行政院國家科學委員會專題研究計畫 成果報告
利用二氧化鈦塗佈於奈米碳管之染料敏化太陽能電池研究 研究成果報告(精簡版)
計 畫 類 別 : 個別型
計 畫 編 號 : NSC 97-2221-E-011-017-
執 行 期 間 : 97 年 08 月 01 日至 98 年 10 月 31 日 執 行 單 位 : 國立臺灣科技大學電子工程系
計 畫 主 持 人 : 李奎毅 共 同 主 持 人 : 葉文昌
計畫參與人員: 碩士班研究生-兼任助理人員:陳嘉桓 碩士班研究生-兼任助理人員:曾光義
報 告 附 件 : 出席國際會議研究心得報告及發表論文
處 理 方 式 : 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢
中 華 民 國 99 年 04 月 25 日
行政院國家科學委員會補助專題研究計畫 ■成果報告
利用二氧化鈦塗佈於奈米碳管之染料敏化太陽能電池研究計畫類別:■ 個別型計畫 □ 整合型計畫 計畫編號:NSC 97-2221-E-011-017-
執行期間: 97 年 8 月 1 日至 98 年 10 月 31 日
計畫主持人:李奎毅 共同主持人:葉文昌
計畫參與人員: 陳嘉桓、曾光義
成果報告類型(依經費核定清單規定繳交):■精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
■出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:國立台灣科技大學電子工程系
附件一
中文摘要
在研究染料敏化太陽能電池(dye-sensitized solar cell, DSSC)陽極的議題中,
許多因素決定了太陽能電池在效能上的表現,其中陽極表面積的大小為主要因素 之一,而二氧化鈦與導電基板的連續性亦是必須考慮之參數,本實驗利用垂直成 長於不銹鋼基板之奈米碳管束做為陽極基底不僅能提供更短之電子傳輸路徑且 成長之多壁碳米碳管具有優良之導電性使得串聯電阻能降到最低,另外高密度奈 米碳管之成長提供更大之表面積可使二氧化鈦奈米粒子連續附著進而達到更大 量染料分子的吸附。
本計畫中,利用熱化學氣相沈積法並以乙炔作為碳源,基板材質為不鏽鋼,
在750℃下成長高方向性及高數密度之奈米碳管束作為陽極基底。為了獲得最佳 效能之染料敏化太陽能電池,具有光觸媒之半導體氧化物層利用德固賽之二氧化 鈦奈米粉體(P25)混合四氧異丙醇鈦(tetra isopropoxide, TTIP)及無水酒精做為塗 佈之前驅物,並調整不同四氧異丙醇鈦之濃度來討論二氧化鈦奈米粒子之連結 性。結果得到在四氧異丙醇鈦濃度為0.072 M 時所製作之太陽電池元件置於100 mW/cm2 光照之下測得開環路電壓(open circuit voltage)為0.34 V,短路電流密度 (short circuit current density)為5.9 mA/cm2,填充因子(fill factor)為0.51及光電轉換 效率(η)為1.49%。
關鍵詞: 染料敏化太陽能電池,奈米碳管,二氧化鈦奈米粒子,光電轉換效率
英文摘要
A large surface area, good electric conductivity and good connection between TiO2 and substrate by combing TiO2 nanoparticles and vertically aligned carbon nanotubes (VACNT) bundles were used to characterize the photoelectric conversion efficiency (η) of dye-sensitized solar cell (DSSC). A novel anode of DSSC has been developed. Vertically aligned carbon nanotube (VACNT) bundles were used as the template. VACNTs were synthesized by thermal chemical vapor deposition. The substrate for VACNTs growth was stainless sheet. C2H2 was used as the carbon source. The growth temperature was 750℃. To obtain the optimum performance, the different concentrations of TiO2 solution were modified to observe the surface morphologies of anode. The best photoelectric characteristics of titanium (IV) isopropoxide (TTIP) concentration for 0.072 M showed the short circuit current density (Jsc), open circuit voltage (Voc), fill factor (FF) and η was 5.9 mA, 0.34 V, 0.51 and 1.49%, respectively.
Keywords: Dye-sensitized solar cell, Carbon nanotube, TiO2 nanoparticles, Photoelectric conversion efficiency
報告內容
前言、目的、文獻探討
Since dye-sensitized solar cell (DSSC) was developed, it has drawn much attention because of its simple fabrication process, low cost, and high conversion efficiency (η).
DSSC was first developed by Tsubomura and Matsumura using aluminum-doped ZnO electrodes [1]. Although the photoelectric conversion efficiency (η) was not too high (about 2.5%), the development of DSSC is still an interesting research topic. A typical DSSC consists of four components, including (1) anode (electrode), (2) nano-crystalline (TiO2 layer), (3) counter electrode, and (4) liquid electrolyte between the two electrodes [2, 3]. The configuration of DSSC is often formed with a special sandwich structure. To commercialize the DSSC devices, it is need to improve the formation skill to achieve relatively longer lifetime and higher η. By increasing the surface area of anode is one of the effective approaches to improve the η.
In 1988, Prof. Grätzel developed a new solar cell by using nano-porous TiO2 film to form the working electrode be sensitized using the Ru-complex dye [4-6]. Among many materials for the anode fabrication, the n-type semiconductor titanium dioxide (TiO2) with nanostructure is usually adopted because of its nanoporous nature [7, 8], which exhibits a relatively larger surface area than TiO2 film [9, 10]. TiO2 has many unique characteristics, such as high photocatalytic activities, chemical and thermal stability and a wide band-gap (~3.2 eV).
The mixture of P25 (TiO2 nanoparticles) and TTIP (Titanium (IV) isopropoxide) is one of the most common approaches for the preparation of nanoporous TiO2 [11, 12].
The large surface area which the nanoporous TiO2 exhibitsis an essential requirement for dye adsorption. On the other hand, carbon nanotubes (CNTs), discovered in 1991 by Iijima [13], have attracted great interest due to their special physical and chemical properties [14, 15]. Because of the good electrical conductivity, thermal stability [16], it was also reported to be used to combine with the mixture of P25 and TTIP as the anode material [17, 18]. The DSSCs with such a fabricated anode has a relatively high η. Nevertheless, this kind of the DSSC was assembled by utilizing random CNTs with the P25 and TTIP composite [19-21]. CNTs have two main surface morphologies:
random CNTs [22] and vertically aligned CNTs (VACNTs) [23, 24]. Between them, it is easier to control the length and calculate the number density of VACNTs. The usable surface area of VACNT is also larger than that of random CNTs. Therefore, VACNTs is more suitable than random CNTs in DSSC applications. In this study, we use the VACNT bundles as a template to let the TiO2 nanoparticles attach and offer a suitable conduction path for electrons transportation in the application of DSSC. The nanoporous TiO2 nanoparticles were attached on the VACNT bundles surface to improve the η.
研究方法
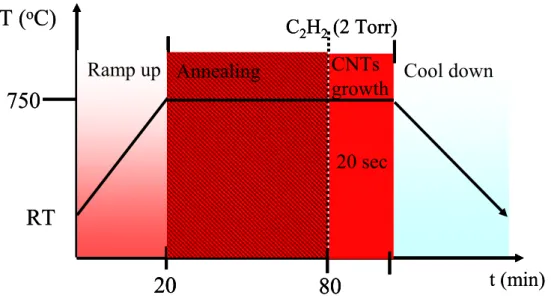
The substrate used for VACNT bundles growth was stainless steel sheets. Before the VACNT bundles growth, the stainless steel sheets were supersonically cleaned with ethanol. A mesh was used to contact with the stainless steel sheets to pattern the Al (20 nm) and Fe (2 nm) layers, which were deposited by electron beam (e-beam) evaporator, respectively. The base pressure of the e-beam evaporator system is about 6×10-7 Torr, and the working pressure is about 1×10-6 Torr. All the e-beam depositions (Al and Fe films) are at room temperature. Subsequently, for the catalyst nanoparticles formation, the stainless steel sheets were loaded into the thermal chemical vapor deposition (TCVD) system and were annealed at 750oC under a pressure of about 4×10-2Torr for 60 min. After annealing, C2H2 gasas the carbon source was introduced into the TCVD system with a flow rate of 150 sccm to grow the VACNT bundles at 750oC for 20 min. Figure 1 shows the procedure of VACNT bundles growth.
TiO2 solution was prepared by mixing P25 (Degussa, BET surface area 50±15 m2g-1, average primary TiO2 particle size 21 nm), TTIP (Titanium (IV) isopropoxide), and ethanol (purity (GC) 99.5%) at a molar ratio of 1: 0.072: 12. The TiO2
nanoparticles of P25 were dispersed by using an ultrasonic agitation for 25 min. After dispersing, the TiO2 solution was poured in drops into the VACNT bundles. The TTIP concentration is varied from 0.018 to 0.090 M. The surfaces of VACNT bundles were adhered with TiO2 nanoparticles which were continuous and adjacent. Subsequently, the TiO2-coated VACNT bundles were heated at 50oC for 120 min to form the DSSC anode. The formed DSSC anode with TiO2-coated VACNT bundles was sensitized by N719 cis-di(thiocyanato)-bis(2,2’-bipyridy1-4-carboxylate-4’-carboxylic acid)-ruthenium(II)] dye (0.71 mg) mixing with anhydrous ethanol (99.5%) for 20 hours at room temperature. After sensitizing, the unnecessary dye attached on the anode was removed and dried in room temperature without light. The counter electrode of DSSC was prepared by amount of copper wires on transparent glass. The DSSC was composed of the anode and counter electrode to be a sandwich structure with silicone. The electrolyte (Esolar ESE-20) was injected into the DSSC structure.
The illumination area of the DSSC sample was 0.2 cm2. Figure 2 shows the schematic illustration of the formed DSSC structure.
The surface morphology of TiO2 nanoparticles adhere onto VACNT bundles was observed by scanning electron microscopy (SEM). The structure of TiO2 nanoparticles was characterized by using X-ray diffraction (XRD). The current density versus voltage (J-V) curve of DSSC was measured by electrochemical analyzer. The light source was a 500 W Xe lamp and the illumination intensity was 70 mW/cm2.
結果與討論
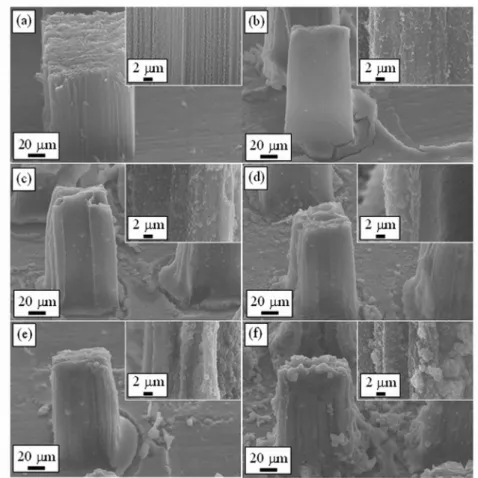
To investigate the influences on the surface morphologies of VACNT bundles with different TTIP concentrations, SEM was used to characterize the surface morphologies of the formed anodes. Figures 3(a)-3(f) show the SEM images of VACNT bundles grown on the stainless steel substrates with various TTIP concentrations. The inset in each image shows the enlarge SEM image. Fig. 2(a) shows a SEM image of pristine VACNT bundles grown on stainless steel substrate.
According to the configuration of mesh, the shape of the synthesized VACNT bundle was cuboid. The length of bottom side was about 60 nm. Each bundle contained VACNTs with a high number density of about 109cm-2. The length of the pristine VACNT bundles was about 80 nm. Because the pristine VACNT bundles were synthesized on the stainless steel substrate directly, it can be observed that the pristine VACNT bundles exhibited good adhesion to the stainless steel substrate. Figures 3(b)-3(f) show a series of SEM images with various TTIP concentrations of 0.018, 0.036, 0.054, 0.072, 0.090 M, respectively. It can be observed that when the concentration of TTIP was increased from 0.018 to 0.072 M, the connection among TiO2 nanoparticles was improved gradually. On the other hand, when the TTIP concentration was higher than 0.072 M, the TiO2 nanoparticles started to aggregate, that is, the surface morphology in this situation was uneven. Therefore, in this study, from the surface morphology observation by SEM, when the TTIP concentration was 0.072 M, an uninterrupted TiO2 thin film was formed, which offered an optimal conduction path for electrons transportation in DSSC application.
To confirm the nanoparticles on the surface of the VACNT bundle surface were TiO2, the XRD measurement system was utilized. Figure 4 shows the XRD spectrum of the VACNTs, which was coated with TiO2 nanoparticles. The angle of 25.1o corresponded to the (101) plane represents the anatase structure of TiO2. The angles, 27.3oand 47.9 o were assigned to (101) and (200) planes, respectively, represent the TiO2 rutile structure. The other planes, designed with SUS, were from the stainless sheet. As a result, from the XRD spectrum results, the nanoparticles were TiO2, and the TiO2 nanopaticles coated on the VACNT bundles in this work were the mixture of the anatase and rutile structures.
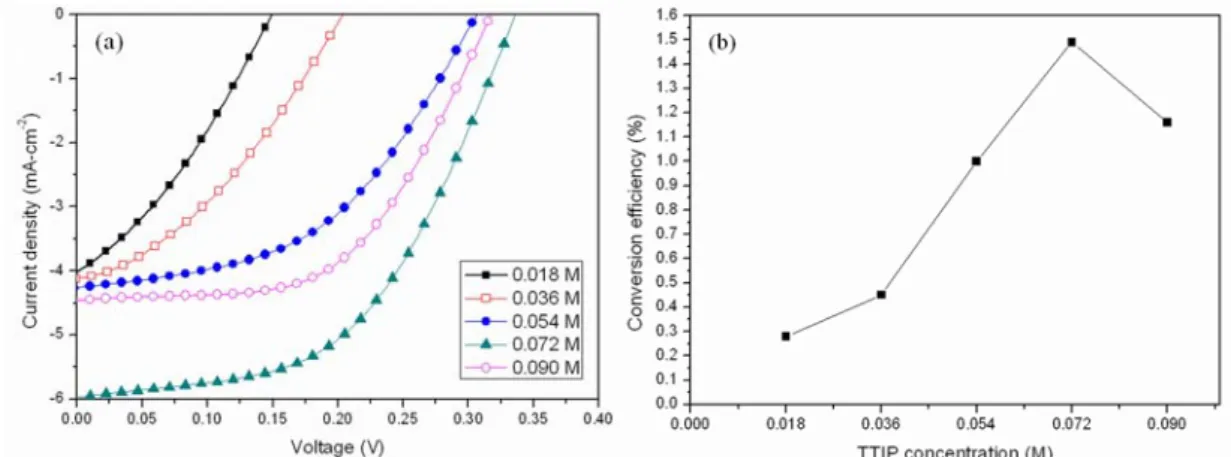
To realize the relationship between the η and TTIP concentration, the η, open circuit, short circuit current, and fill factor (FF) under different TTIP concentrations were measured and summarized in Table 1. Figure 5(a) shows the J-V curve, and Fig.
4(b) shows the relationship between the TTIP concentration and η, respectively. It can be found that the η has a relatively high value at the TTIP solution concentration of 0.072 M. It was because that compared with the other TTIP concentrations, when the TTIP concentration was 0.072 M, this concentration of TTIP made TiO2 nanoparticles
closely connected to each other, resulting in the few loss of electrons during transportation. When the TTIP concentration was lower than 0.072 M, the continuity of TiO2 was not good enough to let electrons transport. On the other hand, when the TTIP concentration was higher than 0.072 M, the aggregation on the surface of VACNT bundles increased the possibility of recombination for electrons, resulting in the reduction in efficiency. The optimum data measured in this work were 1.49% for efficiency, 0.34 V for open circuit voltage, 5.9 mA for short circuit current, and 0.51 for FF, respectively. It was found that there existed an optimal TTIP concentration for enhancing the performance of photoelectric characteristics.
參考文獻
[1] Matsumura M, Matsudaira S, Tsubomura H. Ind. Eng. Chem. Prod. Res. Dev 1980; 19:415-421.
[2] Grätzel, M. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2003;4:145-153.
[3] Horiuchi T, Miura H, Sumioka K, Uchida S. Journal of the American Chemical Society 2004;126:12218-12210.
[4] Vlachopoulos N, Liska P, Augustynski J, Grätzel M. J. Am. Chem. Soc 1988;110:1216-1220.
[5]Hara K, Sugihara H, Tachibana Y, Islam A, Yanagida M, Sayama K, Arakawa H, Kinoshita T. Langmuir 2001;17:5992-5999.
[6] Willinger K, Fischer K, Kisselev R, Thelakkat M. Journal of Materials Chemistry 2009;19:5364-5376.
[7] Hara K, Horiguchi T, Kinoshita T, Sayama K, Sugihara H, Arakawa H. Solar Energy Materials and Solar Cells 2000;64:115-134.
[8] Zaban A, Meier A, Gregg BA. Journal of Physical Chemistry B 1997;101:7985-7990.
[9] Tachibana Y, Moser JE, Grätzel M, Klug DR, Durrant JR. Journal of Physical Chemistry 1996;100:20056-20062.
[10] Adachi M, Murata Y, Takao J, Jiu J, Sakamoto M, Wang F. Journal of the American Chemical Society 2004;126:14943-14949.
[11] O'Regan B, Lenzmann F, Muis R, Wienke J. Chemistry of Materials 2002;14 :5023-5029
[12] Eu S, Hayashi S, Umeyama T, Matano Y, Araki Y, Imahori H. Journal of Physical Chemistry C 2008;112:4396-4405.
[13] Iijima S. Nature 1991;354:56-58.
[14] Ertekin E, Chrzan DC. phys. Rev. B 2005;72:045425-1-045425-5.
[15] Mintmire JW, White CT. Carbon 1995;36:893-902.
[16] Purcell ST, Vincent P, Journet C, Binh VT. Physical Review Letters 2002;88:1055021-1055024.
[17] Gutiérrez-Tauste D, Zumeta I, Vigil E, Hernández-Fenollosa MA, Domènech X.
Ayllón JA. Journal of Photochemistry and Photobiology A: Chemistry 2005;175:165-171.
[18] Stathatos E, Chen Y, Dionysiou DD. Solar Energy Materials and Solar Cells 2008;92:1358-1365.
[19] Yen CY, Lin YF, Liao SH, Weng CC, Huang CC, Hsiao YH, Ma MCC, Chang MC, Shao H, Tsai MC, Hsieh CK, Tsai CH, and Weng B. Nanotechnology 2008;19:375305.
[20] Kado T, Kubota Y, Hayase S. Chemistry Letters 2005;34:1006-1007.
[21] Tan W, Chen J, Zhou X, Zhang J, Lin Y, Li X, Xiao X. Journal of Solid State Electrochemistry 2009;13:651-656.
[22] Holloway AF, Craven DA, Xiao L, Campo JD, Wildgoose GG. Journal of Physical Chemistry C 2008;112:13729-13738.
[23] Guzmán De Villoria R, Figueredo SL, Hart AJ, Steiner Iii SA, Slocum AH, Wardle BL. Nanotechnology 2009;20:405611.
[24] Zhang WD, Thong JTL, Tjiu WC, Gan LM. Diamond and Related Materials 2002;11:1638-1642.
Fig. 1. The procedure of VACNT bundles growth.
Fig. 2. The schematic illustration of the DSSC structure.
Cool down Cool down
750
RT T (
oC)
t (min)
750
RT T (
oC)
t (min) Ramp up
20
Ramp up20
Ramp up Ramp up20
Annealing
80
Annealing80
Annealing80
C2H2(2 Torr) CNTs growth
20 sec C2H2(2 Torr) C2H2(2 Torr)
CNTs growth
20 sec
Fig. 3. The SEM images of the (a) pristine VACNT bundles, and the VACNT bundles
with coated-TiO2 nanoparticles with different TTIP concentration of (b) 0.018, (c) 0.036, (d) 0.054, (e) 0.072, and (f) 0.090 M.
Fig. 4. X-ray diffraction pattern of TiO2 nanoparticles grow on VACNT bundles.
Fig. 5. (a) Current density-voltage characteristics of DSSC based on VACNT bundles with coated-TiO2 and different TTIP concentrations. (b) TTIP concentrations dependence on the η for the DSSC based on J-V measurement.
出席國際學術會議心得報告
計畫編號 NSC 97-2221-E-011 -017
計畫名稱 利用二氧化鈦塗佈於奈米碳管之染料敏化太陽能電池研究
出國人員姓名 服務機關及職稱
李奎毅
國立臺灣科技大學電子工程系 助理教授
會議時間地點 2009 年 7 月 19-24 日 日本神戶
會議名稱 The 14th International Conference on Modulated Semiconductor Structures 發表論文題目 Characterization of supercapacitor of variable content of nitrogen doping in
carbon nanotubes with ruthenium 一、參加會議經過
第 十 四 屆 模 組 半 導 體 結 構 國 際 研 討 會 (The 14th International Conference on Modulated Semiconductor Structures, MSS-14)於 2009 年 7 月 19 日至 24 日於日本神戶 國際會議中心舉行。會議主辦單位為國際純粹及應用聯合物理學會(International Union of Pure and Applied Physics)。本國際研討會的主要宗旨在於發表最新的半導體結構,
包括特性、製程及分析。本次國際會議並與第十八屆二維系統之電子特性國際研討會 (The 18th International Conference on Electronics Properties of Two-Dimensional System, EP2DS-18)合辦,因此增加了研究範圍的發表。近年來由於在半導體、二維系統及奈 米領域相關的科學及工程技術之新發現是相當受到注目。因此,參加會議之研究人員 及團體的主要專長範圍非常的廣。本屆大會除了日本本國之研究學者及團體外,臺灣、
美國、英國、德國、韓國等共約發表二百七十篇論文(oral and poster presentations),以 九個不同主題分別進行研討。
這次發表的論文為利用奈米碳管在大型電容上之研究以 poster 發表,主題雖然與 大型電容有關,但由於奈米碳管與金屬基板結合之應用非常廣,再加上奈米碳管與二 氧化釕結合之特殊型態與結構,論文發表時與參加會議之各國相關研究人員及團隊進 行熱烈之交流與討論,因此獲得不少意見,收穫良多,相信對於將來的相關研究有實 質的幫助。在會議進行期間,對於其他研究人員及團隊之論文發表,亦對其研究領域 進行討論,並收集奈米材料特性、製程與應用之相關研究情形,做為未來進行相關研
究之參考。
二、與會心得
本次參加國際研討會,由於是兩個會議的合作進行,因此有機會與不同領域之研 究人員進行討論,不同之專業背景所引伸出之討論內容增加了許多不同的想法,其相 異之思考模式型態亦展現在處理相關問題上。此次會議論文除了基本理論的探討,對 於相關的工業應用助益良多。在此次的會議中,除了相關之研究領域外,在 poster 發 表時段並利用機會觀摩其他領域,此部分之學習收穫非常大,如何將一些不同領域結 合在一起,利用此次之會議詢問相關之知識與技術需要,並進一步瞭解進入此一領域 所需要之相關設備。對未相關研究啟發出基本想法。
在本次會議中,遇到許多日本的學生發表,其研究內容大多為一流水準,不論其 英文是否流利,皆盡力表達其研究內容,並積極地與參加會議之學者專家討論,許多 學生在相關的準備上不論是口頭或紙面上也非常踏實。在國科會的積極鼓勵下,臺灣 除了博士班學生外,碩士班學生也被鼓勵積極參加國內或國外之國際研討會。相信學 生們看到其他國家同樣碩士生或博士生的發表後,對其本身之研究態度及實驗之規 劃、進行、分析結果到將成果呈現出,必可獲益良多。台灣的碩博士生應該接受這樣 的訓練及洗禮對研究生才是較完整的的訓練。
感謝國家科學委員會的補助,讓本人能夠參加這次的國際會議並發表論文,在與 許多專家學者的討論上,相信所得到的收穫,對未來之研究有實質的幫助。
Characterization of supercapacitor of variable content of nitrogen doping in carbon nanotubes with ruthenium dioxide
K.-Y. Lee*,**, Y.-S. Lin**, Y.-M. Chen**, Y.-S. Huang*,**
* Graduate Institute of Electro-Optical Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan
** Department of Electronic Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan
Keywords: Supercapacitor, Carbon nanotubes, Nitrogen content, Ruthenium dioxide
The supercapacitor, also known as an ultra-capacitor or electrochemical capacitor, has attracted considerable attentions due to its high power density, high energy density, and long cycle life [1]. Electric double layer capacitor (EDLC) and pseudo-capacitor are two forms of supercapacitor to store electrical energy. Among the structure of EDLC, the electrode plays an important role on the performance of EDLC.
Multi-walled vertically aligned carbon nanotubes (VACNTs) are extremely suitable for use as EDLC electrodes because of their good electrical conductivity, large effective surface area, and highly regular structure.
To enhance the performance of EDLCs, some functional atoms [2] and oxides [3] such as nitrogen and ruthenium dioxide (RuO2) are attached on the surface of
the electrodes to improve the charge-exchange behaviour of VACNTs. In this study, we deposited RuO2 nanorods on nitrogen-doped VACNTs to investigate the characteristics of the supercapacitors.
The nitrogen-doped VACNTs were grown, using the thermal chemical vapour deposition (CVD) system, on stainless sheets. C2H2 and NH3 was used the carbon and nitrogen sources during the VACNTs growth, respectively. The RuO2 nanorods were synthesized on the nitrogen-doped VACNTs by metal organic CVD.
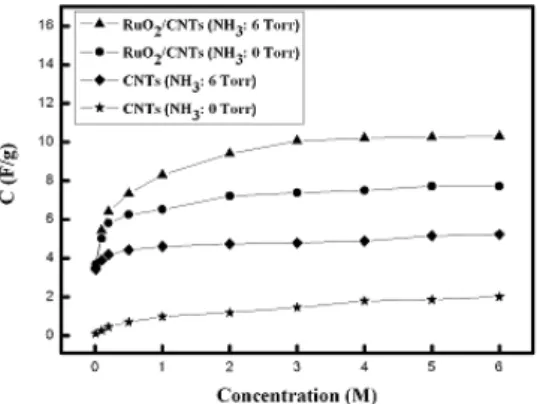
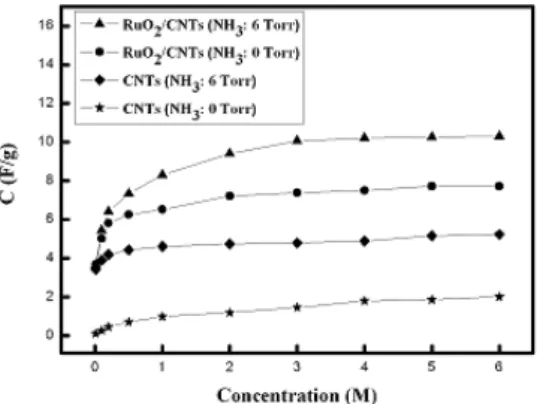
Figure 1 shows the capacitance curves of pristine VACNTs, nitrogen-doped VACNTs, RuO2/VACNTs, and RuO2/nitrogen-doped VACNTs electrodes in different concentrations of KOH electrolyte. It reveals that the addition of RuO2 nanorods to nitrogen-doped VAVNTs surface could efficiently increase the capacitance due to the pseudo-capacitor performance and increased surface area from the growth of the RuO2
nanorod structure.
From the results, the nitrogen-doping and RuO2
nanorods attachment on VACNTs could effectively enhance the characteristics of EDLC. More details of the research will be presented at the conference.
References
1. B. E. Conway J. Electrochem. Soc. 138, 1539 (1991).
2. F. Kapteijn, A. J. Moulijn, S. Matzner and H. P.
Boehm, Carbon 37, 1143 (1999).
3. W.-C. Fang, K.-H. Chen and L.-C. Chen, Nanotechnology 18, 485716 (2007).
Fig.1 Comparison of the capacitances of pristine VACNTs, nitrogen-doped VACNTs, RuO2/VACNTs, and RuO2/nitrogen-doped VACNTs electrodes in different concentrations of KOH electrolyte.
出席國際學術會議心得報告
計畫編號 NSC 97-2221-E-011 -017
計畫名稱 利用二氧化鈦塗佈於奈米碳管之染料敏化太陽能電池研究
出國人員姓名 服務機關及職稱
李奎毅
國立臺灣科技大學電子工程系 助理教授
會議時間地點 2009 年 7 月 19-24 日 日本神戶
會議名稱 The 14th International Conference on Modulated Semiconductor Structures 發表論文題目 Characterization of supercapacitor of variable content of nitrogen doping in
carbon nanotubes with ruthenium 一、參加會議經過
第 十 四 屆 模 組 半 導 體 結 構 國 際 研 討 會 (The 14th International Conference on Modulated Semiconductor Structures, MSS-14)於 2009 年 7 月 19 日至 24 日於日本神戶 國際會議中心舉行。會議主辦單位為國際純粹及應用聯合物理學會(International Union of Pure and Applied Physics)。本國際研討會的主要宗旨在於發表最新的半導體結構,
包括特性、製程及分析。本次國際會議並與第十八屆二維系統之電子特性國際研討會 (The 18th International Conference on Electronics Properties of Two-Dimensional System, EP2DS-18)合辦,因此增加了研究範圍的發表。近年來由於在半導體、二維系統及奈 米領域相關的科學及工程技術之新發現是相當受到注目。因此,參加會議之研究人員 及團體的主要專長範圍非常的廣。本屆大會除了日本本國之研究學者及團體外,臺灣、
美國、英國、德國、韓國等共約發表二百七十篇論文(oral and poster presentations),以 九個不同主題分別進行研討。
這次發表的論文為利用奈米碳管在大型電容上之研究以 poster 發表,主題雖然與 大型電容有關,但由於奈米碳管與金屬基板結合之應用非常廣,再加上奈米碳管與二 氧化釕結合之特殊型態與結構,論文發表時與參加會議之各國相關研究人員及團隊進 行熱烈之交流與討論,因此獲得不少意見,收穫良多,相信對於將來的相關研究有實 質的幫助。在會議進行期間,對於其他研究人員及團隊之論文發表,亦對其研究領域 進行討論,並收集奈米材料特性、製程與應用之相關研究情形,做為未來進行相關研
究之參考。
二、與會心得
本次參加國際研討會,由於是兩個會議的合作進行,因此有機會與不同領域之研 究人員進行討論,不同之專業背景所引伸出之討論內容增加了許多不同的想法,其相 異之思考模式型態亦展現在處理相關問題上。此次會議論文除了基本理論的探討,對 於相關的工業應用助益良多。在此次的會議中,除了相關之研究領域外,在 poster 發 表時段並利用機會觀摩其他領域,此部分之學習收穫非常大,如何將一些不同領域結 合在一起,利用此次之會議詢問相關之知識與技術需要,並進一步瞭解進入此一領域 所需要之相關設備。對未相關研究啟發出基本想法。
在本次會議中,遇到許多日本的學生發表,其研究內容大多為一流水準,不論其 英文是否流利,皆盡力表達其研究內容,並積極地與參加會議之學者專家討論,許多 學生在相關的準備上不論是口頭或紙面上也非常踏實。在國科會的積極鼓勵下,臺灣 除了博士班學生外,碩士班學生也被鼓勵積極參加國內或國外之國際研討會。相信學 生們看到其他國家同樣碩士生或博士生的發表後,對其本身之研究態度及實驗之規 劃、進行、分析結果到將成果呈現出,必可獲益良多。台灣的碩博士生應該接受這樣 的訓練及洗禮對研究生才是較完整的訓練。
感謝國家科學委員會的補助,讓本人能夠參加這次的國際會議並發表論文,在與 許多專家學者的討論上,相信所得到的收穫,對未來之研究有實質的幫助。
Characterization of supercapacitor of variable content of nitrogen doping in carbon nanotubes with ruthenium dioxide
K.-Y. Lee*,**, Y.-S. Lin**, Y.-M. Chen**, Y.-S. Huang*,**
* Graduate Institute of Electro-Optical Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan
** Department of Electronic Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan
Keywords: Supercapacitor, Carbon nanotubes, Nitrogen content, Ruthenium dioxide
The supercapacitor, also known as an ultra-capacitor or electrochemical capacitor, has attracted considerable attentions due to its high power density, high energy density, and long cycle life [1]. Electric double layer capacitor (EDLC) and pseudo-capacitor are two forms of supercapacitor to store electrical energy. Among the structure of EDLC, the electrode plays an important role on the performance of EDLC.
Multi-walled vertically aligned carbon nanotubes (VACNTs) are extremely suitable for use as EDLC electrodes because of their good electrical conductivity, large effective surface area, and highly regular structure.
To enhance the performance of EDLCs, some functional atoms [2] and oxides [3] such as nitrogen and ruthenium dioxide (RuO2) are attached on the surface of
the electrodes to improve the charge-exchange behaviour of VACNTs. In this study, we deposited RuO2 nanorods on nitrogen-doped VACNTs to investigate the characteristics of the supercapacitors.
The nitrogen-doped VACNTs were grown, using the thermal chemical vapour deposition (CVD) system, on stainless sheets. C2H2 and NH3 was used the carbon and nitrogen sources during the VACNTs growth, respectively. The RuO2 nanorods were synthesized on the nitrogen-doped VACNTs by metal organic CVD.
Figure 1 shows the capacitance curves of pristine VACNTs, nitrogen-doped VACNTs, RuO2/VACNTs, and RuO2/nitrogen-doped VACNTs electrodes in different concentrations of KOH electrolyte. It reveals that the addition of RuO2 nanorods to nitrogen-doped VAVNTs surface could efficiently increase the capacitance due to the pseudo-capacitor performance and increased surface area from the growth of the RuO2
nanorod structure.
From the results, the nitrogen-doping and RuO2
nanorods attachment on VACNTs could effectively enhance the characteristics of EDLC. More details of the research will be presented at the conference.
References
1. B. E. Conway J. Electrochem. Soc. 138, 1539 (1991).
2. F. Kapteijn, A. J. Moulijn, S. Matzner and H. P.
Boehm, Carbon 37, 1143 (1999).
3. W.-C. Fang, K.-H. Chen and L.-C. Chen, Nanotechnology 18, 485716 (2007).
Fig.1 Comparison of the capacitances of pristine VACNTs, nitrogen-doped VACNTs, RuO2/VACNTs, and RuO2/nitrogen-doped VACNTs electrodes in different concentrations of KOH electrolyte.