行政院國家科學委員會專題研究計畫 成果報告
利用精準開環複分解聚合(ROMP)之技術合成新型功能性高 分子材料(3/3)
計畫類別: 個別型計畫
計畫編號: NSC93-2216-E-011-001-
執行期間: 93 年 08 月 01 日至 94 年 10 月 31 日 執行單位: 國立臺灣科技大學化學工程系
計畫主持人: 廖德章
計畫參與人員: 黃慶成、許百男、陳文祥;、曾文聰、陳藏斌、林;
郁珊
報告類型: 完整報告
報告附件: 出席國際會議研究心得報告及發表論文
處理方式: 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢
中 華 民 國 95 年 1 月 20 日
行政院國家科學委員會補助專題研究計畫 □ 成 果 報 告
□期中進度報告 利用精準開環複分解聚合(ROMP)之技術
合成新型功能性高分子材料
計畫類別:□ 個別型計畫 □ 整合型計畫
計畫編號:NSC 93-2216- E - 011 - 001 -
執行期間:93 年 8 月 1 日至 94 年 10 月 31 日
計畫主持人:廖德章 共同主持人:
計畫參與人員:黃慶成、許百男、陳文祥、曾文聰、陳藏斌、林郁珊
成果報告類型(依經費核定清單規定繳交):□精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:國立台灣科技大學
中 華 民 國 95 年 01 月 20 日
摘要
本計畫以開環複分解聚合進行嵌段共聚合物的製備,使用環戊二
烯與丙烯丙胺及丙烯醇在高壓反應器中進行 Diels-Alder 反應合成原冰
片烯甲胺及原冰片烯甲醇。在原冰片烯甲胺與酉太酐反應導入保護基團製 備單體原冰片烯甲醯亞胺(NBMPI)。第一年:一種新的可交鏈原冰片烯 單體(NBMOACM)利用原冰片烯甲醇與甲基丙烯基氧乙基異氰酸酯反 應製備而成。此新型單體再與鐒金屬錯體觸媒進行開環複分解聚合,
並且可以藉由改變不同的[M]/[I]值[其中[M]:單體(NBMOACM)的濃 度,[I]: 起始劑的濃度],形成各種不同分子量及鏈段的聚合物,此可 交鏈原冰片烯單體(NBMOACM)在聚合過程中並沒有發生凝膠(Gel)之 現象,另外利用可交鏈基團之聚合物[poly(NBMOACM)] 與甲基丙烯
酸甲酯在自由基起始劑下進行交鏈反應,形成 AB 交鏈共聚合物,此
AB 交鏈共聚合物[含 1 wt% poly(NBMOACM)]在空氣中所測得 10%之 熱裂解溫度(Td10=300 oC)大於聚甲基丙烯酸甲酯之溫度(Td10=276 oC)。
第 二 年 : 製 備 三 種 新 穎 的 可 交 鏈 型 嵌 段 共 聚 合 物 [poly(NBMPI-b-NBMOACM)],可交鏈單體 (NBMA)及水溶性單體 (NBMQA)。因 NBMOACM 單體及/或其聚合物結構中,在酯基與脲基 之間存在有伸乙基,而此基團將可以破壞氫鍵,並大大提高溶解性。
Poly(NBMPI-b-NBMOACM)共聚合物因而可視為是一種新穎的有機可 溶性高分子材料。為了得到水溶性及可交鏈之共聚合物,本計畫製備 兩新穎之單體: 一具末端可交鏈之單鍵,另一單體為水溶性,並進行共
聚合來製備水溶性可交鏈高分子材料。第三年:利用 Diels-Alder 反應
合成末端含環氧基團之新型原冰片烯單體,以鐒金屬錯體觸媒進行開 環複分解聚合製備其聚合,其中此含環氧基團之聚合物[Poly(NBGE)]
顯示出有傑出之熱穩定度(在氮氣環境下,10%之熱裂解溫度為 380 oC)
及溶解性質(可溶於二氯甲烷、四氫呋喃、1,2-二氯苯、苯、甲苯、二 甲苯及 N-甲基-2-吡 咯 酮等溶劑中)。
關鍵詞:開環複分解聚合,環戊二烯,丙烯胺,丙烯醇,丙烯基縮水 甘油醚,原冰片烯甲酞醯亞胺,可交鏈聚合物,聚合反應機 構,分子量及其分佈。
英文摘要
A novel method ring-opening metathesis polymerization (ROMP) is reported for the preparation of polymeric material. The synthesis of norbornene methylene amine and norbornene methylene alcohol is accomplished via the Diels-Alder reaction of freshly cracked cyclopentadiene and the allyl amine and allyl alcohol. Norbornene phthalimide (NBMPI) is prepared by introducing phthalimide group as a protective group for norbornene methylene amine. First year: A novel cross-linkable monomer, 5-(Methacryloyl oxyethylene amino carboxyl)
methylene bicyclo[2.2.1]hept-2-ene, was prepared 5-(Hydroxymethyl)bicyclo[2.2.1]hept-2-ene (norbornene methylene alcohol) and methacryloyloxyethyl isocyanate. Novel cross-linkable polymers of 5-(Methacryloyl oxyethylene amino carboxyl) methylene bicyclo[2.2.1]hept-2-ene (NBMOACM) have been synthesized by ring-opening metathesis polymerization (ROMP) using a Ru catalyst {Cl2Ru(CHPh)[P(C6H11)3]2}. We will change different [M]/[I] values by using ring-opening metathesis polymerization ([M]: monomer concentration and [I]: initiator concentration), and produce different molecule weight and segments polymers. No gel formation occurred during ROMP of NBMOACM. Poly(NBMOACM) was incorporated into poly(methyl methacylate) [poly(MMA)] to produce AB cross-linked materials. These cross-linked materials [1 wt% poly(NBMOACM), Td10=300 oC in air] had higher thermal stability than pure poly(MMA) (Td10=276 oC in air). Second year: Ring-opening metathesis polymerization (ROMP) was performed with {RuCl2(CHPh)[P(C6H11)3]2} catalyst (I) to prepare either low polydispersity block copolymers of 5-(phthalimide methyl)bicyclo[2.2.1]hept-2-ene(NBMPI) and 5-(methyl
methacryloyl ethyl amino carboxyl )bicyclo[2.2.1]hept-2-ene (NBMOAC)
or homopolymer of cross-linkable 5-(acryloyl methyl)bicyclo[2.2.1]hept-2-ene (NBMA). Moreover, ring-opening metathesis polymerization of the quaternized 5-(ammonium methyl)bicyclo[2.2.1]hept-2-ene (NBMQA) was carried out with catalyst (II). Three diblock copolymers of NBMPI and NBMOAC were prepared, varying both molecular weight and percent of each block. The novel diblock copolymers were characterized by means of NMR and solubility.
The diblock copolymers exhibited good solubility in various solvents. In order to obtain water-soluble cross-linkable copolymer, we have prepared another two monomers: one was with the cross-linkable end group, and the other was water-soluble. Third year: a functional norbornene monomer containing epoxy group, i.e. 5-(glycidyl ether) bicyclo[2,2,1] hept-2-ene}
[NBGE], was synthesized by the Diels-Alder reaction of cyclopentadiene and allyl glycidyl ether. The synthesis of polynorbornene carrying pendent epoxy group [poly(NBGE)] was polymerized by the well-defined ruthenium complex {RuCl2(CHPh)[P(C6H11)3]2} as catalyst via ring-opening metathesis polymerization (ROMP). Poly(NBGE) showed good thermal stability(Td,10%=380 oC in nitrogen) and good solubility in a variety of solvents such as methylene chloride, tetrahydrofuran, 1,2-dichlorbenzene, benzene, toluene, xylene and N-methyl-2-pyrrdione.
Keywords: ring-opening metathesis polymerization (ROMP), cyclopentadiene, allyl amine, allyl alcohol, allyl glycidyl ether, norbornene phthalimide, cross-linkable monomer and polymer, polymerization mechanism, [M]/[I]
計畫緣由與目的
烯類複分解聚合(Olefin metathesis polymerization)在高分子合成 上是一個很重要的方法。近年來在高分子材枓的合成上,環烯類開環
複分解聚合與非環二烯類及炔類的複分解聚合日趨重要1)。隨著各種新
型觸媒開發,對於帶有各式官能基的高分子材枓合成也越來越多,成 為重要的功能性高分子材枓合成方法之一。
有機金屬觸媒用於複分解聚合已有一段時日,但對有官能基之單 體在聚合常會有所限制,同時水份或氧氣對聚合也相當敏感。例如鎢
(W),鈦(Ti),鉬(Mo)及鐒(Ru)等金屬化合物為較常用的環烯 類開環複分解聚合之觸媒,其中鐒(Ru)金屬觸媒對水份或氧氣的安 定性(stability)或相容度(tolerance)較佳,甚至可於水溶液中進行
聚合反應 2)。例如 1996 年 Grubbs 等人新開發的鐒碳烯配位觸媒
{Cl2Ru(CHPh)[P(C6H11)3]2}3, 4),對環烯類開環複分解聚合非常有效,尤 其在空氣中還算安定,可進行具有官能基單體的聚合。另外有高的聚 合 速 率 及 高 分 子 量 之 特 性 , 一 般 而 言 均 具 有 活 性 聚 合 (living polymerization)的現象。
利用環烯類衍生物為單體所進行的開環複分解聚合之相關研究越來 越多,主要有側鏈型液晶5, 6, 7),兩步法(two step)合成三嵌段(triblock)
共聚合物8),各種官能基導入之聚合物 9, 10)。其中官能基的導入使聚合
物具有光電性質11),生化活性 12)。
聚原冰片烯(polynorbornene)及其衍生物是第一個藉開環複分解
聚合之商業化產品,為重要的工程塑膠之一 13)。因其透明性良好,耐
衝擊性佳(橡膠添加劑),廣泛之溫度使用範圍,良好之機械物性,加
工性。廣用於照明器具,機械,電子零件,管件,食品包裝等。又其
衍生物如酸及酯類的聚合物更被當作電子產業的光阻劑14, 15, 16)。國內
有關開環複分解聚合的研究,據筆者所知還算很少,尤其在大學之學 術機構更少,對於此一極具潛力的高分子材料之合成方法更顯示出其 重要性。
由於近年來奈米技術與奈米材料的重要性提升,高分子奈米材料 中嵌段聚合物又扮演重要角色,而開環複分解聚合技術有利於發展嵌 段聚合物,若材料具兩親媒性更可形成自組裝性奈米微粒材料。
本計畫主要是在高壓反應器中藉由 Diles-Alder 反應合成出多種新
的原冰片烯衍生物,進一步合成出新的原冰片烯聚合物及新的原冰片 烯共聚合物,並研究其聚合行為與物性,對原冰片烯衍生物的開環複 分解聚合相關領域有更深入的了解與貢獻。此外,將所得的原冰片烯 聚合物經過改質成水溶性原冰片烯衍生物,並研究其在水溶液中的巨 觀與微觀行為。本研究室過去在複分解聚合(metathesis polymerization)
及相關配位聚合初步已有一些具體成果與經驗 17-36,以及在水溶液高
分子之溶液物性及螢光特性研究初步也已有成果 37-56,本計劃的期末
報告均按照計劃之內容成功地達成進度,並得到良好的結果。
一、 儀器與設備
(1) 紅外線光譜儀(IR),型號為 JASCO IR-9700 測試範圍為 4000-
400cm-1,測試法為利用KBr 打片測試而得。
(2) 13C 及1H NMR 核磁共振光譜儀型號為 JOEL EX-400,測試範圍 為 100.4 MHz 到 399.65 MHz 之間。所使用的溶劑為重氫氯仿 (CDCl3)。
(3) 示差熱分析儀,型號為 Du pont 9000 DSC system,設定升溫速度 為每分鐘10℃。
(4) 毛細管熔點測定儀,型號為 Model BüCHI 535。
(5) 元素分析儀,型號為 Perkin-Elmer 2400。
(6) 熱重分析儀,型號為 ULVAC (Sinku-Riko) model 7000,升溫速率
為每分鐘 10℃,氮氣流率為每分鐘 60 立方公分。
二、 實驗:
第一年度
1. Preparation of 5-(methacryloyloxyethylaminocarboxylmethyl )bicyclo [2.2.1]hept-2-ene (NBMOACM) (Scheme I)
Methacryloyloxyethyl isocyanate (MOI) (3.07 g, 0.011 mol, 10%
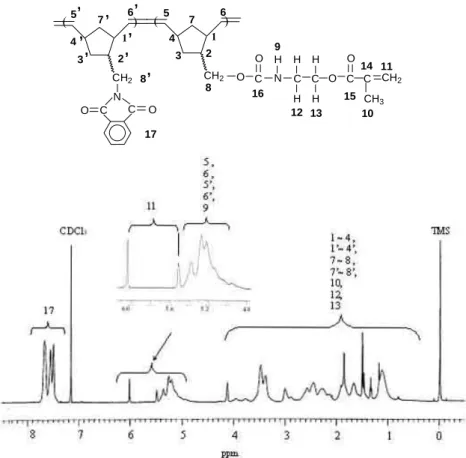
excess) and 5-(hydroxymethyl)bicyclo[2.2.1]hept-2-ene (bp. 93~95℃/13 mmHg; endo / exo = 80 / 20)(NBCH2OH, 1.24 g, 0.010 mol) were added to CH2Cl2 and kept at room temperature for 4 hrs. The product, 5-(methacryloyloxyethylamino carboxylmethyl)bicyclo[2.2.1]hept-2-ene (NBMOACM), thus obtained was a white solid. Figure 1 shows the DSC thermogram of NBMOACM. The endotherm at 63 ℃ corresponds to the melting point. This is followed by an exotherm (193.4. kJ/mole) that has an onset at ca. 140℃ and a peak at 178℃. The yield was 75 % and endo / exo = 72 / 28. ANAL. Calculated for C15H21O4N: C, 64.52 %; H, 7.52 %; N, 5.02 %; found: C, 64.07 %; H, 7.50 %; N, 4.98 %. The 1H and 13C NMR spectra of NBMOACM are shown in Figures 1(A) and 1(B), respectively.
The spectra agree satisfactorily with the proposed structure. IR (KBr pellet, cm-1): 3360 (υN-H), 3050 (υC-H , olefinic C-H stretch), 2960 (υC-H, C-H stretch), 1715(υC=O), 1640(υC=C), 1540 (υC-H , aliphatic C-H), 1300 (υC-O).
2. Ring-opening metathesis polymerization of NBMOACM
The norbornene-containing derivative was dissolved in methylene chloride. After a freeze-pump-thaw cycle, a solution of Ru catalyst (I) and/or Ru catalyst (II) in methylene chloride was injected to the monomer solution. Then the solution was stirred at ambient temperature. The polymerization was terminated by the addition of trace ethyl vinyl ether.
The solution was continuously stirred for another 10 min, and then polymer
was precipitated in excess of methanol. Functional polynorbornenes were obtained. The 1H and 13C NMR spectra of NBMOACM are shown in Figures 2(A) and 2(B), respectively. The spectra agree satisfactorily with the proposed structure. IR(KBr pellet, cm-1): 3340 (υN-H), 3020 (υC-H , olefinic C-H stretch), 2955 (υC-H, C-H stretch), 1700 (υC=O), 1630 (υC=C), 1525 (υC-H , aliphatic C-H), 1295 (υC-O).
第二年度
1. Living ring-opening metathesis copolymerization of NBMPI and NBMOACM
A solution of catalyst was prepared by dissolving {RuCl2(CHPh)[P(C6H11)3]2} (8.00 mg, 9.72×10-2 mmol) in 1 mL of anhydrous methylene chloride under argon-filled drybox. The monomer of 5-(phthalimide methyl)bicyclo[2.2.1]hept-2-ene (NBMPI) (2.53 g, 1.00×10-2 mol) was dissolved in 9 mL of methylene chloride and then degassed thrice via a freeze-pump-thaw cycle. After complete degassing, the catalyst solution was injected into the monomer solution by syringe.
The pink solution was vigorously stirred at 30 ℃ for 20 minutes.
NBMOACM (0.88 g, 4.63×10-3 mol) was injected into the still-living reaction mixture and the solution was stirred for another 12 hrs at room temperature. After addition of NBMOACM, the color of the solution changed from pink to yellow. The polymer, poly(NBMPI-b-NBMOACM)-2, was precipitated in excess of methanol and dried overnight in a vacuum system at room temperature to give a flaky white solid. The 1H NMR spectrum of poly(NBMPI-b-NBMOACM)-2 is shown in Figure 6.
2. Hydrolysis and quaternization of poly(NBMPI-b-NBMOACM)
Poly(NBMPI-b-NBMOACM)-2 (0.50 g) was suspended in 50 mL of ethanol in a Schlenk tube. To the above mixture, 5 mL hydrazine monohydrate and a trace of 2,6-di-tert-butyl-4-methylphenol were added.
The mixture was degassed thrice via a freeze-pump-thaw cycle, and the tube was heated to 100 ℃. The polymer had a cotton-like appearance and the mixture gradually changed to a light yellowish homogeneous solution after 1 hr. Hydrolysis was continued for 5 hrs and then the solution was cooled to room temperature. Quaternization of polymer was continuously carried out by reacting it with gaseous dry hydrochloride at room temperature for 6 hrs. The resulting copolymer [poly(HCQNBMA-b-NBMOACM)] was precipitated during the reaction.
The precipitated copolymer was dissolved in water, filtered and then precipitated from acetone. Poly(HCQNBMA-b-NBMOACM) collected and dried under vacuum at room temperature, and the yield was 90 %. The polymer, poly(HCQNBMA-b-NBMOACM), was soluble in water, but insoluble in alcohol (methanol and ethanol) and other organic solvents.
3. Synthesis of acryloyl chloride
Acrylic acid (36.0 g, 0.50 mol), thionyl chloride (59.5 g, 0.50 mol) and a few drops of DMF (as a catalyst) were mixed and reacted at 60 ℃.
After 9 hrs, the reaction mixture was distilled at 70 ℃ and the acryloyl chloride was obtained. The yield of acyloyl chloride was 40 %.
4. Synthesis of cross-linkable 5-(acryloyl methyl) bicyclo[2.2.1]hept-2-ene (NBMA)
5-(hydroxyl methyl) bicyclo[2.2.1]hept-2-ene (16.4 g, 0.13 mol) and triethyl amine (TEA, 14.8 g, 0.14 mol) were added to tetrahydrofuran (THF, 80 mL) and stirred for 30 minutes. Then, acryloyl chloride (12.0 g, 0.13 mol) was dissolved in tetrahydrofuran (THF, 20 mL) and solution was
added to the reaction mixture in drops and the temperature was maintained at 0℃. After completion of addition of the acryloyl chloride solution, the temperature was raised to 45 ℃ for another 4 hrs (Scheme III). The resulting product, 5-(acryloyl methyl)bicyclo[2.2.1]hept-2-ene (NBMA) (b.p. 89 - 91 oC / 7 mmHg), was shown in following structure:
CH2 O C 8 O
H 10 H H H
H
H H
H H
1 2
4 3 5 n6
7a 7s
n
n n
n n
n n
n 3x nn
CH CH2
11 n9
x x x x x x
x
x x
x
CH2 O C 8 O
H H H H
H H H
H
H 1
2 4 3
5 6
7a 7s
x3x n
CH2 CH 9 10 11
NBMA
The product was purified by column chromatography (SiO2, ethyl acetate / n-hexane = 1 / 3). NBMA was obtained as a viscous, colorless liquid. The yield was 70 % . [endo/exo = 84/16, 1H NMR (500 MHz, CDCl3): (ppm) = 5.91, 6.11 (m, cyclic, =CH, endo), 6.05 (m, cyclic,
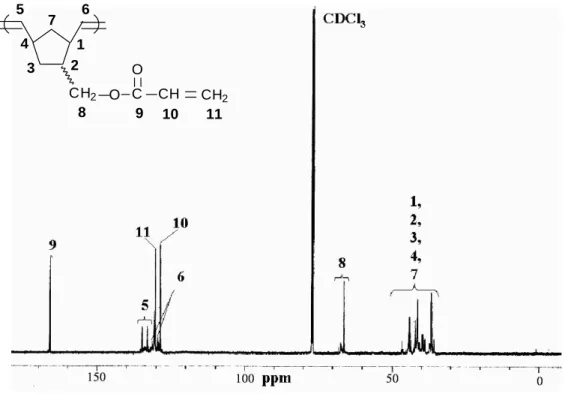
=CH, exo), 6.12 (m, vinylic, -O(O)C-CH=CH2), 5.78, 6.37 (m, vinylic, -O(O)C-CH=CH2) (Figure 7); 13C NMR (125 MHz, CDCl3): (ppm) = 28.8 (Cn3), 29.4 (Cx3), 37.7 (Cn2), 37.9 (Cx2), 41.5 (Cn4), 42.1 (Cx4), 43.5 (Cx1), 43.7 (Cn1), 44.8 (Cx7), 49.2 (Cn7), 67.8 (Cn8), 68.4 (Cx8), 128.5 (C10), 130.3 (C11), 132.0 (Cn6), 136.1 (Cx6), 136.8 (Cx5), 137.4 (Cn5), 166.0 (Cn9), 166.1 (Cx9)]. IR (KBr pellet, cm-1): 1727(υC=O), 1621(υC=C).
5. Synthesis of quaternized 5-(ammonium methyl) bicyclo[2.2.1]hept-2-ene (NBMQA)
The synthesis of quaternized 5-(ammonium methyl)bicyclo[2.2.1]hept-2-ene was accomplished via the Diels-Alder reaction of freshly cracked cyclopentadiene and allyl amine (b.p. 60-61 ℃ /
endo exo
11 mmHg). After dissolving 5-(ammonium methyl)bicyclo[2.2.1]hept- 2-ene (10.0 g, 81.3 mmol) in absolute ethanol (50 mL), the dry gaseous hydrochloride (HCl(g)) generated by reaction of sodium chloride (NaCl, 100 g) with conc. sulfuric acid(H2SO4, 400 mL) was purged into the amine solution at ambient temperature. The reaction was continued till gaseous foggy layer over reaction mixture was disappeared. The precipitated product, quaternized 5-(ammonium methyl)bicyclo[2.2.1]hept-2-ene (NBMQA) was filtered and washed repeatedly with n-hexane 50 mL each time. Washing was continued till no unreacted amine detected in the filtrate.
Then, the quaternized 5-(ammonium methyl) bicyclo[2.2.1]hept-2-ene (NBMQA) was filtered off and dried for 6 hrs at 50 ℃ under vacuum. The resulting product was shown in following structure:
7
3 6
5
4 3 2 1
H H H H H
H H
H H 8 CH2
9 NH3 Cl 7
NBMQA
The yield was 70 %. 1H NMR (500 MHz, D2O): δ (ppm) = 0.59-0.62 (H3), 1.30-1.44 (H7), 1.93-1.98 (H3), 2.35-2.37 (H2), 2.72-2.73 (H8), 2.87-3.07 (H1, H4), 6.00-6.28 (H5, H6), 7.40-7.60 (H9)5); 13C NMR (125 MHz, D2O):δ (ppm) = 30.1, 30.9 (C3), 37.2, 37.4 (C2), 42.1-44.4 (C1, C4, C8), 45.0, 49.7 (C7), 131.8, 136.6 (C6), 138.1, 139.5 (C5).
6. Ring-opening metathesis polymerization of 5-(acryloyl methyl)bicyclo[2.2.1]hept-2-ene (NBMA) using catalyst (I)
NBMA (700 mg, 3.93 mmol) was dissolved in 19 mL methylene chloride. After a freeze-pump-thaw cycle, the solution of catalyst (I) (0.60
mg, 0.79×10-3 mmol) in 1 mL methylene chloride was injected to the monomer solution. Then the solution was stirred at 30 ℃ for 1.5 hrs. After polymerization, the polymer was precipitated in excess methanol. The resulting product poly(NBMA) was shown in following structure:
11 9 10
8
7 6
5 4
3 2
1
O O C H2
C CH CH2
Poly(NBMA)
1H NMR (500 MHz, CDCl3):δ(ppm)= 1.05-3.02 (H1-4, H7), 3.73-4.11 (H8), 5.22-5.34 (H5, H6), 6.06-6.12 (H10), 5.79, 6.34-6.38 (H11) (Figure 7); 13C NMR (125 MHz, CDCl3):δ(ppm) = 35.74-46.58 (C1-4, C7), 66.22 (C8), 128.57 (C10), 129.34-134.76 (C5, C6), 130.35 (C11), 166.14-166.30 (C9) (Figure 8).
7. Ring-opening metathesis polymerization of quaternized 5-(ammonium methyl)bicyclo[2.2.1]hept-2-ene (NBMQA) using catalyst (II)
NBMQA (352 mg, 2.35 mmol) was dissolved in methylene chloride and methanol (co-solvnet, 25 mL, CH2Cl2 : MeOH = 1:3). After a freeze-pump-thaw cycle, the solution of catalyst (II) (5.00 mg, 5.89×10-3 mmol) in 1 mL methylene chloride was injected to the monomer solution by syringe. Then, the solution was stirred at 60 ℃ for 4 hrs. After polymerization, the polymer was precipitated in excess acetone. The resulting product poly(NBMQA) was shown in following structure:
CH2 1 3 2 4
5 6
7
8 9
NH3 Cl
Poly(NBMQA)
The product poly(NBMQA) was soluble in water and methanol. 1H NMR (500 MHz, D2O):δ(ppm)= 1.10-3.50 (H1-4, H7, H8), 5.16-5.47 (H5, H6), 7.57 (H9)5) (Figure 9); 13C NMR (125 MHz, D2O):δ(ppm) = 35.86-44.14 (C1-4, C7), 74.02-74.34 (C8), 130.11-136.46 (C5, C6).
第三年度
1. Synthesis of 5-(glycidyl ether) bicyclo[2,2,1] hept-2-ene}(NBGE)
(Scheme IV)
A mixture of endo- and exo-5-glycidyl ether derivatives of bicyclo[2, 2, 1]hept-2-ene (norbornene) was prepared by the Diels-Alder reaction of cyclopentadiene and allyl glycidyl ether. An ampoule was charged with cyclopentadiene (30 g, 0.455 mol), allyl glycidyl ether (69 g, 0.6 mol) and hydroquinone (0.3 g), and held at 180 oC for 24 hrs. From the reaction mixture was isolated 5-(glycidyl ether)bicyclo[2,2,1] hept-2-ene (NBGE) (37 g, 0.2 mol, yield = 45 %) by distillation 92-93 oC/2 mmHg. IR: 1560 (CH=CH, vinylic), 1099 (C-O-C, ether), 905 (oxirane ring). The NMR spectra of the monomer are shown in Figure 10. The spectra agree satisfactorily with the proposed structure.
2. Polymerization of poly{5-(glycidyl ether)bicyclo[2,2,1]hept-2-ene}
[Poly(NBGE)]
A solution of catalyst was prepared by dissolving {RuCl2(CHPh)[P(C6H11)3]2} (0.001g, 1.22×10-6 mol) in 1 mL of anhydrous
methylene chloride under argon-filled dry box. The monomer (0.22 g, 1.22×10-3 mol) was dissolved in 5 mL of methylene chloride and then degassed via a freeze-pump-thaw cycle. After complete degassing, the catalyst solution was injected into the monomer solution by syringe. The pink solution was vigorously stirred at room temperature for 24 hr, and the color changed from pink to yellow. The reaction was terminated by the addition of a small amount of ethyl vinyl ether (0.5 mL). After termination, the solution was stirred for an additional 5 min, and the polymer, poly[5-(glycidyl ether)bicyclo[2,2,1] hept-2-ene] , was precipitated in excess of methanol and dried overnight in a vacuum system at room temperature to give a flaky white solid. The NMR spectra of the polymer are shown in Figures 11(A) and 11(B). 1H NMR (CDCl3): δ 5.28-5.17 (H2, H3), 2.53-3.59(H8, H9, H10,H11), 2.38-1.12(H1, H4, H5, H6, H7). 13C NMR (CDCl3): δ 135.14, 133.68, 131.51, 130.38(C2, C3), 74.70(C10), 73.43(C9), 71.50(C8), 50.53(C11), 46.09-37.15 (C1, C4, C5, C6, C7). These spectra agree satisfactorily with the proposed structure.
3. Hydrogenation of poly{5-(glycidyl ether) bicyclo[2,2,1] hept-2-ene}
Poly(NBGE) (0.5 g) was dissolved in 50 mL of xylene in an ampoule. To the above solution were added 2.75 g (7.5 equiv. relative to the repeating unit) of p-toluenesulfonylhydrazide as a hydrogenation agent and a trace of 2,6-di-tert-butyl-4-methylphenol. The ampoule containing the polymer, solvent and hydrogenation agent was then degassed thrice via a freeze-pump-thaw cycle and sealed. Then it was gradually heated to 120 oC. At 100℃, a homogeneous solution resulted and nitrogen began to evolve. The solution was stirred at 120 oC for 3 hr until the evolution of nitrogen stopped. The solution was cooled to room temperature and precipitated with methanol. The hydrogenated product, poly(HNBGE), was dried in vacuum overnight at room temperature.
三、 結果與討論:
第一年度
1. Characterization of the poly(NBMOACM)
In the 1H NMR spectrum of poly(NBMOACM), as the vinylic proton peaks of norbornene ring at 5.98 and 6.04 ppm disappeared, new vinyl protons appeared as broad signals between 5.10 and 5.40 ppm [Figure 2(A)]. These broad signals correspond to the vinylic protons of the cis and trans double bond of the ring-opened polymer, respectively. It allows us to estimate a cis and trans ratio of 33 / 67 for this polymer. The proton of -NH- for poly(NBMOACM) was observed as a broad signal between 4.90 and 5.05 ppm. The vinylic proton peaks of cross-linkable side chain were observed at 5.55 ppm and 6.08 ppm. In the 13C NMR spectrum [Figure 2(B)], the signals at 5.67-44.04 ppm are ascribed to the cyclic structure of the polymer. The various vinylic carbon peaks of the ring-opened polymer backbone appeared between 129.13 and 134.60 ppm.
The signals at 125.71 and 135.84 ppm are due to the vinylene units of the methacryloyl group in the side chains. Considerable micro structural variety is possible in these systems owing to the possibility of the head-tail additions, cis and trans vinylene units. 13C NMR was complex with many overlapping multiples or broad unresolved peaks and it was impossible to interpret them in terms of micro structural detail, as is often the case for polymers produced by ROMP.
2. Free radical photopolymerization of novel doubly polymerizable functional norbornenes
Rate of photopolymerization of novel doubly polymerizable norbornene, NBMOACM, in different solvents with varying dielectric constant was determined by dilatometry.58) Photopolymerization was carried out using 2,2’-azobis(cyclohexane-1-carbonitrile) as initiator at 30
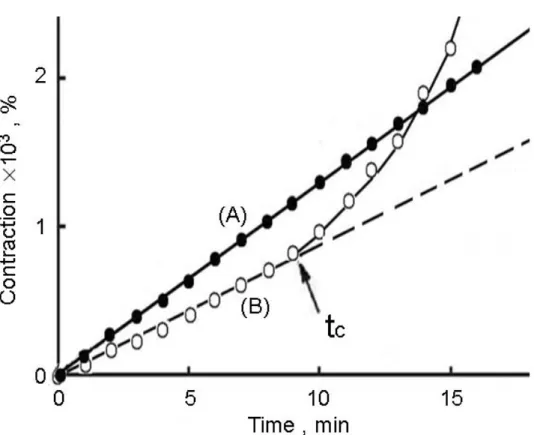
oC, using an Ushio 250 W high-pressure mercury lamp together with filters (Toshiba UV-DIC and IRQ-80) for 3650 Å.58) Free radical photopolymerization of the doubly polymerizable norbornenes was carried out under UV irradiation.58) Interestingly, studies with doubly polymerizable norbornene had shown that there existence of a critical time (tc) for the cross-linking reaction and the insoluble cross-linked microgel was obtained after a period of UV irradiation, which might be due to the free radical reaction of norbornene group and (meth)acrylic group of doubly polymerizable NBMOACM.59,60) From Figure 3, although volume contraction (%) of starting cross-linking reaction was calculated to be smaller than 1×10-3 percent, tc values of NBMOACM was observed remarkably, ca. 9 min. Sen et al. and Sveum et al. also reported the norbornene group could be possible to react with (meth)acrylic group under free radical mediated polymerization condition although the free radical reaction could not be carried out efficiently. 59,60) In this study, insoluble cross-linked microgel was obtained when photopolymerization of NBMOACM (0.25 mol.L-1) in DMF after about 9 min (time > tc) of UV irradiation. Before commencing time of cross-linking reaction (time < tc), the relationship of the volume contraction of the reaction solution and irradiation time was linear. From contraction of volume of the reaction mixture before onset of cross-linking reaction, the initial rate of photopolymerization was calculated.58) When the cross-linking reaction occurs (time > tc), the contraction of volume of the reaction solution increased remarkably. Comparing slopes of volume contraction curves of NBMOACM and MMA, the rate of photopolymerization for various monomers is in the following order MMA > NBMOACM (before tc).
From Table 1, it can be seen that the rate of photopolymerization (Rp) of NBMOACM in less polar solvent such as benzene is higher than the rate of photopolymerization (Rp) in polar solvents such as DMF(Run No. 3~6,
Table 1). The influence of solvent on rate of photopolymerization (Rp) might be explained in terms of difference in the environment around the propagating radical of polymer chain and monomer. As the polarity of the solvent increases, there will be effective hydrogen-bonding between monomer and solvent. As polar solvent might be interacted readily with the propagating polymer chain and/or monomer (for example, hydrogen-bonding) in propagation stage and thus probably resulting in dormant condition of the propagating polymer chain and/or monomer.
This may be reason for observing lower rate of polymerization (Rp) in polar solvent.58,61) Since the rate of polymerization(Rp) is dependent on polarity of solvent, the dielectric constant (ε) of the solvent may be used as a rough measure of such interactions (Table 1). The lower rate of polymerization (Rp) of functional norbornenes such as NBMOACM having more polar groups like urethane than MMA in more polar solvents clearly demonstrates the dormant stage of propagation due to the hydrogen-bonding of NBMOACM. This also further confirms the strong interactions between solvent molecule and the monomer, and/or propagating polymer chain, which contains more polar urethane or amide group by hydrogen bonding.58,61) On comparing the UV spectra of NBMOACM and MMA in DMF solution, it can be seen that the UV absorption (λmax ) is red-shifted by 4 nm from 268 nm(MMA) to 272 nm (NBMOACM). Such red shift could be observed when there is a hydrogen-bonding between solute and solvent/solute. The proposed model of interaction by hydrogen-bonding between solvent (DMF molecule)/ monomer (NBMOACM) (Scheme II) and, monomer (NBMOACM) (Scheme II) and/or propagating polymer chain is shown in Scheme II. Polymerization of NBMOACM led to the formation of a novel organo-soluble polynorbornene with cross-linkable side chains [poly(NBMOACM)]. Gel formation did not occur during the
polymerization of NBMOACM whether p-methoxyphenol (MEHQ) was added or not.62) Increasing the [M]/[cat] ratio resulted in the formation of higher molecular weight polymers(Table 2). The molecular weight distribution of poly(NBMOACM) was narrow even though the molecular weight was high (Mn= 3.3× 105, Mw / Mn = 1.8)(Table 2). Despite their high molecular weight (Mn= 3.3× 105 and [M]/[cat] = 1.2× 105 ), they showed excellent solubility in various organic solvents such as DMSO, DMF, DMAc, THF, pyridine, methylene chloride and chloroform(Table 2).
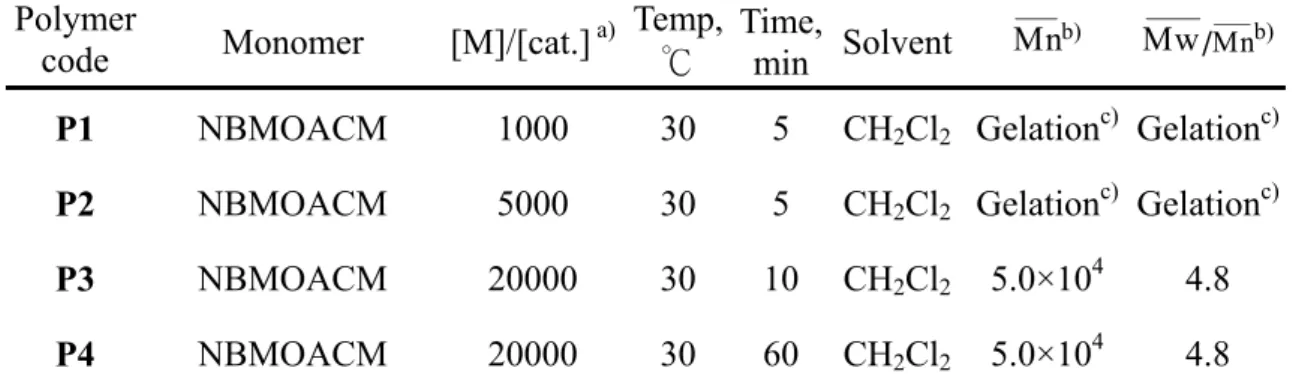
Thus, poly(NBMOACM) obtained was as an organo-soluble polymeric material. When xylene, chloroform or methylene chloridewas used as a solvent and Ru catalyst (II)(2.45 ×10-5 mol.L-1)was used as a catalyst, gel formation was observed after 10 min at 30 during ROMP of ℃ NBMOACM with [M]/[I] ratio of 103 and 5×103(P1 and P2, Table 3).
Enhancing the [M]/[I] ratio could be a strategy to obtain high molecular weight poly(NBMOACM) and avoid the gelation in Ru catalyst (II) solution. ROMP of NBMOACM with [M]/[I] ratio of 2×104 was
performed by using Ru catalyst (II) [(NHC)(PCy3)(Cl)2Ru=CHPh](1.12×10-5 mol.L-1) at 30 (℃
P3 and P4,
Table 3). A novel organo-soluble poly(NBMOACM) could be obtained with the high molecular weight of 5.0×104 and PDI of 4.8(P3 and P4, Table 3). The polydispersity indice (PDI) of poly(NBMOACM) obtained from Ru catalyst (II) is broad(PDI=4.8), which arises from an unfavorable rate of initiation relative to propagation as well as considerable secondary metathesis(“back-biting”).63,64-66) That is, Ru catalyst (II) is an effective polymerization catalyst, the rate of propagation is much higher than the rate of initiation leaving a high concentration of uninitiated carbene available upon complete monomer consumption, resulting in nonliving behavior and broad polydispersity indices.63,65)
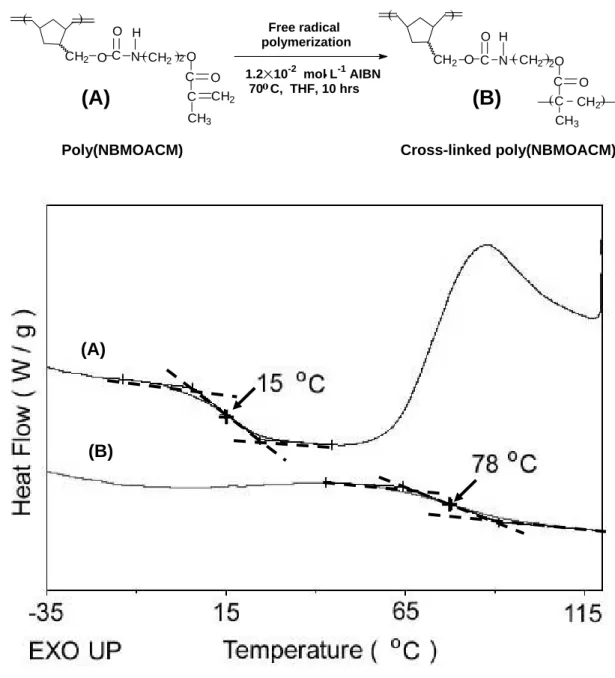
3. Novel macromolecular architectures from dual cure by using NBMOACM
Novel macromolecular architectures were prepared from mutifunctional NBMOACM by dual cure. It was possible to carry out ring-opening metathesis polymerization and conventional free radical polymerization of NBMOACM as it contains a norbornene group and a methacryloyl group. A novel organo-insoluble functional polynorbornene could be obtained after ROMP of NBMOACM. The resulting functional polynorbornene, poly(NBMOACM), still contains cross-linkable side chains and shows a glass transition temperature (Tg)value of 15 oC[Figure 4(A)]. Since resulting poly(NBMOACM) has cross-linkable methacryloyl groups in side chain, poly(NBMOACM) was cross-linked by thermal free radical polymerization. Glass transition temperature (Tg) of the resulting cross-linked poly(NBMOACM) was observed at 78 oC[Figure 4(B)]. Cross-linked polymeric material was obtained due to cross-linking of the methacryloyl side chains of the poly(NBMOACM). Also, free radical polymerization of NBMOACM could be carried out because it contains a methacryloyl group.59,60) The incorporation of poly(NBMOACM) which was obtained through chemical cross-links in poly(MMA) (polymethylmethacrylate) can be considered to improve thermal stability, and chemical resistance of poly(MMA). The exothermal curve (ca. 176 oC) of poly(NBMOACM) indicate that the initiation of thermally induced cross-linking reaction of the methacryloyl group.
Thermal stability of interpenetrating (IPN) polymer of poly(MMA) and poly(NBMOACM) was higher than that of pure poly(MMA).67) Increase in thermal stability by 24 oC [1 wt% poly(NBMOACM), Td10=300 oC in air]
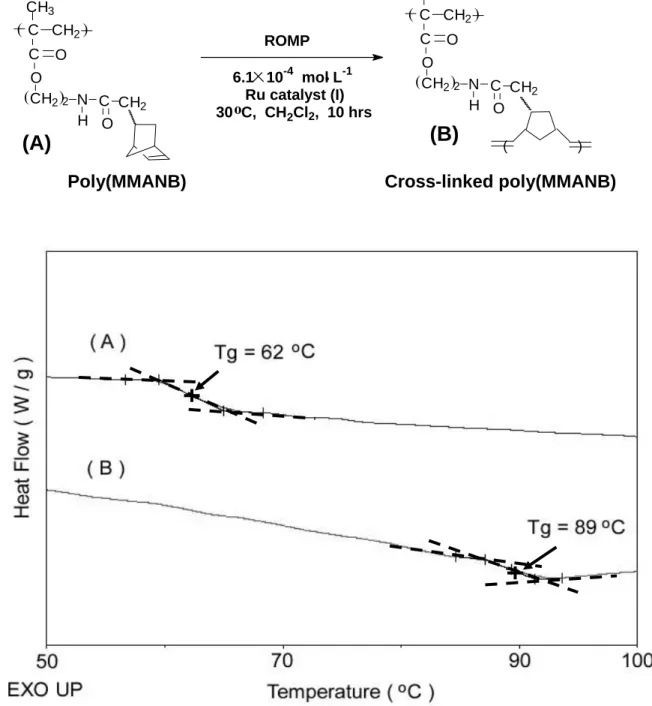
was observed for the new AB cross-linked materials compared to pure poly(MMA)(Td10=276 oC in air). Also, an intended polymer with norbornene side chains [poly(MMANB)] has been prepared. In the 1H
NMR spectrum of poly(MMANB), the signals of vinylic proton peaks of methyl methacryloyl group at δ 5.55 and 6.08 ppm completely disappeared and the signals of vinylic proton peaks of norbornene ring between δ 5.95 and 6.16 ppm still remained. It has been identified the structure of poly(MMANB) from the NMR spectrum, which was exhibited an endo/exo ratio (endo/exo = 72/28) same as that of NBMOACM monomer.
Additional cross-linking was observed because of the norbornene-containing side chains of the poly(MMANB)(Tg=62 oC) [Figure 5(A)]. The resulting cross-linked poly(MMANB) was insoluble in common organic solvents and had a Tg value at 89 oC[Figure 5(B)].
第二年度
1. Characterization of the diblock copolymer
poly(NBMPI-b-NBMOACM) and homopolymer poly(NBMA)
The 1H NMR spectra of diblock copolymers of 5-(phthalimide methyl) bicyclo[2.2.1]hept-2-ene (NBMPI) and 5-(methyl methacryloyl ethyl amino carboxyl)bicyclo[2.2.1]hept-2-ene (NBMOACM) are presented in Figure 6. The vinylic proton peaks of norbornene ring at δ(ppm) 5.81 and 5.99 disappeared. The 1H NMR spectra of the copolymers showed new vinyl protons as broad signal betweenδ (ppm) 5.15 and 5.18. In the 13C NMR spectra, the signals atδ (ppm) 30-50 are ascribed to the peaks of cyclic structure of the copolymers. The various vinylic carbon peaks of the ring-opened polymer backbone appeared between δ (ppm) 134 and 135.
The signals at δ (ppm) 133 and 136 are due to the vinylene units of the methacryloyl group in the side chains. In the 1H NMR spectrum of water-soluble poly(NBMPI-b-NBMOACM), the vinylic proton peaks of norbornene ring at δ (ppm) 7.31 and 7.80 disappeared, and showed new vinyl protons around 2.2 - 2.3 ppm in the 1H NMR spectrum of poly(NBMPI-b-NBMOACM). 5-(Acryloylmethyl)bicyclo[2.2.1]hept-2-ene
(NBMA) with cross-linkable end group was characterized by NMR and DSC. The Tg of poly[5-(acryloyl methyl)bicyclo[2.2.1]hept-2-ene] was measured by DSC (10 •min℃ -1) and revealed the Tg of 11-12 . ℃ Ring-opening metathesis polymerization of NBMA was carried out.
In the 1H NMR spectrum of poly(NBMA), the vinylic proton peaks of norbornene ring at δ (ppm) 5.91, 6.05 and 6.11 disappeared, the 1H NMR spectrum of the polymer showed new vinyl protons as broad signal between δ (ppm) 5.22 and 5.34. These broad signals correspond to the vinylic protons of the cis and trans double bond of the ring-opened polymer, respectively. The vinylic proton peaks of cross-linkable side chain were observed at δ (ppm) 5.79 and 6.34-6.38. In the 13C NMR spectrum, the signals at δ (ppm) 76.57-77.43 are ascribed to the peaks of CDCl3 and the signals at δ (ppm) 35.74-46.86 are ascribed to the cyclic structure of the polymer. The various vinylic carbon peaks of the ring-opened polymer backbone appeared between 129.34 and 134.76 ppm. The signals at δ (ppm) 128.57 and 130.35 are due to the vinylene units of the methacryloyl group in the side chains. Considerable micro structural variety is possible in these systems owing to the possibility of the head-tail additions, cis and trans vinylene units. In the 1H NMR spectrum of water-soluble poly(NBMQA), the vinylic proton peaks of norbornene ring betweent δ (ppm) 6.00 and 6.28 disappeared, the 1H NMR spectrum of the polymer showed new vinyl protons as broad signal between δ (ppm) 5.16 and 5.47. The peak of amine salt 57) atδ (ppm) 7.57. The molecular weights and molecular weights distributions of polymers: poly(NBMPI-b-NBMOACM) and poly(NBMA) are shown in Table 4.
2. Solubility of polymers
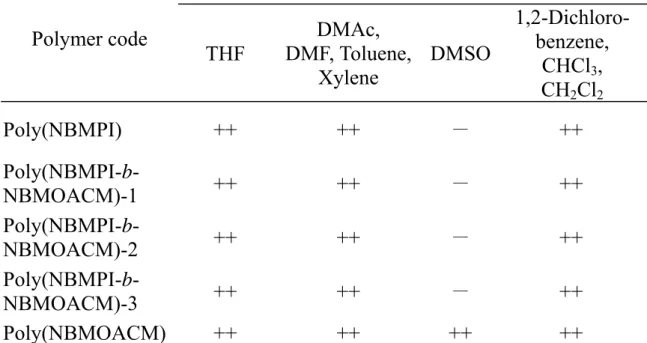
Poly(NBMOACM) was soluble in dimethylsulfoxide (DMSO), N, N-dimethyl-formamide (DMF), N, N-dimethylacetamide (DMAc),
tetrahydrofuran (THF), methylene chloride, chloroform, 1,2-dichlorobenzene, xylene and toluene. The resulting poly(NBMPI-b-NBMOACM)s were soluble in N, N-dimethylformamide (DMF), N, N-dimethylacetamide (DMAc), tetrahydrofuran (THF), methylene chloride, chloroform, 1,2-dichlorobenzene, xylene and toluene.
NBMPI-containing diblock copolymers are insoluble in dimethylsulfoxide (DMSO). The same phenomenon was also observed for NBMPI homopolymer (Table 5). Poly(HCQNBMA-b-NBMOACM), which was obtained after hydrolysis and quaternization of poly(NBMPI-b-NBMOACM), was soluble in water.
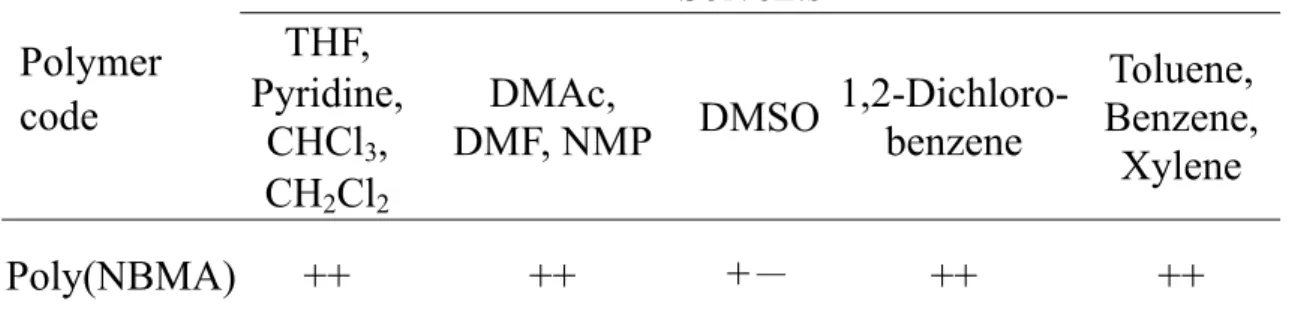
The solubility of the poly(NBMA) in various solvents is shown in Table 6. Poly(NBMA) was soluble in pyridine, N, N-dimethylformamide (DMF), N, N-dimethylacetamide (DMAc), tetrahydrofuran (THF), methylene chloride, chloroform, 1,2-dichlorobenzene, xylene, toluene, N-methyl-2-pyrrolidinone (NMP) and benzene. Poly(NBMQA) was soluble in water and methanol (Table 7).
第三年度
Characterication of poly{5-(glycidyl ether) bicyclo[2,2,1] hept-2-ene}
[Poly(NBGE)]
In Table 8 the results are listed for the ring-opening metathesis polymerization of the monomer by varying the ratio of monomer concentration to catalyst concentration [cat.]. When the mole ratio of monomer to catalyst was increased, the higher molecular weight polymers were obtained.
1H NMR, 13C NMR and IR analyses were carried out to prove the chemical structure of the polymer. As the polymerization proceeded (Figure 11), the vinylic proton peaks at 5.73-5.92 ppm disappeared. The 1H NMR spectrum of the polymer showed new vinyl protons as broad signals
between 5.20 and 5.32 ppm in the ratio of approximately 25:75. These broad signals correspond to the vinyl protons of the cis and trans double bond of the ring-opened polymer, respectively. There is a band at 710 cm-1 and a broad band at 966 cm-1 in the polymer. The strong band about 710cm-1 is due to the cis arrangement about a double bond, in which the
=CH bond can undergo an out of plane bending vibration, while a trans double bond usually absorbs near 966 cm-1 in such a vibration mode. The above IR in formation also supports the assumption that the ring-opened polynorbornene main chain has both cis and trans double bonds. In the
13C NMR spectroscopy, the monomer gave vinylic carbon peaks at 132.6, 136.7, 136.8 and 137.3 ppm. On the other hand, the polymer did not show these peaks; instead, the various vinylic carbon peaks of the ring-opened polymer backbone 68) appeared between 130.3 and 135.4 ppm.
If the p-toluenesulfonylhydrazide was employed to carry out hydrogenation of poly(NBGE), the gel-like product [poly(HNBGE)] was obtained. The behavior would be due to the crosslinking reaction resulted from ring-opening of epoxide group. The solubility characteristics of the poly{5-(glycidyl ether) bicyclo[2,2,1] hept-2-ene} [poly(NBGE)] and the gel-like product[poly(HNBGE)] are shown in Table 9. It was found that poly{5-(glycidyl ether methyl)bicyclo[2,2,1] hept-2-ene} [poly(NBGE)]]
exhibits good solubility in a variety of solvents such as methylene chloride, tetrahydrofuran, 1,2-dichlorbenzene, benzene, toluene, xylene and N-methyl-2-pyrrdione. However, poly(HNBGE) was found to be insoluble in common organic solvents. The excellent solubility of active epoxy-containing poly(NBGE) could be designed with high potential of application for photoresist, UV curing, and elastomers.
The thermal stability of the ring-opened polymers was evaluated by thermogravimetric analysis (TGA) in air at a heating rate 20 oC.min-1. Figure 12 shows the TGA curve for poly{5-(glycidyl ether)bicyclo[2,2,1]
hept-2-ene} [poly(NBGE)]. From Figure 12, only one stage was observed and mass loss starts at 350 oC and continues to 380 oC with ca. 10 % mass loss in the TG curve for poly(NBGE).
四、 結論:
第一年度
A novel doubly polymerizable norbornene was prepared. Rate of photopolymerization of novel doubly polymerizable norbornene in different solvents with varying dielectric constant was determined by dilatometry. Rates of photopolymerization were depend on interaction such as hydrogen bonding between propagating polymer chain and monomer and/or solvent. No gelation and/or insoluble elastomer formation occurred during ROMP of NBMOACM in Ru catalyst(I) system.
Two methylene units between urethane group and ester group of poly(NBMOACM) disturbed hydrogen bonding and enhanced solubility.
The polydispersity indices (PDIs) of polymers obtained from Ru catalyst (II) are broad. Free radical polymerization of NBMOACM could be carried out because it contained a methacryloyl group and poly(MMANB) could be obtained. Due to the norbornene-containing side chains of the poly(MMANB), cross-linking was observed. Novel macromolecular architectures using NBMOACM was obtained by a combination of ROMP and conventional free radical polymerization. Interpretation of characterization and polymerization of the novel doubly polymerizable norbornenes are being studied and will be communicated shortly. The methodology used here is expected to open up the possibility of producing entirely novel materials with new and potentially interesting properties.
第二年度
Novel diblock copolymers having different molecular weight from 5-(phthalimide methyl)bicyclo[2.2.1]hept-2-ene (NBMPI) and 5-(methyl methacryloyl ethyl amino carboxyl)bicyclo[2.2.1]hept-2-ene(NBMOACM) have been successfully synthesized by ROMP using {RuCl2(CHPh)[P(C6H11)3]2} catalyst. The molecular weight distributions of the diblock copolymers were narrow (Mw/Mn~1.5-1.6). Furthermore, ROMP of cross-linkable 5-(acryloyl methyl) bicyclo[2.2.1]hept-2-ene (NBMA) and quaternized 5-(ammonium methyl)bicyclo-[2.2.1]hept-2-ene (NBMQA) was successful and afforded polymers in good yields.
第三年度
A new norbornene containing epoxy group, 5-(glycidyl ether) bicyclo[2,2,1] hept-2-ene (NBGE), was successfully synthesized; and can be polymerized by {RuCl2(CHPh)[P(C6H11)3]2} catalyst via ring-opening metathesis polymerization (ROMP). Results of IR, 1H NMR, and 13C NMR agree with the proposed structure for monomer and polymer.
Increasing the ratio of [M] to [cat.], the formation of higher molecular weight polymers was obtained. The polynorbornene containing active epoxy groups, poly(NBGE), showed good thermalstability(Td,10%=380oC).
High performance polynorbornenes with active epoxy groups could be designed with high potential of application for photoresist, UV curing and elastomers.
五、 計畫成果自評:
本 計 畫 完 全 依 照 計 畫 內 容 執 行 , 執 行 之 成 果 已 發 表 在 PolymerPreprints (Polym. Prepr. Am. Chem. Soc Div. Polym. Chem., Vol.
44(1), pp. 943-944 (2003)) 及專利(廖德章, 黃慶成, 洪壽懋, 架橋基を 有するノルボルネソ系化合物及びそれに誘導される重合体, 日本專 利, 特開 2005-126372 及廖德章, 黃慶成, 洪壽懋, NORBORNENES WITH CROSS-LINKABLE GROUPS AND THEIR POLYMERIC MATERIALS, 美國專利申請中)中。本計畫之新型含原冰片烯單體皆使 用儀器鑑定其結構,鑑定結果均符合結構,另外其聚合物皆具有優良
物性(如溶解性、熱性質及機械性質),可供學術界及工業界參考,為
一具有發展潛力之高分子材料。
六、 致謝:
感謝行政院國家科學委員會補助專題計劃NSC 93-2216-E-011-001 之經費補助,使本三年期計劃之期末報告得以順利執行。
七、 參考文獻:
1. A. Demonceau, A. W. Stumpf, E. Savive, and A. F. Noels, Macromolecules, 30, 3127 (1997)
2. P. Schwab, R. H. Grubbs, and J. W. Ziller, J. Am. Chem. Soc., 118, 100 (1996)
3. D. M. Lynn, S. Kanaka, and R. H. Grubbs, J. Am. Chem. Soc., 118, 784 (1996)
4. P. Schwab, M. B., J. W., R. H. Grubbs, Angew. Chem., Int. Ed. Engl.,
34, 2039 (1995)
5. C. Pugh, J. Dharoa, and S. V. Arehart, Macromolecules, 30, 4520 (1997)
6. B. R. Maughon, M. Weck, B. Mohrand R. H. Grubbs, Macromolecules,
30, 257 (1997)
7. S. V. Arehart and C. Pugh, J. Am. Chem. Soc., 119, 3027 (1996)
8. M. Weck, P. Schwab, and R. H. Grubbs, Macromolecules, 29, 1789 (9996)
9. B. R. Maughon and R. H. Grubbs, Macromolecules, 30, 3459 (1997) 10. A. Deffieux and M. Schappacher, Polym. Adv. Tec., 7 , 122 , (1996) 11. J. Gratt and R. E. Cohen, Macromolecules, 30, 3137 (1997)
12. V. C. Gibson, E. L. Marshall, M. North, D. A. Robson, and P. J.
William, Chem. Commun.,1095 (1997) 13. Japan Plastic , 8 , 11 (1974)
14. D. J. Liaw, A. Soum, M. Fontanille, A. Parlier and H. Rudler, Makromol. Chem. Rapid Comm., 6, 309 (1985)
15. 木尾田徹 日本合成橡膠 第七回光反應電子用材料研究會講座
(東京)1998 年 1 月 21 日
16. U. Okoroanyanwu, T. Shimokawa, J. Byers, D. Medeiros, C. G.
Willson, Q. J. Niu, J. M.J. Frechet, SPIE , 3049, 92
![Table 8. Effect of [Monomer]/[Catalyst] ratio on molecular weight and molecular weight distribution in the polymerization of poly(NBGE)](https://thumb-ap.123doks.com/thumbv2/9libinfo/9128530.412630/51.892.140.775.155.357/table-effect-monomer-catalyst-molecular-molecular-distribution-polymerization.webp)
![Figure 10 NMR spectra of 5-(glycidyl ether) bicyclo[2,2,1]hept-2-ene mononer taken in CDCl 3 : (A) 1 H NMR and (B) 13 C NMR](https://thumb-ap.123doks.com/thumbv2/9libinfo/9128530.412630/52.892.191.698.116.960/figure-spectra-glycidyl-ether-bicyclo-mononer-taken-cdcl.webp)