammonia. The amount of electron holes in the carrier channel decreased the electric characteristic of the OTFT sensor.
II.5.2. Charge trapping
Ammonia is highly soluble in water and forms ammonium (NH4+). When ammonium
attached to the surface of the OTFT sensor, the negative charges were induced. Electron holes were attracted to neutralize the negative charge. The decreased electron hole carriers affected the poor electric characteristics of the OTFT sensor.
II.5.3. Dielectric layer interaction
A pentacene active layer is like a sponge structure, consisting of grains and grain boundaries. Ammonia gas molecules and water vapor easily diffused through grain boundaries to the dielectric layer. Ammonia gas molecules dissolve in water and form ammonium ((NH4+). Ammonium ion molecules interact with dielectric layers. The
interaction occupied the electron holes of the carrier channel during interface.
We predicted that when the device exposed to a vacuum environment, there is no water and ammonia sensing mechanisms tend to be electron doping and dielectric layer interaction. When the device is exposed to water vapor, the major mechanisms of ammonia sensing tend to dielectric layer interaction and charge trapping.
II.6. Research Motivation
Based on the importance of ammonia sensing, we developed a selectivity and high sensitivity OTFT as ammonia sensor, which does not respond to carbon dioxide, ethanol, formaldehyde or methane [Figure II.5 and Figure II.6]. We propose that this ammonia sensor can be applied to breath ammonia detection. Expiratory ammonia includes water vapor. Ammonia is highly
bound with water molecules to form ammonium. We consider water molecules an important factor affecting ammonia sensing. In this thesis, we discuss how the concentrations of water molecules affect ammonia sensing.
Figure II.1. Chemical structure of pentacene.
Table II.1. Reviewed of OTFT sensors [26].
Active layer Recognition element Analytes
Phthalocyanines Oxygen, iodine, bromine, NO2, ozone, alcohols, ketones, thiols, nitriles, esters, ring compounds, lactic acid, pyruvic acid
Naphthalene tetracarboxylic derivatives
Nitrogen, oxygen, water vapor, alcohols, ketones, thiols, nitriles, esters, ring compounds
Pentacene Water vapor, 1-pentanol, aqueous
analytes,
Oligothiophenes Alcohols, ketones, thiols, nitriles, esters, ring compounds, lactic acid, glucose
Polythiophenes Alkyl or alkoxy side chains Ammonia, water vapor, chloroform, alcohols, ketones, thiols, nitriles, esters, ring compounds, alkanes Poly(phenylene
ethynylene)
Enantioselective pendant groups
Figure II.3. Surface morphology of pentacene based PMMA and PVP sensor. .The roughness of surface of PMMA and PVP are 0.31 and 0.3 nm, respectively. Grain size of pentacene based PMMA and PVP are both around 1.2 μm [Zan, 2009].
Figure II.4. Schematic diagram of mechanisms of ammonia sensing. Ammonia gas molecules diffused to OTFT sensor, there are three proposed mechanisms: a). electron doping, induced electron holes were trapped by electron of nitrogen of ammonia. b). charge trapping, electron holes were attracted to neutralize the negative charges induced by ammonium. c). dielectric layer interaction, ammonium ion molecules interact with dielectric layer. The interaction occupied the electron holes of carrier channel in interface.
0
1
CH 4 CH3COCH3 C 2H5OH CO 2 NH 3|
V
th(V
)|
Standard Devices UV-treated Devices N 20
5
10
15
20
25
(
-
)
/
(%
)
Standard Devices UV-treated Devices CH4 CH 3COCH3 C 2H5OH CO 2 NH 3 N 2Figure II.5. Kinds odors dependent OTFT responses. Carbon dioxide (CO2), alcohol
0 1 2 3 4 5 -5 -4 -3 -2 -1 0 1 0.5ppm Vt h (V) NH3 concentration (ppm) 0 sec 1000 sec 2000 sec Standard 0 1 2 3 4 5 0.00 0.25 0.50 0.75 1.00 1.25 Standard NH3 concentration (ppm) 0 sec 1000 sec 2000 sec 0.5ppm (a) (b)
Figure II.6. Concentration dependent ammonia sensing response by time measurement of OTFT-1200 (a) Threshold voltage shift and (b) mobility variations are shown when devices were exposed to NH3 with concentrations varied from 0 ppm to 5 ppm [Zan, 2009].
Chapter III.
Materials and Methods
III.1. Chemicals
Nitrogen and ammonia gas are purchased from 新復發 and 洽隆, respectively.
III.2. Facilities
4200 semiconductor characterization system and Model 2636 Dual-Channel System Source Meter Instrument (Low Current) were purchased from Keithley. Gas chamber was manufactured by EVERBEING (奕葉). PC-540 Four-channel MFC readout power supply and PC-615 vacuum gauge controller were purchased from PROTEK. Mass flow controller 5850E was purchased from BROOKS.
III.3. Sensing-system
Three major components were organized to sensing-system [Figure III.1]: gaseous controller, gas chamber and semiconductor characteristics analyzer. The PC-540 MFC [Figure III.2] was introduced to gas chamber via mass flow controller [Figure III.3].Total volume of chamber is around 50 L [Figure III.4]. Inside pressure of chamber was monitored by vacuum gauge [Figure III.5]. Chamber system was equipped with probes and device station (probe station). The probes were connected to and regulated by semiconductor characteristics analyzer (Keithley 4200 and 2636A) [Figure III.6 and 7].
III.4. The Measurement of Electrical Characteristics of OTFT
Electric properties, the gate potential and source/drain bias voltage, of OTFT devices were monitored by using probe station and semiconductor analyzer (Keithley 2636A and 4200).
III.4.1. Id-Vg curve
The Id-Vg was measured for devices selection. In the general Id-Vg measurement, the drain current (Id) was detected at constant bias voltage of drain (Vd = -5V) while sweeping the gate voltage (Vg) was from 20V ~ -40V. When on/off ratio is above 3-4 oder and on current is among 10-7 A, the device was selected for ammonia sensing. PMMA and PVP device selection was show in Figure III.8.
III.4.2. Id-time measurement
After Id-Vg selection, Vg was chose from the linear region which is the largest variation of Id and the nearest to on current. Id-time measurement, the Id was measured at a constant Vd (-5V) and Vg (-10V or -15V).
III.4.3. I/I0 normalization
Before any gas introduced to chamber, the device was kept in vacuum environment, in the mean time, drain current was measured. We took the vacuum status drain current as a base line. Since, any change of drain current was compared to base line.
III.5. Experimental Design
We considered ammonia solubility in water molecule to form ammonium, which means the more water molecule approach to ammonia; the more ammonium ions are formed. First, we selected three concentrations of water vapor, 0%, 50%, 100% to presume the amount of ammonium ions increased and ammonia sensing ability raised [Figure III.9.]. Second, we chose two materials, PMMA and PVP as dielectric layer of OTFT. As Figure III.10, the contact angle of PVP (51.8°) is smaller than PMMA (61.7°), which referred the material of PVP is more hydrophilic than the PMMA. It means PVP of OTFT more attract water molecules, which could improve ammonia sensing ability.
Figure III.1. Sensing-system.
Figure III.3. Mass flow controller 5850E.
(a) Uncapped gas-sensing chamber (b) Capped gas-sensing chamber
(a)
(b)
Figure III.5. PC-615 vacuum gauge controller.
Figure III.8. The electric characteristics of PMMA and PVP OTFTs. Drain current (Id) was detected at constant bias voltage of drain (Vd = -5V) while sweeping the gate voltage (Vg) was from 20V ~ -40V. Field effect mobility ( (cm2/Vs) and threshold voltage (V
th (V)) were
calculated in the linear regime (Vd=-5V) defined by standard metal-oxide-semiconductor
Chapter IV.
Results and Discussion
IV.1. Under Nitrogen Gas Environmental as An < 5% Relative Humidity
After a nitrogen gas injection, neither the PMMA nor PVP devices responded to the drain current which represents that nitrogen gas did not effect the device sensing. When the chamber was full of nitrogen gas, we introduced ammonia gas with different concentrations, 0.5 and 2 ppm. In the PMMA sensing, ammonia caused drain current decay with a time of 200 seconds. The decline at 0.5 and 2 ppm of ammonia were 3.5 % (black) and 13.7 % (red), respectively. In PVP, the drain current was decayed to saturate in 100 seconds. The decline at both 0.5 and 2 ppm of ammonia were 6.8% (blue) and 15.5% (green), respectively. [Figure IV.1]. Without the existence of moisture, PVP was more active to ammonia sensing; reaction time and saturation status were half-time reduced when compared with PMMA; drain current reduced by time cost.
Without the existence of moisture, PMMA and PVP are sensitive to ammonia. We propose that in this state, the sensing mechanism might be electron doping. Minakata (1994) studied ammonia sensors formed with thin pentacene films doped with iodine that could detect ppm concentrations of ammonia gas, causing the reduction of conductivity and resistivity increasing linearly with time [35]. Aside from what we propose, the dielectric layer can attract ammonia attachment to a response; the active layer, pentancene, is also responsive to ammonia. Ammonia is a dipolar molecule adsorbed on the active layer of sites in between gain boundaries that might be capable of creating an effective hole trap while adsorbing to the grain boundaries [34]. The presence of polar molecules is known to change the rate of charge transportation in organic materials by increasing the amount of energetic disorder through charge–dipole interactions[36]. Polar molecules behave as acceptor-like deep trap states for the charge carriers moving at the interface between the
organic semiconductor layer and insulator [37, 38].
Although we had a vacuum (2×10-1 torr), gas chamber, and introduced nitrogen gas, there still may have existed a low concentration of humidity which formed a thin layer water vapor membrane. Another effect of ammonia sensing we propose is ammonia gas physical attachment through pentacene to dielectric surfaces, with forming ammonium interacting with PMMA or PVP, causing a decrease of electric performance(what we propose as dielectric layer interaction).
IV.2. Under Air Containing 50% Relative Humidity
Before ammonia, we introduced air as relative humidity 50±5 % to the chamber. Organic thin film transistor (OTFT) sensors responded to air with water vapor content. In PMMA, the response of air was approximately 10% on average; introduction of air caused a response saturation after 100 seconds. The responses of ammonia in 0.5 and 2 ppm were 8.7 % and 17.3 %, respectively. In PVP, the response of air was 6.5 % on average; instead of PMMA, PVP responded to air with a delay of approximately 100 seconds. The responses of ammonia in 0.5 and 2 ppm were 11.7 % and 19.8 %, respectively [Figure IV.2]. Response rates of ammonia in PMMA and PVP did not yield differences, which may be because the response of PVP was delayed to air and not saturated to the device capacity of air. Therefore, we elongated the PVP device expose time (double) to air until the response was saturated [Figure IV.3]. The results showed the response of air in PVP to be 13 % on average. The time of saturation was approximately 400 seconds, including the initial 100-second delay time. After that, we introduced ammonia of 0.5 and 2 ppm to compare with the results in Figure IV.2. The responses were 31.7 % and 76.1 %, respectively. Ammonia sensing ability
of PVP was increased four times to PMMA and air was unsaturated to the state of PVP.
The ammonia sensing ability of PVP is of higher quality than PMMA when water vapor existence must be saturated. We suggest that with PVP, the dielectric was exposed to air/moisture, and the surface was forming and accumulating a more –OH chemical functional group. That could be attractive to ammonium, which forms by ammonia contacting with water vapor. We discovered that the sensing mechanisms involve weak interactions between the analyte functionalities and the polymers’ side chains[34]. Kim et al. (2008) studied the pyridine group of PVP which interacts strongly with water molecules through hydrogen-bonding. Kim’s results indicate that the surface polarity arose from the functional group in the polymer [39]. When PVP was exposed to ambient air, the surface interacted with water molecules leading PVP to form more –OH groups. We discovered that water molecules in humid air diffuse into the grain boundary of the polycrystalline semiconductor layer and/or the interface between the semiconductor and dielectric gate, where they created both donor- and acceptor-like traps, leading to significant degradation of device performance [40]. The diffusion of water molecules is intimately related to the density of grain boundaries in the pentacene film because small molecules migrate into the channel region through defects[27]. The results showed that the sensing mechanisms are suitable to charge trapping and dielectric layer interaction.
IV.3. Under >90% Relative Humidity of Water Vapor Environment
We introduced water vapor, relative humidity >90 %, into the chamber before introducing ammonia. In PMMA, the response of water vapor was 20.5 % on average. The responses of ammonia in 0.5 and 2 ppm were 36.9 % and 63.7 %, respectively. In PVP, the response of
water vapor was 28.2 % on average. The responses of ammonia in 0.5 and 2 ppm were 18.8 % and 43.7 %, respectively [Figure IV.2]. Response rates of ammonia were observed in PMMA and PVP. In the response rate and decline rate, PMMA was more sensitive than PVP. The response rate showed no significance difference between PVP and PMMA with a high ammonia concentration, 2 ppm, but observed PMMA sensing showed a higher quality twice that of PVP at 0.5 ppm. Our results showed that the ammonia sensing ability of PVP was of no higher quality than PMMA in high concentrations of water vapor. The performance was not what we expected in IV.2. The device was exposed to air/moisture and PVP sensing ability was of higher quality than PMMA.
IV.4. Ammonia in Nitrogen Gas
Different from IV.1 performance, we introduced nitrogen gas and ammonia simultaneously. Nitrogen gas is not sensible to OTFT sensors; therefore, we observed the change of drain current as only an ammonia effect. During the first 200 seconds, responses of ammonia of PMMA were straight declines of drain current and the decreasing rates were 6.9 %, 8.9 %, 11.4 %, 20.1 %, and 15.3 % using ammonia concentrations of 0.5, 1, 2, 5, and 10 ppm, respectively [Figure IV.2]. Instead, responses of PVP during the initial 50 seconds revealed delay phenomena, then received a straight decline of drain current. Within 200 seconds, the decreasing rates of drain current for PVP were 8.9 %, 9.5 %, 6.4 %, 12.2 %, and 18.1 % using ammonia concentrations of 0.5, 1, 2, 5, and 10 ppm, respectively [Figure IV.5]. As long as time treatment was elongated, both PMMA and PVP sensing to ammonia were saturated to device capacity.
Although we supposed that PVP with more –OH chemical functional group would be more sensitive to ammonia, instead, the results in ammonia in nitrogen gas showed that there are no differences between PMMA and PVP devices over long time periods, or even that PMMA is slightly more sensitive to ammonia [Figure IV.6]. When nitrogen and ammonia were introduced simultaneously, the devices were not in stable ambient gas, which may influence the dielectric surface polarity.
IV.5. Ammonia in Air
Introducing air and ammonia simultaneously, we observed the responses of PMMA and PVP. During the first 200 seconds, ammonia responses of PMMA were 20.8 %, 32.4 %, 42.0 %, 51.8 %, and 61.3 % (using ammonia concentrations of 0.5, 1, 2, 5, and 10 ppm, respectively) [Figure IV.8]. Responses of PVP were 15.9 %, 19.5 %, 26.1 %, 41.62 %, and 55.2 % (using ammonia concentrations of 0.5, 1, 2, 5, 10 ppm, respectively) [Figure IV.9]. The changing rate of PMMA was of higher quality than PVP. As previously discussed in Section IV.3, PVP has a delay to water vapor that influences ammonia sensing sensitivity. As long as time was increased, responses of ammonia sensing were saturated both in PMMA and PVP. We compared the changing rate of saturation regions within 500 seconds. Responses of PMMA using concentrations 0.5, 1, 2, 5, and 10 ppm were 28.9 %, 41.7 %, 55.7 %, 67.2 %, and 76.4 %, respectively; responses of PVP were 33.0 %, 37.5 %, 53.0 %, 70.2 %, and 82.9 %. There were no significant differences between PMMA and PVP [Figure IV.10]. We suggest that PVP was not admitted water vapor completely and ammonia attracted to the device was limited to similar to PMMA.
ambient air without elongated time leading to the PVP surface contacting with moisture. Without the saturated surface of chemical functional groups or formation of native oxide to interact with water molecules, the sensing ability was poorer than under atmospheric environments but of much higher quality than ammonia in nitrogen gas.
Figure IV.2. Responses of ammonia after introduced air, content water vapor (relative humidity 50±5%).
Figure IV.4. Responses of ammonia after introduced water vapor, content water vapor (relative humidity >90%).
Chapter V. Conclusions
When moisture exists, PMMA or PVP are more sensitive to ammonia and interact with water vapor to form ammonium and attract to dielectric layers of OTFT sensors, especially in PVP. Although PVP has a delay phenomenon of moisture response, when it is admitted to saturation, the response of ammonia can increase four times. However, in high water vapor concentrations, PVP sensing ability was not observed to be of higher quality than PMMA. When carrier gas and ammonia were introduced simultaneously, we did not observe PVP sensing ability to be of higher quality than PMMA. The responding changes showed no significant differences in long time detection. For future applications, we suggest that, prior to ammonia sensing, the device should be exposed to air/moisture until it is saturated for higher responses.
References
1. Timmer, B., W. Olthuis, and A.v.d. Berg, Ammonia sensors and their applications--a
review. Sensors and Actuators B: Chemical, 2005. 107(2): p. 666-677.
2. Nelson, D. and M. Cox, Lehninger Principles of Biochemistry, Fourth Edition. 2004: {W. H. Freeman}.
3. Shimamoto, C., Hirata, I., Katsu, K, Breath and blood ammonia in liver cirrhosis. Hepato-gastroenterology, 2000. 47(32): p. p. 443-445.
4. DuBois, S., Eng, Sue, et al., Breath Ammonia Testing for Diagnosis of Hepatic
Encephalopathy. Digestive Diseases and Sciences, 2005. 50(10): p. 1780-1784.
5. Conkle JP, C.B., Welch BE., Trace composition of human respiratory gas. Arch Environ Health, 1975. 30(6): p. 290-295.
6. Tietz, N.W., Clinical Guide to Laboratory Tests 3ed. 1995: W.B. Saunders
7. Mondzac A, E.G., Seegmiller JE., An enzymatic determination of ammonia in
biological fluids. J Lab Clin Med, 1965. 66(3): p. 526-531.
8. Jacobs, H.A.M. and F.M.F.G. Olthuis, A kinetic determination of ammonia in plasma. Clinica Chimica Acta, 1973. 43(1): p. 81-86.
9. Kimble, K., J. Walker, D. Finegold, and S. Asher, Progress toward the development of
a point-of-care photonic crystal ammonia sensor. Analytical and Bioanalytical
Chemistry, 2006. 385(4): p. 678-685.
10. Rao, G.S.T. and D. Tarakarama Rao, Gas sensitivity of ZnO based thick film sensor to
NH3 at room temperature. Sensors and Actuators B: Chemical, 1999. 55(2-3): p.
166-169.
11. Karthigeyan, A., et al., A room temperature HSGFET ammonia sensor based on
iridium oxide thin film. Sensors and Actuators B: Chemical, 2002. 85(1-2): p. 145-153.
12. Li, C., et al., Surface Treatment and Doping Dependence of In2O3 Nanowires as
Ammonia Sensors. The Journal of Physical Chemistry B, 2003. 107(45): p.
12451-12455.
13. Wang, Y.-D., et al., Ammonia-sensing characteristics of Pt and SiO2 doped SnO2
materials. Solid-State Electronics, 2001. 45(2): p. 347-350.
14. Xu, C.N., et al., Selective detection of NH3 over NO in combustion exhausts by using
Au and MoO3 doubly promoted WO3 element. Sensors and Actuators B: Chemical,
2000. 65(1-3): p. 163-165.
15. Palmqvist, E., et al., DC-resistometric urea sensitive device utilizing a conducting
polymer film for the gas-phase detection of ammonia. Biosensors and Bioelectronics,
1995. 10(3-4): p. 283-287.
16. Lähdesmäki, I., A. Lewenstam, and A. Ivaska, A polypyrrole-based amperometric
17. Chabukswar, V.V., S. Pethkar, and A.A. Athawale, Acrylic acid doped polyaniline as
an ammonia sensor. Sensors and Actuators B: Chemical, 2001. 77(3): p. 657-663.
18. Svehla, G., Vogel's Qualitative Inorganic Analysis. 7 ed. 1996: Prentice Hall.
19. Searle, P.L., The berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A review. The Analyst, 1984. 109(5): p. 549-568.
20. Mount, G.H., et al., Measurement of atmospheric ammonia at a dairy using differential
optical absorption spectroscopy in the mid-ultraviolet. Atmospheric Environment,
2002. 36(11): p. 1799-1810.
21. Tsumura, A., H. Koezuka, and T. Ando, Macromolecular electronic device:
Field-effect transistor with a polythiophene thin film. Applied Physics Letters, 1986.
49(18): p. 1210-1212.
22. Burroughes, J.H., C.A. Jones, and R.H. Friend, New semiconductor device physics in
polymer diodes and transistors. Nature, 1988. 335(6186): p. 137-141.
23. D. J. Gundlach, Y.Y.L., T. N. Jackson, S. F. Nelson, and D. G. Schlom, Pentacene
organic thin-film transistors-molecular orderig and mobility. IEEE Electron Device
Letters, 1997. 18(3): p. 87-89.
24. D. Knipp, R.A.S., A. Völkel, and J. Ho, Pentacene thin film transistors on inorganic
dielectrics: Morphology, structural properties, and electronic transport. JOURNAL
OF APPLIED PHYSICS, 2003. 93(1): p. 347-355.
25. Yang, Y.S., et al., Deep-level defect characteristics in pentacene organic thin films.
Applied Physics Letters, 2002. 80(9): p. 1595-1597.
26. Mabeck, J. and G. Malliaras, Chemical and biological sensors based on organic
thin-film transistors. Analytical and Bioanalytical Chemistry, 2006. 384(2): p.
343-353.
27. Crone, B., et al., Electronic sensing of vapors with organic transistors. Applied Physics Letters, 2001. 78(15): p. 2229-2231.
28. Someya, T., et al., Integration and Response of Organic Electronics with Aqueous Microfluidics. Langmuir, 2002. 18(13): p. 5299-5302.
29. Dunlap, D.H., P.E. Parris, and V.M. Kenkre, Charge-Dipole Model for the Universal
Field Dependence of Mobilities in Molecularly Doped Polymers. Physical Review
Letters, 1996. 77(3): p. 542.
30. Gundlach, D.J., et al., Solvent-induced phase transition in thermally evaporated
pentacene films. Applied Physics Letters, 1999. 74(22): p. 3302-3304.
31. Someya, T., et al., Vapor sensing with alpha,omega-dihexylquarterthiophene
field-effect transistors: The role of grain boundaries. Applied Physics Letters, 2002.
81(16): p. 3079-3081.
32. Torsi, L., et al., Multi-parameter gas sensors based on organic thin-film-transistors. Sensors and Actuators B: Chemical, 2000. 67(3): p. 312-316.
Sons.
34. Locklin, J. and Z. Bao, Effect of morphology on organic thin film transistor sensors. Analytical and Bioanalytical Chemistry, 2006. 384(2): p. 336-342.
35. Minakata, T., Fabrication of a gas sensor using pentacene thin films as a detector.
Polymers for Advanced Technologies, 1995. 6(9): p. 607-610.
36. Jeong, J.W., et al., The response characteristics of a gas sensor based on
poly-3-hexylithiophene thin-film transistors. Sensors & Actuators: B. Chemical, 2010.
146(1): p. 40-45.
37. Pacher, P., et al., Chemical Control of Local Doping in Organic Thin-Film Transistors:
From Depletion to Enhancement. Advanced Materials, 2008. 20(16): p. 3143-3148.
38. Etschmaier, H., et al., Continuous tuning of the threshold voltage of organic thin-film
transistors by a chemically reactive interfacial layer. Applied Physics A: Materials
Science & Processing, 2009. 95(1): p. 43-48.
39. Kim, S.H., et al., Effect of water in ambient air on hysteresis in pentacene field-effect
transistors containing gate dielectrics coated with polymers with different functional groups. Organic Electronics, 2008. 9(5): p. 673-677.
40. Knipp, D., et al., Influence of impurities and structural properties on the device
stability of pentacene thin film transistors. Journal of Applied Physics, 2007. 101(4): p.
![Figure I.1. Nitrogen cycle [1].](https://thumb-ap.123doks.com/thumbv2/9libinfo/8564698.188590/19.892.133.748.113.818/figure-i-nitrogen-cycle.webp)
![Table I.2. Current ammonia sensors [1].](https://thumb-ap.123doks.com/thumbv2/9libinfo/8564698.188590/23.892.145.762.131.845/table-i-current-ammonia-sensors.webp)
![Table II.1. Reviewed of OTFT sensors [26].](https://thumb-ap.123doks.com/thumbv2/9libinfo/8564698.188590/31.892.131.790.429.1012/table-ii-reviewed-of-otft-sensors.webp)
![Figure II.5. Kinds odors dependent OTFT responses. Carbon dioxide (CO 2 ), alcohol (C 2 H 5 OH), methane (CH 4 ), and acetone (CH 3 COCH 3 ) [Zan, 2009]](https://thumb-ap.123doks.com/thumbv2/9libinfo/8564698.188590/35.892.126.797.114.814/figure-dependent-responses-carbon-dioxide-alcohol-methane-acetone.webp)