行政院國家科學委員會專題研究計畫 成果報告
創新三維離子奈米通道固體電解質界面膜用於動力鋰離子 電池與其電化學反應動力學機制之研究
研究成果報告(精簡版)
計 畫 類 別 : 個別型
計 畫 編 號 : NSC 99-2218-E-011-008-
執 行 期 間 : 99 年 06 月 01 日至 100 年 05 月 31 日 執 行 單 位 : 國立臺灣科技大學工程技術研究所
計 畫 主 持 人 : 王復民
計畫參與人員: 博士班研究生-兼任助理人員:鄭賀名
報 告 附 件 : 出席國際會議研究心得報告及發表論文
處 理 方 式 : 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢
中 華 民 國 100 年 06 月 16 日
Study on Novel Solid-electrolyte-interface (SEI) Layers with Three-dimensional Ionic Nano-tunnels for Power Lithium Ion Batteries and Its Kinetic Mechanism
during Electrochemical Reaction (1/3)
Fu-Ming Wang (王復民)
Graduate Institute of Engineering, National Taiwan University of Science and Technology, Taipei 106, Taiwan, R.O.C.
(NSC 99-2218-E-011-008-)
Abstract
In this study, we chose maleimide-based additives which are isomer for each other (the maleimide substituent on the benzene are para-, meta-, and ortho- position) to investigate the structural effect in solid electrolyte interface (SEI) and the battery performance. First, the cyclic voltammetry (CV) measurement showed that the reduction potential of maleimide-based additive was prior to other components. Then, the structure of maleimide product was further found retaining the same connected sites to benzene and keeping C=C configuration after reduction based on the results of AC impedance. The possible product structure for different maleimide-based additive was proposed in this study. In the analysis of impedance spectra, the resistance of SEI was influenced by steric hindrance of the additive. The X-ray photoelectron spectroscopy (XPS) demonstrated the content of a specific inorganic compound, LiF, was decreased due to the existence of maleimide-based additive in the electrolyte.
Finally, in the battery performance test, the structural effect of additive showed remarkable divergence in discharge capacity loss in the initial 1C/1C cycle test. As the cycle proceeded, the discharge capacity of batteries with maleimide additives reached a steady state and did not reduce anymore. Besides, the performance of the additive with large steric hindrance even excelled the one without any additive.
Keywords: Lithium ion battery, solid electrolyte interface, maleimide, additive,
performanceObjective
Many vital factors contribute to properties of the SEI. There is no single parameter controlling the SEI. It is the combination and concomitant effect of all these factors which dictates the properties, quality, and efficiency of SEI. Hence, the resulting influence of many factors listed in this section is collective and interdependent rather than independent. Since SEI is an interface layer between the active material and the
electrolyte, it is certain that properties of both these phases determine the composition of SEI. SEI is essentially formed on the surface of the carbonaceous negative active material, thus the type of carbon significantly affects the SEI. Winter et al. showed that irreversible capacity loss in the first cycle attributed to SEI formation is linearly proportional to the BET specific surface area of the carbon [1]. Zheng et al. found that the crystallographic structure and particle morphology are as influential as BET specific surface area because coke and graphite powders having same BET specific surface area exhibited different ICL [2]. In addition, edges and surface imperfections like defects, crevices, and active sites act as catalytic sites for solvent reduction.
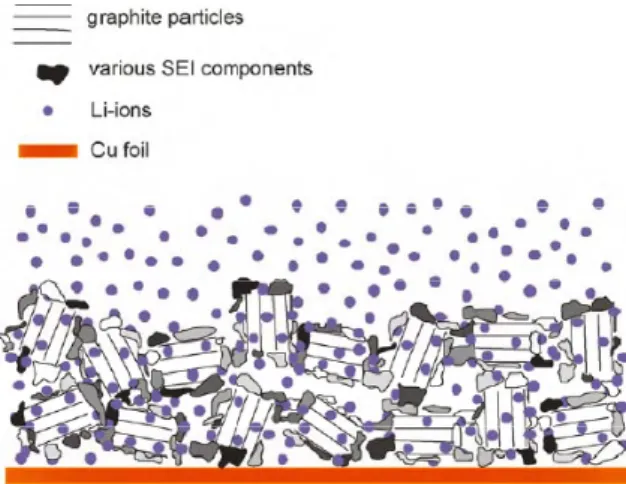
Hence, these regions play an important role in solvent reduction kinetics. Dangling bonds and high current density on these sites prefer electrolyte reduction. Thus concentration and nature of defects, and edge to basal plane ratio [3] are also critical factors affecting SEI properties. In general, the SEI on the cross section of the electrode and the edges of the graphite particles contain more inorganic components like LiF [46] while the SEI on the graphene sheets contain soft organic compounds as shown in a schematic sketch of SEI in Fig. 1. [4]
Even though the surface is very important for SEI formation, the importance of the crystallographic structure of the carbon should not be underestimated. Highly ordered carbons are more vulnerable to exfoliation and are thus more sensitive to electrolyte composition. Therefore, crystallographic order significantly affects the extent of exfoliation and co-intercalation of solvents [5-6].
Fig. 1 Sketch of a lithiated graphite composite electrode covered by inhomogeneous
SEI. The SEI components shown in darker shades of grey are mainly inorganic while those shown in lighter shades of grey are organic. [4]Once the importance of the surface properties of the material was understood, many efforts were made to modify the surface morphology and chemical properties of the carbons by various methods. Scott et al. [7] performed reduction of their electrodes in
butyl-lithium solutions for varying durations of time to decrease the ICL. The SEI formed was thicker but more brittle. Ein-Eli and Koch [8] oxidized the graphite powders with HNO3 and (NH4)2S2O8 and enhanced the specific charge capacity of graphite. This was attributed to the production of cavities or nanovoids during oxidation (etching). Instead of altering the surface groups already present on the carbon surface, Pan et al. immobilized aryl functional groups onto carbon to facilitate the SEI formation on these groups, but the improvement was limited in terms of suppression of the ICL [9-10]. As for electrochemical treatments like electroless plating of graphite by Cu were also tried and the discharge capacity and columbic efficiency were found to improve [11]. Another kind of the surface treatments of carbons is the thermal treatments. The main target of thermal treatments is to obtain high specific charge capacity carbons, which can accommodate more than one Li+ per C6. It was mainly completed by pyrolysis of different organic precursors at various temperatures. Work done in this field has been reviewed by Zheng et al. [12]. There are also treatments combining two or more methods. For example, Ohzuku et al. [13]
improved the performance of graphite fiber anode by heat treating acetylene black and graphite at 700 ◦C to remove hydroxyl groups and water from the surface.
According to the above literature review, the choice of additive to improve the SEI in the Li-ion batteries usually requires some specific inherent properties of the chemicals, such as conjunction structure, functional group with strong RA-delocalizing ability, etc. All of the investigations focus on the nature of the chemical element or compound.
However, few additives were studied based on their substituent position. Therefore, in this study, the structural isomers of maleimide-based additive were studied and the effect of the same functional group with different linking position in the SEI and battery performance was investigated. Our lab has previously investigated maleimide-based compounds as novel additives for electrolytes in lithium-ion batteries [14]. The study first found that maleimide-based additives were reduction-type additive based on the CV measurement. Secondly, according to XPS results, maleimide-based additives formed a novel SEI formation preventing the formation of LiF and Li2O compounds during the charge/discharge process. Thirdly, the battery with maleimide-based additives increased 4.9% capacity and 16.7%
capacity retention when the battery was cycled at 1C/1C [14]. According to Wang et al., additive with maleimide group is ideal for SEI formation in lithium-ion batteries.
Hence, the additives used in this research all possess maleimide substituent but on varied positions—para, meta and ortho sites, as shown in Table 2 and labeled as Mi-2, -3 and -4. How the positions of function groups affect the SEI formation and the capability of lithium-ion batteries are major issues of this study, which may provide important guidance for future additive selection.
Experiments
The electrode materials were obtained from the disassembled commercial batteries (EXA Co.) The cathode consisted of 91wt% of LiCoO2, 3wt% KS6 and 6wt% of PVDF as binder with an Al current collector. The anode consisted of 93wt%
MCMB-2528, 3wt% KS4 and 4wt% PVDF as binder with a Cu current collector. The separator (Celgard 2320) was PP/PE/PP tri-layer with a thickness of 20μm (PP:
polypropylene; PE: poly ethylene). The basic electrolyte, designated as the blank in this study, was 1.1M lithium hexafluorophoshate (LiPF6) in Propylene carbonate (PC, Novolyte tech. Ltd., 99.98%)/ ethylene carbonate (EC, Novolyte tech. Ltd., 99.98%)/
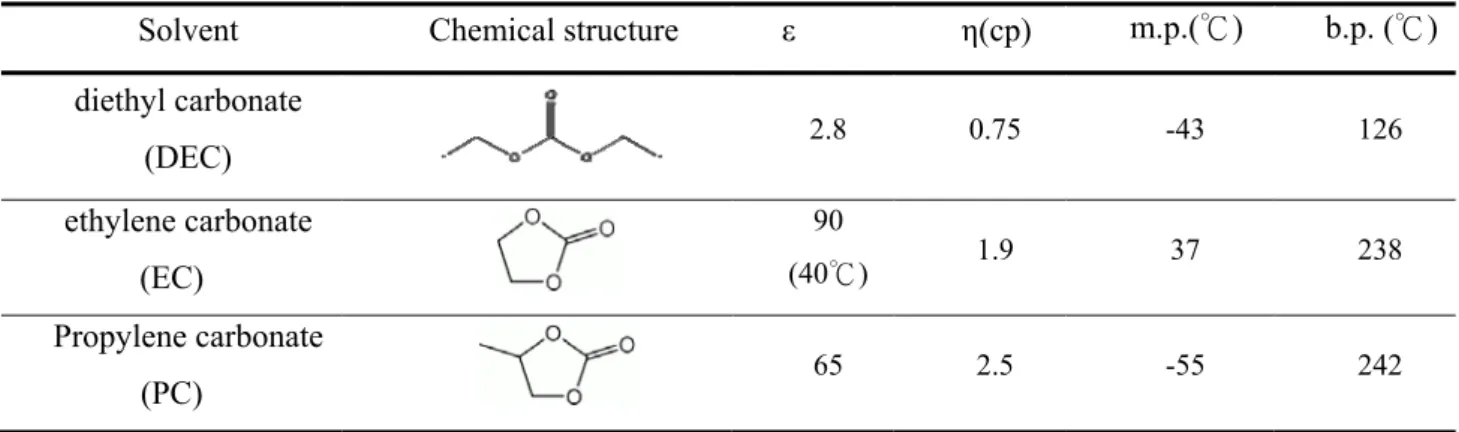
diethyl carbonate (DEC ,Novolyte tech. Ltd., 99.98%) in the ratio of 2:3:5 by volume, with 2 wt.% vinylene carbonate (VC Novolyte tech. Ltd., 99.98%), which was the commercial electrolyte composition adopted from EXA Co. in Taiwan.[15] The preparation of electrolyte was conducted in the glove box. The physical properties of these 3 solvent are shown in Table 1. Although the dielectric constant of DEC is fairly low, the relatively low viscosity can decrease the viscosity of the PC-EC mixed solvent and DEC can promote the wettability of the separator.
Table 1 Physical properties of solvents at 25℃.
Solvent Chemical structure ε η(cp) m.p.(℃) b.p. (℃) diethyl carbonate
(DEC) 2.8 0.75 -43 126
ethylene carbonate (EC)
90
(40℃) 1.9 37 238
Propylene carbonate
(PC) 65 2.5 -55 242
The content of maleimide-based additive (abbreviated to Mi-based additive) in the blank electrolyte all is 0.1 wt.% in this study. Table 2 presents the chemical structure of Mi-based additives (all from Aldrich Co., >98%). The water content of the electrolytes determined by Karl Fisher titration was less than 35 ppm.
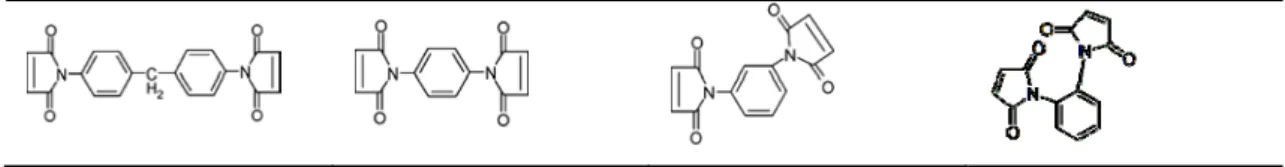
Table 2 The chemical structure of maleimide-based additives.
1,1’-(methylenedi-4,1-p henylene) bismaleimide (MI-1)
N,N’-1,4-phenylenedi maleimide
(MI-2)
N,N’-1,3-phenylenedi maleimide
(MI-3)
N,N’-1,2-phenylenedi maleimide
(MI-4)
Cyclic voltammetry analysis
Fig. 2 shows cyclic voltammetry result of Li/C 3-electrode half cell with different
electrolytes at 0.8-3.0V vs. Li+/Li, and the trends of reduction peaks for all cells are similar: one peak at ca. 1.85V vs. Li+/Li, another peak at 1.35V vs. Li+/Li, the other around 1.2V vs. Li+/Li. However, cells with Mi-based additives all had an extra reduction peak at 2.3V vs. Li+/Li, which did not appear in the cell without maleimide-based additive. This could be attributed to the reaction of Mi-based additives. Therefore, all of the additives used in this study can be verified to be reduction-type additive.
Fig. 2 (a) the blank (b) Mi-2 0.1wt.% in the blank electrolyte (c) Mi-3 0.1wt.% in the
blank electrolyte (d) Mi-4 0.1wt.% in the blank electrolyte. The arrows point at the possible reaction peaks.The most likely explanation for peaks occurring in all samples (with/without additives) comes from the reaction of the solvents (PC, EC and DEC) and VC. It is reported that PC and EC/DEC mixed solvent usually react at 0.5-1V vs. Li+/Li with two peaks [16-17], and VC reaction occurs around 1.3V [18]. In this CV results, the reduction peak appearing at 1.85V, 1.35V and 1.2V (all versus Li+/Li) could be attributed to reaction of VC and PC/EC/DEC respectively, summarized in Table 3, though the
actual potentials were slightly higher than the above reported ones. The shift of the peak was probably caused by variations in activation polarization because the type of carbon and the system of electrolyte used in this study were different from the literature described above. Although those Mi-based additives are constitutional isomers, and consequently the difference in steric hindrance should cast an impact on reactivity, affecting the reaction voltage in the CV measurement, yet it turned out that there was no variation in the reduction voltage for all samples with different Mi-based additives, this implying that the reduction part of the additives occurred at the outermost structure, such as the oxygen of the C=O or the carbon double bond, both of which are in the nitrogen-containing heterocyclic ring, so steric hindrance had no influence in reactivity for those additives. Hence, the product of those Mi-based additives would retain the original configuration after reduction.
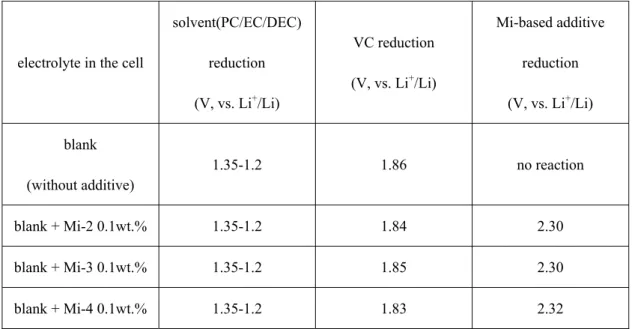
Table 3 The reduction potential and the corresponded reaction for all samples.
electrolyte in the cell
solvent(PC/EC/DEC) reduction (V, vs. Li
+/Li)
VC reduction (V, vs. Li
+/Li)
Mi-based additive reduction (V, vs. Li
+/Li) blank
(without additive)
1.35-1.2 1.86 no reaction
blank + Mi-2 0.1wt.% 1.35-1.2 1.84 2.30
blank + Mi-3 0.1wt.% 1.35-1.2 1.85 2.30
blank + Mi-4 0.1wt.% 1.35-1.2 1.83 2.32
AC Impedance analysis
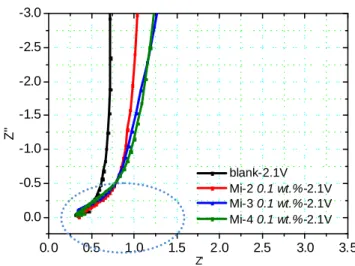
Electrochemical impedance spectra of 3-electrode full cell (carbon/LiCoO2 with Li metal as the reference electrode) were taken at specific voltages during the first intercalation of Li+ into carbon at 0.2C current rate. The specific voltages for impedance spectra measurement were decided from the CV results (Fig. 3): the reduction peaks occurring at 2.3 and 1.85V (both versus Li+/Li) represent the reaction of additives and VC, so 2.1 and 1.75V vs. Li+/Li were regarded as the completion of additives and VC reaction. As for solvents, their reaction started at 1.35V vs. Li+/Li and kept reacting as the voltage decreased; hence, 1.25 and 0.8V vs. Li+/Li were chosen to measure EIS for three reasons: (1) part of solvents had already decomposed to form the SEI film; (2) due to the fact that the amount of product was different at
1.25 and 0.8V vs. Li+/Li, the transition of SEI formation can be observed by EIS; (3) the Li+ did not intercalate into carbon at that voltage so Li+ would not intervene the impedance results. Therefore, the specific voltages were 2.1, 1.75, 1.25, and 0.8V (all vs. Li+/Li). Fig. 4 presents the impedance results at 2.1V vs. Li+/Li, at which the additive reaction completed. The semicircle of additives at high frequencies was larger than that of the blank, indicating that the surface of carbon had been modified by Mi-based additives so the resistance increased. In addition, according to J. Y. Song et al., the straight line at medium frequencies corresponds to the capacitive reactance instead of the diffusion effect because there are no intercalated lithium atoms in the carbon matrix. Therefore, the carbon matrix serves as the blocking electrode for lithium atoms. [19] Fig. 5 shows the impedance spectra for every individual sample at 2.1, 1.75 as well as 1.25V (vs. Li+/Li), and we focused on the part of straight sloping line at medium frequencies. For the blank, the gradient of the line was almost vertical at 2.1 and 1.75V vs. Li+/Li, as shown in Fig. 5(a). However, the gradient of the line for Mi-based additives became smaller at 2.1 and 1.75V vs. Li+/Li in Fig. 5(b) ~ (d).
The difference between the blank and Mi-based additives was attributed to products on the carbon surface – for the blank, there was only VC reaction during this period between 2.1 and 1.75V vs. Li+/Li, but for those samples with extra additives, there were Mi-based additive and VC reaction in the same voltage interval.
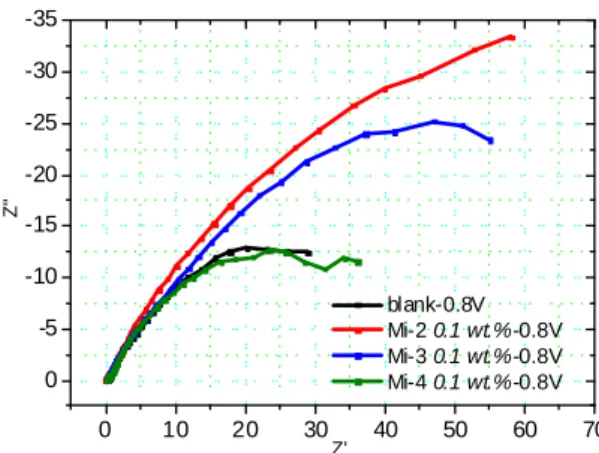
As the voltage decreased, the amount of product on the carbon surface increased, causing the gradient of the straight line to become smaller. The phenomenon means an interface gradually appeared and the interphase was exactly the SEI film generated by electrolyte on the carbon surface. At 0.8V vs. Li+/Li (Fig. 6), the solvent in the electrolyte had reacted and the original straight line at moderate frequency region became another semicircle due to the formation of the SEI. This semicircle included the impedance of solvent and Mi-based additives; assuming that the impedance of solvent kept constant with different additive, the difference among the semicircle can be attributed to divergence of additive. It is obvious that the cell with Mi-2 additive had the highest resistance, and for the cell with Mi-4 additive or without any additive, the resistance was the lowest. In addition, above impedance spectra proved that the Mi-based did participate in the formation of the SEI film.
Fig. 4 Impedance spectra of carbon as working electrode in 3-electrode system during
the first Li+ intercalation process. V=2.1V vs. Li+/Li for every sample.
Fig. 5 Impedance spectra of carbon as working electrode in 3-electrode system during
the first Li+ intercalation process at 2.1, 1.75 and 1.25V vs. Li+/Li for (a) the blank, (b) Mi-2 0.1 wt.% in the blank, (c) Mi-3 0.1 wt.% in the blank, (d) Mi-4 0.1 wt.% in the blank.0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 0.0
-0.5 -1.0 -1.5 -2.0 -2.5 -3.0
Z"
Z'
blank-2.1V Mi-2 0.1 wt.%-2.1V Mi-3 0.1 wt.%-2.1V Mi-4 0.1 wt.%-2.1V
0 2 4 6 8 10
0 -5 -10 -15 -20 -25
0 2 4 6 8 10
0 -5 -10 -15 -20 -25
0 2 4 6 8 10
0 -5 -10 -15 -20 -25
0 2 4 6 8 10 12 14 16 18 20
0 -10 -20 -30 -40 -50 blank-2.1V
blank-1.75V blank-1.25V
Z"
Z'
LA (blank)
Z"
Z'
Mi-2 0.1wt.%-2.1V Mi-2 0.1wt.%-1.75V Mi-2 0.1wt.%-1.25V
Mi-2
Mi-3 0.1wt.%-2.1V Mi-3 0.1wt.%-1.75V Mi-3 0.1wt.%-1.25V
Z"
Z'
Mi-3
Mi-4 0.1wt.%-2.1V Mi-4 0.1wt.%-1.75V Mi-4 0.1wt.%-1.25V
Z"
Z'
Mi-4
Fig. 6 Impedance spectra of carbon as working electrode in 3-electrode system during
the first Li+ intercalation process. V=0.8V vs. Li+/Li for every sample.
Fig. 7 is the impedance spectrum of carbon as working electrode in 3-electrode
C/LiCoO2 system with/without Mi-based additives after the formation cycle, when most of the SEI film is formed. The carbon electrode was measured in the delithiation state after the first cycle so the semicircle of charge transfer does not occur in the impedance as described previously. The result shows that the extent of semicircle related to SEI varied with different additives. By fitting the equivalent circuit proposed by Zhang, S.S et al. [20] the value RSEI can be obtained: 0.456Ω for cell without any additive, 1.238, 1.333, 0.503Ω for cell with Mi-2, Mi-3 and Mi-4, respectively. The order of RSEI is Mi-2~Mi-3>Mi-4>blank.
Fig. 7 Impedance spectra of carbon as working electrode in 3-electrode system
with/without Mi-based additives, and carbon was measured in the delithiation state after the first cycle.According to the literature [21-22], because a “dried” SEI itself is neither ionic conductive nor electronic conductive, the ionic conduction in the SEI results from the
0 10 20 30 40 50 60 70
0 -5 -10 -15 -20 -25 -30 -35
Z"
Z'
blank-0.8V Mi-2 0.1 wt.%-0.8V Mi-3 0.1 wt.%-0.8V Mi-4 0.1 wt.%-0.8V
0 2 4 6 8 10
1.0 0.5 0.0 -0.5 -1.0 -1.5 -2.0 -2.5 -3.0
Z" (ohm)
Z' (ohm)
blank Mi-2 0.1 wt.%
Mi-3 0.1 wt.%
Mi-4 0.1 wt.%
migration of solvated Li+ through the micro-pores of SEI. Hence, the ionic conductivity of SEI can be measured by impedance to evaluate compactness and stability of the SEI film. Generally, high resistance corresponds to a compact and stable SEI. In this study, the RSEI of Mi-4 additive was lowest and the one of Mi-2 was the highest, so Mi-2 could generate the most compact SEI film.
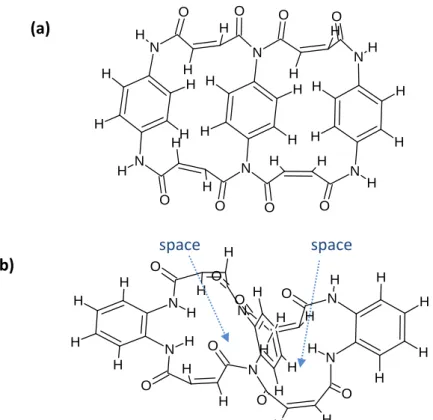
In the previous CV and AC analysis, it is concluded that the product of those Mi-based compounds would keep the original configuration after reduction, causing the product to have different degree of bulkiness and twist. The product generated from Mi-2 would be less bulky and less bending because of little steric hindrance.
Besides, the structure of Mi-2 product would be more planar compared to those of Mi-3 and -4 products, as shown in Fig. 8(a), so the stacking of the Mi-2 product is more compact, causing difficulty for Li+ passage, which in turn resulted in the highest resistance in impedance spectra. On the contrary, owing to the largest steric hindrance, the product derived from Mi-4 tends to be bulky and winding (Fig. 8(b)), so there could be lots of space within molecular structure or the stacking structure between the Mi-4 products, leading Li+ to pass through the space readily and resulting in a lower resistance in impedance spectrum. As for the structure of Mi-3 product, its result settling between those of Mi-2 and Mi-4 products is reasonable.
O
N O
N
O O
N
O N
O
N O O
N H
H
H H
H H H
H H
H
H H
H
H
H
H H
H H
H H
H
H H
O N O N O
O N
O N
O
N
O O N
H H
H H
H H H
H H
H H
H H
H H
H
H H H
H
H H H H
Fig. 8 Part of possible structure of (a) Mi-2 product, and (b) Mi-4 product. These
molecules do not show exactly real structure but they still can make a comparison with each other.(a)
(b)
space space
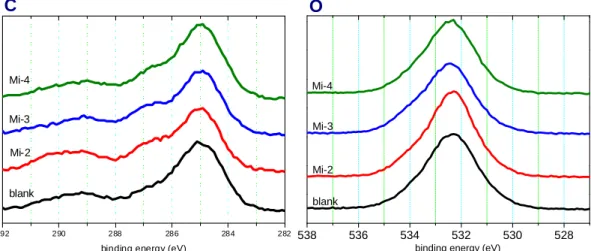
XPS analysis
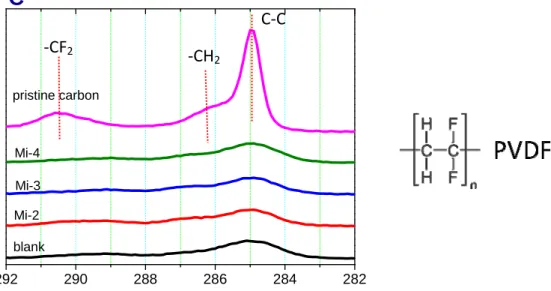
After the analysis of the difference in the constitutional structures, the influence of the N-containing maleimide substitution in the chemical composition of the SEI film is analyzed by XPS in the following section. Compared with pristine carbon which means the electrode does not undergo any electrochemical treatment, it is noted that the intensity of characteristic peak at 285eV related to graphite and the peak at 286.3 and 290.5 corresponding to –CH2 and -CF2 respectively from PVDF binder obviously decrease after the formation of the SEI film, as shown in Fig. 9, indicating that the SEI film is certainly generated and covers on the carbon surface. [4, 23]
Fig. 9 C1s XPS spectra on the de-lithiated carbon e lectrode with different electrolyte:
the blank, Mi-2 0.1 wt.% in the blank, Mi-3 0.1 wt.% in the blank, Mi-4 0.1 wt.% in the blank, and pristine carbon.
Fig. 10 shows C 1s and O 1s XPS spectra for the SEI film formed on the carbon
electrode after the formation process. The peaks at 283-287 eV in C 1s spectrum contains many organic compounds frequently appearing in the SEI film, including CH2CH2O at 285.8 eV, PEO (C-O) at 286.5 eV, other polymer species at 285.5-286.5, CH2O at 287.5 eV, etc. In another higher binding energy, around 290 eV, carbonate derivatives are believed to locate at this region, such as ROCO2Li at 289-290 eV, LiCO3 at 290 eV. As for the O ls XPS spectrum, the peak occurring at 531-534 eV contains C=O at 532.2 eV, COC at 533.5 eV, LiCO3 at 533 eV, most of which are the species appearing in the C ls XPS spectrum as well [4, 23-26]. Typically, the chemical composition distribution of the SEI film is a dense inorganic matrix consisting mainly of LiF and LiCO3 close to the electrode surface and a porous organic or polymeric layer contacting with electrolyte [27]. Therefore, it is interesting that LiCO3 exists at292 290 288 286 284 282
C
blank Mi-2
Mi-3 Mi-4
pristine carbon
‐CF2 ‐CH2
C‐C
the surface of the SEI film based on the above XPS spectra. The existence of LiCO3
results from the reaction of metastable semicarbonate to form stable LiCO3 during rinsing solvent to get rid of influence of residual electrolyte (sample treatment before XPS observation). [26] The reaction is as follows:
Therefore, the existence of LiCO3 implicitly represents the existence of organic species, i.e. semicarbonate. Although the exact chemical composition was not obtained from the above preliminary analysis, the tendency of all samples with different electrolyte was similar, suggesting that the chemical composition, or specifically speaking, the organic composition (not considering LiCO3) was not affected by the N-containing maleimide substituent.
Fig. 10 C 1s and O 1s XPS spectra for the SEI film formed on the carbon electrode
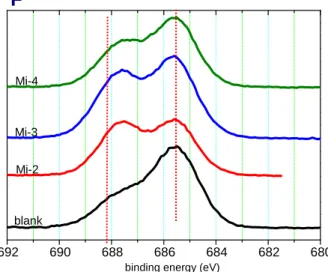
after the formation process. The carbon electrode is de-lithiated condition.After organic distribution was found similar for all samples, inorganic compound was investigated. Typically, the compound relating to F element in the SEI film always exists in an inorganic form, and F 1s XPS spectrum (Fig. 11) is analyzed. All samples show two main peaks: one at 685.6, corresponding to LiF, and the other at 688 eV, related to LiPF6 and its derivatives. The detection of the LiPF6 and its derivatives on the outermost SEI film is probably attributed to the remaining salt LiPF6 that was not washed out completely by DEC before XPS analysis. [28] The existence of LiF is attributed to degradation product of LiPF6 (LiPF6 ↔ LiF + PF5). Moreover, PF5, Lewis acid and very reactive gas, can further have a reaction with the organic composition in the SEI film, forming another more LiF, as shown in Fig. 12. [21, 29]
Hence, stabilizing PF5 is important to avoid the degradation of the SEI. Because
292 290 288 286 284 282
C
binding energy (eV) blank
Mi-2 Mi-3 Mi-4
538 536 534 532 530 528
O
blank Mi-2 Mi-3 Mi-4
binding energy (eV)
gaseous PF5 cannot be detected in XPS analysis due to vacuum operation condition, the content of LiF is the indication of stability of lithium salt in the electrolyte.
Fig. 11 F 1s XPS spectra for the SEI film formed on the carbon electrode after the
formation process. The carbon electrode is de-lithiated condition.Fig. 12 Related reactions of LiPF
6. [29, 119]F1s XPS spectrum (Fig. 11) shows that the intensity of these peaks had a difference.
For blank, the intensity of LiF and the content ration of LiF to LiPF6 was the highest compared to the samples with Mi-based additives; for different kinds of Mi-based additive, the ratio of LiF to LiPF6 from high to low follows the order: Mi-4 > Mi-3 >
Mi-2. Accordingly, Mi-based additive certainly influenced the inorganic composition in the SEI film, and different structure of Mi-based compounds cast varied degree of impact in the content of LiF. Then, the main reason for different content of LiF is investigated. Examining carefully the structure of the maleimide part in the additives, there are two amide functional groups in the maleimide unit (Fig. 12), and amide is proven to effectively stabilize PF5, a reactive product from the decomposition of LiPF6. Consequently, Mi-based additives can be regarded as lithium salt stabilizer.
Wang et al. use Mi-1 as additive (Fig. 13) and show similar results that the intensity
692 690 688 686 684 682 680
F
blank Mi-2 Mi-3 Mi-4
binding energy (eV)
of the LiF peak without the Mi-1 additive exceeds that with the additive after the batteries were cycled.[14] The reason for amide to stabilize PF5 probably is the lone pair at N or O atom.[20, 30-31]
O O O
O N N
R
Fig. 13 The general structure of Mi-based compound consisting of two maleimide
unit. In addition, there are two amide functional groups in a maleimide unit.Fig. 14 The chemical structure of Mi-1 additive used in the other literature.[14]
Because every sample could differ from each other due to individual error, the ability to stabilize PF5 should be investigated by the relative intensity of LiF to LiPF6 instead of absolute value. From F 1s XPS results, the ratio of LiF to LiPF6 from high to low follows the order: Mi-4 > Mi-3 > Mi-2. The difference in the ratio was caused by the varied number of amide functional group reacting with PF5. The number of reacting amide functional group in Mi-4 was less than that in Mi-2, so Mi-2 can effectively inhibit PF5 from reacting and generating LiF. The difference in ability to reduce the formation of LiF resulted from the difference in steric hindrance. In the previous discussion, the structure of Mi-2 product was deduced to be less bulky and less bending due to its little steric hindrance, and the structure of Mi-4 product tended to be bulky and winding because of large steric hindrance. For Mi-4, the winding structure could block the contact between PF5 and lone-pair electron at N or O atom.
Despite of much more space inside the structure of Mi-4 product, the size of space was not enough for PF5 to interact with lone-pair electron at N or O atom, as shown in
Fig. 14 and 15.
one maleimide unit one amide functional
maleimide unit
O N O N O
O N
O N
O
N
O O N
H H
H H
H H H
H H
H H
H H
H H
H
H H H
H
H H H H
Fig. 15 Part of possible structure of Mi-4 product. These molecules do not show
exactly real structure. The red cycle indicates that part of N and O atom are hard to stabilize PF5.By the above analysis, Mi-based additive are found having two factors affect the cell.
The first factor is that it is different structures that have a difference in the impedance spectra. The second factor is that the lone-pair electron at N or O atom in the maleimide unit possesses the ability to stabilize the reactive PF5 compound, but the level to suppress the generation of LiF is dependent of the structure after all. These two factors have opposite impact in the battery. Take Mi-2 additive as an example. In the impedance analysis, RSEI of Mi-2 was the largest, meaning that the SEI was more compact and migration of solvated Li+ through the micro-pores of SEI was more difficult; however, in the XPS analysis, the content of LiF in the Mi-2 was the lowest, suggesting that the SEI film with Mi-2 should had better ion conductivity because LiF can reduce ionic conductivity of the SEI. These two opposite impacts can exist simultaneously and therefore their effects competed to each other. Which factor is dominant is discussed by battery performance in the following section.
Battery performance
After several runs of experiments, the discharge capacity in the first cycle did not particularly increase with the use of additive in the electrolyte, as shown in the Fig. 16.
However, the statistics presents that the discharge capacity of battery with Mi-4 additive still have a chance to exceed that of battery without it because the higher average.
Fig. 16 The statistics of discharge capacity in the first cycle. The battery was charged
and discharged at 0.2C rate (0.002A) between 4.2V and 2.75.In the first cycle, despite the fact that most of SEI film was formed, the condition of SEI film did not reach a steady state, so the contribution of Mi-based additives was masked by other components (such as VC and PC). Therefore, the performance in the first cycle was barely improved and the variation among all batteries owing to the position of functional group was not significant. However, when the cycle number was extended longer and the SEI gradually achieved a more stable condition, the influence of additive became remarkable in 1C/1C cycle performance, as shown in
Fig. 17 (discharge capacity versus cycle number). The discharge capacity of batteries
with all Mi-based additives dropped abruptly at initial 10 cycles, and then faded away smoothly. The main cause of sudden capacity drop can be attributed to higher impedance in the battery with Mi-based additive because the capacity loss had the same tendency with the order in the impedance analysis (Mi-2~Mi-3>Mi-4>blank).Since SEI in as interphase between carbon and electrolyte, the SEI with more blocking property would stick the Li+ in the layer causing the loss of capacity, especially for high current rate. Therefore, the cycle performance was is in agreement of the impedance results, and the structure of Mi-based additive was predominant in the initial cycles. After several cycles, the battery condition reached a steady state.
The curve with Mi-based additive became smooth but the one without additive (i.e.
the blank) kept decaying in the following cycle. Two possible causes contributed to the phenomena: (1) although the higher impedance had negative influence in capacity loss in the initial cycles, it can protect the electrode from solvent co-intercalating [32]
due to its compactness; (2) PF5 was stabilized by maleimide functional group so it did
blank BX CX DX
10.0 10.2 10.4 10.6 10.8 11.0 11.2 11.4
di sc har g e c apaci ty ( m A h )
Mi-2 Mi-3 Mi-4
not degrade the SEI film.[30] The results presented the integration of both factors in the prolonging cycles.
Among all kinds of Mi-based additive, the performance of the battery with Mi-4 excelled that of other additives. The discharge capacity of the battery with Mi-4 additive was lower than the blank at first, but the cycle retention of Mi-4 was better than that of the blank so the battery with Mi-4 even surpass the results of the blank battery. It is noteworthy that the amount of additives was merely 0.1 wt.% in the electrolyte, and with such minute amount, the cycle performance was significantly improved for batteries with Mi-4. Finally, it can be concluded that different structure of Mi-based did have varied results in the battery performance. Mi-based additives can protect the SEI film due to higher impedance. However, the larger impedance did not guarantee a better performance because the larger impedance cause terrible capacity loss at initial cycles, such as Mi-2 and -3. Besides, in the extending cycles, the performance of the battery with Mi-4 exceeded that of the blank battery thanks to the protection of Mi-4 and the stability of PF5 by the functional group.
Fig. 17 The 1/1C cycle performance of batteries with/without additives. Discharge
capacity versus cycle number.Conclusion
To investigate the structural effect in SEI and the battery performance, maleimide-based additives which are isomer for each other (the maleimide substituent on the benzene are para-, meta-, and ortho- position) was studied. First, the CV measurement showed that the reduction potential of maleimide-based additive occurring at 2.3V was prior to other components in the electrolyte. Moreover, there was no significant variation in reduction potential among different maleimide-based additives, indicating that the structure did not influence electrochemical reaction.
-10 0 10 20 30 40 50 60 70 80 90 100 110
0 1 2 3 4 5 6 7 8 9 10 11
blank Mi-2 0.1 wt.%
Mi-3 0.1 wt.%
Mi-4 0.1 wt.%
discharge capacity (mAh)
cycle no.
Mi-2 product could be less bulky and winding so the stacking of Mi-2 product in the SEI film should be more compact; Mi-4 product was in opposite way, containing more space for Li+ to pass through the SEI film readily. The difficulty for Li+ travelling through SEI showed in the impedance spectra. In the analysis of impedance spectra, the resistance of SEI containing Mi-4 additive with large steric hindrance was smaller than the one containing Mi-2 additive with little steric hindrance. By simulation of equivalent circuit, the RSEI follows this order: Mi-2~Mi-3>Mi-4>blank.
The above result presented additives with different structure cast an impact on the SEI film. As for chemical composition, the XPS demonstrated similar organic distribution whether the electrolyte contained maleimide-based additive or not. However, the content of a specific inorganic compound, LiF, was decreased due to the existence of maleimide-based additive in the electrolyte because the functional group with maleimide-based additive can stabilize highly reactive PF5. Finally, in the battery performance test, although the additive had no notable variation in the first discharge capacity, the structural effect of additive showed remarkable divergence in discharge capacity loss in the initial 1C/1C cycle test. As the cycle proceeded, the discharge capacity of batteries with maleimide additives reached a steady state and did not reduce anymore. On the contrary, the discharge capacity of the battery without additive kept declining as the cycle number increased because of lack of stability by maleimide-based additive. Besides, only the performance of Mi-4 additive with large steric hindrance can excel the one without any additive. This study suggests that the structural effect of maleimide-based additive plays an important role in the SEI layer and further influences the battery performance, even though the additives have the same functional group. Therefore, the choice of additive should not only consider specific chemical properties but also the structure.
References
1. Winter, M., P. Novak, and A. Monnier, Graphites for Lithium-Ion Cells: The Correlation of the First-Cycle Charge Loss with the Brunauer-Emmett-Teller Surface Area. Journal of the Electrochemical Society, 1998. 145(2): p. 428-436.
2. Zheng, T., A.S. Gozdz, and G.G. Amatucci, Reactivity of the Solid Electrolyte Interface on Carbon Electrodes at Elevated Temperatures. Journal of the Electrochemical Society, 1999. 146(11): p. 4014-4018.
3. Peled, E., et al., Effect of carbon substrate on SEI composition and morphology.
Electrochimica Acta, 2004. 50(2-3): p. 391-395.
4. Verma, P., P. Maire, and P. Novák, A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochimica Acta, 2010.
55(22): p. 6332-6341.
5. Aurbach, D., et al., The Correlation Between the Surface Chemistry and the Performance of Li-Carbon Intercalation Anodes for Rechargeable
`Rocking-Chair' Type Batteries. Journal of the Electrochemical Society, 1994.
141(3): p. 603-611.
6. Aurbach, D., et al., Failure and Stabilization Mechanisms of Graphite Electrodes. The Journal of Physical Chemistry B, 1997. 101(12): p. 2195-2206.
7. Scott, M.G., A.H. Whitehead, and J.R. Owen, Chemical Formation of a Solid Electrolyte Interface on the Carbon Electrode of a Li-Ion Cell. Journal of the Electrochemical Society, 1998. 145(5): p. 1506-1510.
8. Ein-Eli, Y. and V.R. Koch, Chemical Oxidation: A Route to Enhanced Capacity in Li-Ion Graphite Anodes. Journal of the Electrochemical Society, 1997. 144(9):
p. 2968-2973.
9. Pan, Q., H. Wang, and Y. Jiang, Covalent modification of natural graphite with lithium benzoate multilayers via diazonium chemistry and their application in lithium ion batteries. Electrochemistry Communications, 2007. 9(4): p. 754-760.
10. Pan, Q., H. Wang, and Y. Jiang, Natural graphite modified with nitrophenyl multilayers as anode materials for lithium ion batteries. Journal of Materials Chemistry, 2007. 17(4): p. 329-334.
11. Liu, Z., A. Yu, and J.Y. Lee, Modifications of synthetic graphite for secondary lithium-ion battery applications. Journal of Power Sources, 1999. 81-82: p.
187-191.
12. Zheng, T., et al., Lithium Insertion in High Capacity Carbonaceous Materials.
Journal of the Electrochemical Society, 1995. 142(8): p. 2581-2590.
13. Ohzuku, T., Y. Iwakoshi, and K. Sawai, Formation of Lithium-Graphite Intercalation Compounds in Nonaqueous Electrolytes and Their Application as a Negative Electrode for a Lithium Ion (Shuttlecock) Cell. Journal of the Electrochemical Society, 1993. 140(9): p. 2490-2498.
14. Wang, F.-M., et al., Novel SEI formation of maleimide-based additives and its improvement of capability and cyclicability in lithium ion batteries.
Electrochimica Acta, 2009. 54(12): p. 3344-3351.
15. Jan, Y.-S., Ho, L.-C., Li, S.-M., Hsieh, T.-T., Chuan, W.-Y, U.S. Patent 0,121,356 (2006).
16. Inaba, M., et al., Electrochemical scanning tunneling microscopy analysis of the surface reactions on graphite basal plane in ethylene carbonate-based solvents and propylene carbonate. Journal of Power Sources, 1997. 68(2): p. 221-226.
17. Abe, K., et al., Functional electrolytes: Synergetic effect of electrolyte additives for lithium-ion battery. Journal of Power Sources, 2008. 184(2): p. 449-455.
18. Aurbach, D., et al., On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries. Electrochimica Acta, 2002. 47(9): p.
1423-1439.
19. Song, J.Y., et al., Two- and three-electrode impedance spectroscopy of lithium-ion batteries. Journal of Power Sources, 2002. 111(2): p. 255-267.
20. Zhang, S.S., K. Xu, and T.R. Jow, Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochimica Acta, 2004. 49(7): p. 1057-1061.
21. Zhang, S.S., A review on electrolyte additives for lithium-ion batteries. Journal of Power Sources, 2006. 162(2): p. 1379-1394.
22. Zhang, S.S., K. Xu, and T.R. Jow, EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochimica Acta, 2006. 51(8-9): p. 1636-1640.
23. Ota, H., et al., Analysis of Vinylene Carbonate Derived SEI Layers on Graphite Anode. Journal of the Electrochemical Society, 2004. 151(10): p. A1659-A1669.
24. Andersson, A.M. and K. Edstrom, Chemical Composition and Morphology of the Elevated Temperature SEI on Graphite. Journal of the Electrochemical Society, 2001. 148(10): p. A1100-A1109.
25. Tasaki, K., et al., Solubility of Lithium Salts Formed on the Lithium-Ion Battery
Negative Electrode Surface in Organic Solvents. Journal of the Electrochemical Society, 2009. 156(12): p. A1019-A1027.
26. Andersson, A.M., et al., The influence of lithium salt on the interfacial reactions controlling the thermal stability of graphite anodes. Electrochimica Acta, 2002.
47(12): p. 1885-1898.
27. Edström, K., M. Herstedt, and D.P. Abraham, A new look at the solid electrolyte interphase on graphite anodes in Li-ion batteries. Journal of Power Sources, 2006. 153(2): p. 380-384.
28. El Ouatani, L., et al., The Effect of Vinylene Carbonate Additive on Surface Film Formation on Both Electrodes in Li-Ion Batteries. Journal of the Electrochemical Society, 2009. 156(2): p. A103-A113.
29. Li, W., et al., Additives for Stabilizing LiPF6-Based Electrolytes Against Thermal Decomposition. Journal of the Electrochemical Society, 2005. 152(7): p.
A1361-A1365.
30. Li, W. and B.L. Lucht, Inhibition of the Detrimental Effects of Water Impurities in Lithium-Ion Batteries. Electrochemical and Solid-State Letters, 2007. 10(4): p.
A115-A117.
31. Xu, M., et al., Experimental and Theoretical Investigations of Dimethylacetamide (DMAc) as Electrolyte Stabilizing Additive for Lithium Ion Batteries. The Journal of Physical Chemistry C, 2011. 115(13): p. 6085-6094.
32. Vetter, J., et al., Ageing mechanisms in lithium-ion batteries. Journal of Power Sources, 2005. 147(1-2): p. 269-281.
5
國科會補助專題研究計畫項下出席國際學術會議心得報告
日期:100 年 6 月 30 日
一、 參加會議經過
本次會議為 2011 年春季材料科學會議,會議地點位於美國舊金山舉行,共計五天 (4/25~4/29)。MRS 會議中共分為 51 項主題,並且分為包括能源用材料(Material for energy sustainability)、光電用材料(Electronic and photonic materials)、奈米材料與科技 (Nanomaterials and nanotechnology)、有機生物用材料(Organic and biomaterials)與一般 材料科學(General material science)等大項目,而本人則實際參與 L 分項的電化學介面 現象與臨場光譜應用於儲能材料之研究(Interfacial Phenomena and In-situ Techniques for Electrochemical Energy Storage and Conversion)的演講聆聽與海報張貼報告。本會議
計畫編號 NSC 99-2218-E-011-008-
計畫名稱 創新三維離子奈米通道固體電解質界面膜用於動力鋰離子電池與其電化學 反應動力學機制之研究
出國人員
姓名 王復民 服務機構
及職稱
國立臺灣科技大學 工程技術研究 所 助理教授
會議時間 100 年 4 月 25 日至
100 年 4 月 29 日 會議地點 美國 舊金山
會議名稱
(中文) 2011 年春季材料科學會議 (英文) 2011 MRS Spring Meeting
發表論文 題目
(中文)
官能基效應對於馬來醯亞胺添加劑用於鋰電池影響之研究 鋰電池需要水分嗎?(英文) Substitute Functional Group Effects of Novel Maleimide Based Additives in Lithium Ion Battery
Does Lithium Ion Battery Need Moisture ?
6
第一天(4/25)僅為會議登記與晚宴,提供與會人士與研究學者互相交流認識的機會。
第二天(4/26)則開始進行本次會議的主題,學術演講的議題包括臨場 X 光光譜(XANES 與 XPS)用於分析材料結構、第一原理理論計算用於分析臨場光譜的技術、新型正負極 材料的合成與碳表面介面分析技術之開發與分析等等,同時會中也針對世界各國鋰離 子電池產業的發展與政府政策、電動車用鋰離子電池需要努力的課題進行分析與分 享。4/26 的晚上則進行鋰離子電池負極材料介面現象的材料與分析技術海報論文張貼 與展示,而本人此次共發表兩篇文獻(如附件一~二)。第三天(4/27)的會議為進行新開 發介面分析技術的學術演講,為因應動力鋰電池市場的來臨,相關高功率放電電池的 產量可能急劇上升,因此本次會議中大部分皆以高經濟效應的快速檢驗方式或是新技 術之展現為主,包括 AP-XPS、In-situ TEM 用於磷酸鋰鐵等材料的研究。第四天(4/28) 的會議共分為上午與下午的活動,上午是進行鋰離子電池用電解液與添加劑的學術演 講,包括針對鋰離子電池中非常重要的鈍性膜(SEI),深入的探討與解析。下午則為高 分子電解質的開發介紹,包含 Soluble Polysulphide 與 PEO 用於電池設計的能力與現行 所發展出來的電池性能,同時間會議也針對電池模組的充放電行為和安全更進一步學 術探討。會議的第五天(4/29)針對電容器(M 分項)未來可能可以與鋰電池材料與系統進 行整合作學術演講,期中談到包括能量密度更高的鋰硫電池、二次鋰空氣電池新型正 材料合成等,因為高功率整合模組發展的瓶頸問題,使得有必要提早進行新系統的研 究。
二、 與會心得
參加此次會議經與多位國際學者與聆聽學術演講後,認為台灣在此鋰離子電池領域的
7
研究具一定競爭力,惟政策必須更明確與學術資源的集中,包括國內可以學習日本產 學研究整合成立鋰離子電池相關研究部門,並搭配工研院、中科院等財團法人的協助,
投與固定與長久的經費支持國內無論是研究學術還是產業界的提升,此才長久之計,
並可與國際並駕齊驅。
三、 考察參觀活動(無是項活動者略) 無
四、 建議
感謝國科會全力支援本次出席 2011 MRS Spring Meeting,並發表兩篇論文,同時可以 增進對於不同領域材料的知識,非常值回票價。我國應可結合各大專院校的學術資源 與政府的力量,申請舉辦類似的會議於台灣,此舉除可以讓國內學術研究知名度提升 外,對於國際合作的可能性也會大幅提升。
五、 攜回資料名稱及內容
Program and Exhibit Guide of 2011 MRS Spring Meeting (一本) Abstract CD-ROM (一片)
六、其他
無
Page 1 of 1
Abstract Viewer
Program Number: L3.2 Day / Time: Tuesday, Apr. 26, 5:00 PM - 8:00 PM
Substitute Functional Group Effects of Novel Maleimide Based Additives in Lithium Ion Battery.
F.Wang 1
; M.Yu 2
; C.Wan 2
1. Graduate Institute of Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan; 2. Department of Chemical Engineering, National Tsing Hua University, Hsinchu, Taiwan
Numerous researchers used various compounds and spectroscopic techniques have modified and identified of solid electrolyte interface (SEI) are the chemically decompose products of electrolyte solvents and salts.
Two years ago, we had published the maleimide (MI) based are examined as new additives for electrolytes in lithium ion batteries. We had attempted to increase capacity whereby irreversible
phenomena reduced during charge and discharge process and cyclic ability are also strengthen due to a new SEI chemical structure. In this new publication, we go a step further to display the substituent positions effects of novel SEI formation of maleimide based additives. In addition, we also found these new candidate additives with suitable addition into electrolyte will reveal an interesting SEI of
morphology on the MCMB surface and enhance rate ability of battery.
Citation: F.Wang, M.Yu, C.Wan. Substitute Functional Group Effects of Novel Maleimide Based Additives in Lithium Ion Battery.. Program No. L3.2. 2011 Abstract Viewer. San Francisco, CA:
Materials Research Society
Application Design and Programming Copyright ScholarOne, Inc. All Rights Reserved. Patent
Pending.
Page 1 of 1
Abstract Viewer
Program Number: L3.3 Day / Time: Tuesday, Apr. 26, 5:00 PM - 8:00 PM
Does Lithium Ion Battery Need Moisture?
C.Cheng 2,3
; F.Wang 1
1. Graduate Institute of Engineering, National Taiwan University of Science and Technology, Taipei, Taiwan; 2. Department of Chemical Engineering, National Tsing Hua University, Hsinchu, Taiwan; 3.
Material and Chemical Research Laboratories, Industrial Technology Research Institute, Hsinchu, Taiwan
Numerous researchers studied the water uptake influences which contain in the electrolyte or the electrodes, suggesting these influences have enormous pernicious effects on lithium ion battery performance such as capacity, cycle life and safety. It is commonly accepted that the moisture in LiPF6-based electrolyte caused by the irreversible side reaction making capacity loss in the first cycle due to the water has been reduced by the unwilling reaction with LiPF6 of the electrolyte and formed inorganic solid electrolyte interfaces (SEIs) on the anode surface or HF in the electrolyte.. Therefore, it is difficult to dominate the forming mechanism of SEIs such as chemical composition, film thickness and surface morphology on electrode concerning significantly affect battery performance, especially with moisture. Mijung et al.(1) state that the rate capability and capacity retention of positive electrode were improved with water-adsorption of lithium ion batteries by using specific optimized LiCoO2 and LiNi1/3Co1/3 Mn1/3O2 composite cathode material. In addition, Saharan et al.(2) studied that the active materials can be a major source of moisture contributors to the overall cell moisture and suggest to dry in minimizing the amount of moisture uptake from the environment
In our investigation, the improvement regarding water uptake on lithium ion batteries by using an novel additive in electrolyte has been examined. The systematically analysis of increasingcapacity retention and cycle-ability enhancement under normal manufactoring process (relative humidity > 95%) without dry room and the reacion mechanism of additive with water are also revealed in this work. Ref. [1] N.
Mijung, Y. Lee, J. Cho, J. Electrochem. Soc. 153 (2006) A935 [2] V. Saharan, J. Roberts, V. Manev, Y.H. Chia, G. Maclean, S.R. McMullen, J. Power Sources 146 (2005) 809
Citation: C.Cheng, F.Wang. Does Lithium Ion Battery Need Moisture?. Program No. L3.3. 2011 Abstract Viewer. San Francisco, CA: Materials Research Society
Application Design and Programming Copyright ScholarOne, Inc. All Rights Reserved. Patent
Pending.
國科會補助計畫衍生研發成果推廣資料表
日期:2010/11/23
國科會補助計畫
計畫名稱: 創新三維離子奈米通道固體電解質界面膜用於動力鋰離子電池與其電化學反 應動力學機制之研究
計畫主持人: 王復民
計畫編號: 99-2218-E-011-008- 學門領域: 電化學
研發成果名稱 (中文) 添加劑
(英文) Additive
成果歸屬機構 國立臺灣科技大學 發明人 (創作人)
王復民,鄭錦淑
技術說明
(中文) 鋰離子電池的製程在過去一直必須於高乾燥低水含量環境下才能進行,主因在於 水會於電池電化學反應中電解成為氧氣與氫氣,此容易造成電池膨脹與性能衰退。
另外,電池中的高水含量也會與鋰鹽進行副反應形成氫氟酸,此產物將會造成電 極材料的過鍍金屬離子溶出與集電層之腐蝕,非常容易使電池發生危險。本技術 之創新在於可以解決水於電池中之問題,並且不需要高乾燥低水含量環境下進行,
可以有效降低製作成本與提升電池性能,研究結果指出在特定水含量或是高濕度 環境製程下,添加劑的加入可以有效提升鋰電池電容量5~10%。
(英文) The manufactoring of lithium ion battery must proceeds in the low moisure and high drying surrounding according to the electrochemical reaction of water, which forms oxygen and hydrogen. The two gases makes battery performance decay and volume expansion. In addition, the high water content proceeds side reaction to HF with lithium salt, suggesting dissolube transition metal from electrode material and current collector corrsion.
This novel investigation and technique concludes that the manufactoring of lithium ion battery no longer untilizes highly dry surrounding and eliminates the cost and enhances battery performance. The results show that the battery with additive at typical water content increases the capacity of 5~10%.
產業別 化學業;其他專業、科學及技術服務業
技術/產品應用範圍 電化學元件,鋰電池,染料敏化太陽電池,電容器
技術移轉可行性及 預期效益
鋰電池必須在高乾燥低水含量環境下才能進行製程是目前所習知的技術,因此絕大部分 廠商皆建造乾燥是與乾燥廠房以因應製程,或是利用氣室(Air Chamber)方式進行二次封 裝加工程序,原因在於高含量水會在第一次電化學反應中電解生成氣體,因此電池會先 預留空間使氣體可以流至氣室,然後再將氣室移除進行二次封裝,然而此程序會多出一 段氣室過程,極不便利且製造額外成本。
另外,在利用添加劑來移除水的部分則在目前所有文獻皆無類似想法與研究成果發表,
此證明本技術具有突破與創新性,急需立即申請成為本校指標性專利與技術移轉至國內 外,提升我國鋰電池研發能見度。
註:本項研發成果若尚未申請專利,請勿揭露可申請專利之主要內容。
99 年度專題研究計畫研究成果彙整表
計畫主持人:王復民 計畫編號:99-2218-E-011-008-
計畫名稱:創新三維離子奈米通道固體電解質界面膜用於動力鋰離子電池與其電化學反應動力學機制 之研究
量化
成果項目
實際已達成
數(被接受 或已發表)
預期總達成 數(含實際已
達成數)
本計畫實 際貢獻百
分比 單位
備 註
(
質 化 說 明:如 數 個 計 畫 共 同 成 果、成 果 列 為 該 期 刊 之 封 面 故 事 ...等
)
期刊論文 0 0 100%
研究報告/技術報告
0 0 100%研討會論文 0 0 100%
論文著作 篇
專書 0 0 100%
申請中件數 1 0 100%
專利 已獲得件數 0 0 100% 件
件數 0 0 100% 件
技術移轉
權利金 0 0 100% 千元
碩士生 0 1 100%
博士生 1 0 100%
博士後研究員 0 0 100%
國內
參與計畫人力
(本國籍)
專任助理 0 0 100%
人次
期刊論文 3 2 100%
研究報告/技術報告
0 0 100%研討會論文 5 2 100%
論文著作 篇
專書 0 0 100% 章/本
申請中件數 4 1 100%
專利 已獲得件數 0 0 100% 件
件數 0 0 100% 件
技術移轉
權利金 0 0 100% 千元
碩士生 0 0 100%
博士生 0 0 100%
博士後研究員 0 0 100%
國外
參與計畫人力
(外國籍)
專任助理 0 0 100%
人次
其他成果
(
無法以量化表達之成果如辦理學術活動、獲 得獎項、重要國際合 作、研究成果國際影響 力及其他協助產業技 術發展之具體效益事 項等,請以文字敘述填 列。)
無
成果項目 量化 名稱或內容性質簡述
測驗工具(含質性與量性)
0課程/模組
0電腦及網路系統或工具
0教材
0舉辦之活動/競賽
0研討會/工作坊
0電子報、網站
0科 教 處 計 畫 加 填 項
目 計畫成果推廣之參與(閱聽)人數
0國科會補助專題研究計畫成果報告自評表
請就研究內容與原計畫相符程度、達成預期目標情況、研究成果之學術或應用價 值(簡要敘述成果所代表之意義、價值、影響或進一步發展之可能性) 、是否適 合在學術期刊發表或申請專利、主要發現或其他有關價值等,作一綜合評估。
1. 請就研究內容與原計畫相符程度、達成預期目標情況作一綜合評估
■達成目標
□未達成目標(請說明,以 100 字為限)
□實驗失敗
□因故實驗中斷
□其他原因 說明:
2. 研究成果在學術期刊發表或申請專利等情形:
論文:□已發表 □未發表之文稿 ■撰寫中 □無 專利:□已獲得 ■申請中 □無
技轉:□已技轉 ■洽談中 □無 其他:(以 100 字為限)
3. 請依學術成就、技術創新、社會影響等方面,評估研究成果之學術或應用價 值(簡要敘述成果所代表之意義、價值、影響或進一步發展之可能性)(以 500 字為限)
本研究成果成功將添加劑鈍性膜電化學合成於負極表面上,並且形成特殊的三維立體結構 之奈米通道,期不只可以提供電池容量約 5~10%外,也可以增加電池的循環壽命約 15%!
中國石油工業股份有限公司(中石化)對於本計劃所開發的奈米通道鈍型膜技術具有非常 高的興趣,目前正在洽談產學合作案與技術移轉的可能性!