國立臺灣大學生命科學院生化科學研究所 碩士論文
Graduate Institute of Biochemical Sciences College of Life Science
National Taiwan University Master Thesis
Rasd1 在斑馬魚幼魚內之功能及表現調控
Function and Expression Regulation Control of Rasd1 in Zebrafish Larvae
潘和彥 He-Yen Pan
指導教授:管永恕 博士 Advisor: Yung-Shu Kuan, Ph.D.
中華民國 105 年 7 月 July, 2016
致謝
最先要感謝的,是管永恕老師。感謝管老師在百忙之中抽空,帶領我以及 N202 實驗室同仁們完成各自的實驗及目標。
兩年前,由於自身的不成熟,選擇離開了姚季光老師的實驗室。在各個實驗 室間徘徊了一陣子後,終於來到現在的實驗室。來到這實驗室後,從老師以及學 長們學到許多操作分子生物學的相關技術及技巧,以及斑馬魚的養殖及解剖等的 知識。
再一次的,我要感謝管永恕老師,感謝管老師的心地善良,感謝管老師的耐 心教導;我還要感謝姚季光老師,感謝姚老師的嚴格的標準,以及細心的指導;
我也要感謝姚老師實驗室的學長學姐們,教我各種實驗技術以及實驗室管理相關 知識;我要感謝林邦齊學長,感謝您陪我一起討論我的實驗內容及數據;我要感 謝吳柏宗學長,感謝您抽空看我的實驗過程是否哪邊有問題;我要感謝呂旻諭,
感謝您每天中午陪我買午餐、吃飯,還有閒話家常;我要感謝溫世賢,感謝您跟 我分享 in situ hybridization 以及 western 的做法以及注意事項;我要感謝沈明諺,
感謝您決定加入 N202 實驗室,讓這個實驗室欣欣向榮;我還要感謝張泰琪士官 長,沒有您的一句話,我現在可能還在軍中渾渾噩噩的過日子;最後我要感謝我 的父母,謝謝您們默默的支持我,默默的付出。
我很幸運的在我人生中能遇到這麼多貴人,即使是罵我或是批評我,都能讓 我看到許多事物,讓我學習並成長。感謝跟我相遇的每一個人,即使只是萍水相 逢,我還是會珍惜與你們相遇的每個瞬間。
List of Abbreviations
AC: Adenylyl cyclase
ATCH: Adrenocorticotropic hormone
CREB: cAMP response element binding protein CP: Choroid plexus
CSF: Cerebrospinal fluid DEX: Dexamethasone
DMT1: Divalent metal transporter 1 dpf: Days-post-fertilization
ESR1: Estrogen receptor 1
GEF: Guanylyl nucleotide exchange factor GSK3β: Glycogen synthase kinase 3β hpf: Hours-post-fertilization
IPN: Interpeduncular nucleus LHA: Left habenula
miR-375: microRNA-375
NHEJ: Nonhomologous end-joining nNOS: Neuronal nitric oxide synthase Nrp1a: Neuropilin-1a
PAP7: Peripheral benzodiazepine receptor associated protein 7 PBS: Phosphate buffer saline
PCR: Polymerase chain reaction PDE4: Phosphodiesterase 4 PFA: Paraformaldehyde PKA: Protein kinase A PKG: Protein kinase G Sema3A: Semaphorin3A Sema3D: Semaphorin3D sgRNA: Small guide RNA
STK11: Serine/threonine-protein kinase 11
Table of Content
中文摘要 ………...……...VI
Abstract ………..……...VII
Chapter 1 Introduction………...1
1-1 Dorsal diencephalon conduction system: the habenula-interpeduncular circuit………....…...1
1-2 Molecules that involve in habenular axonal target recognition...…...2
1-3 Sema3D-Nrp1a signaling pathway in axon attraction…………...…....3
1-4 Function and expression control of rasd1………...4
1-5 Rab6b is Rab6bb homology and regulates retrograde transport from Golgi to ER...……...…...…...5
Chapter 2 Material and Method……….……7
2-1 Primer and guiding RNA sequences………...…….7
2-2 Zebrafish strains and maintenance………...…….7
2-3 Whole mount RNA in situ hybridization………..………...7
2-4 Microinjection………...…….…………..………...8
2-5 Whole mount immunofluorescence staining…...…….………..………9
2-6 rab6bb mutant generation…...…….………...……..9
2-7 T7 Endonuclease I assay...…….………...…….………10
2-8 Restriction enzyme genotyping.………...…….……….……10
2-9 Dexamethasone treatment.………...…….………..……10
Chapter 3 Result………...…….………...………12
3-1 rasd1 mutants do not show significant axon projection defect in habenula………...…….………...……..………12
IV
cells...12 3-3 Efficiency of ectopic rasd1 expression induced by DEX is decreased
gradually after DEX treatment….………...…….…….…14 3-4 rab6bb mutants are generated by CRISPR/Cas9 system...……..…14 Chapter 3 Conclusions and Discussions..…….………..…….……….….……15 References .………..…….………..………..….…….………..…….………..……18 Figures ………..…….………..………..….…….………..….….………..……24
Table of figure content
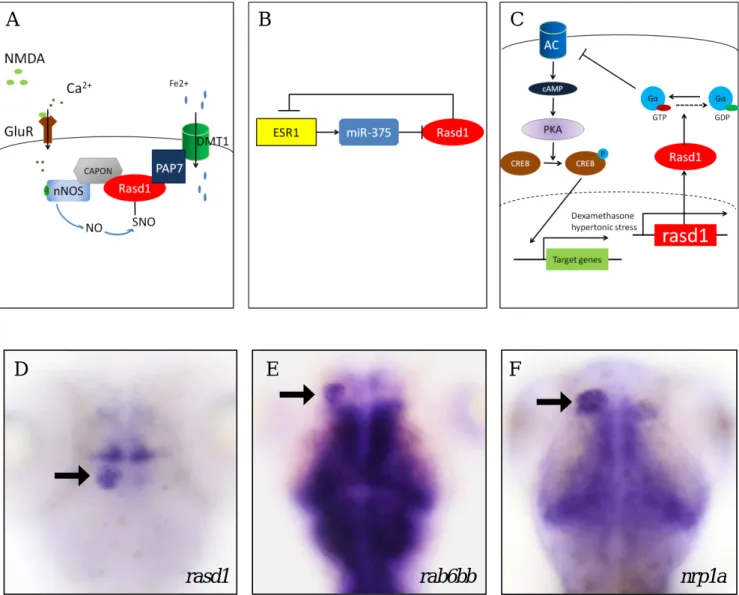
Figure 1………..…….………..…..….…….………..…….………...…..………..……25 (A-F) Rasd1 function and mRNA expression pattern
Figure 2………..…….………..…..….…….………..…….………...…..………..……26 (A-D) Expression patterns of rasd1 in various developmental stages
Figure 3………..…….………..…..….…….………..…….…...………..………..……27 (A-C) CRISPR-Cas9-mediated rasd1 knockout line generation
Figure 4………..…….………..…..….…….………..…….…………..………...……28 (A-H) Loss- and gain-of-Rasd1 functional analyses
Figure 5………..…….………..…..….…….………..…….…………..……...…..……29 (A-C) Rasd1 antibodies can detect overexpressed Rasd1
Figure 6………..…….………..…..….…….………..…….…………..……...…..……30 (A-D) Tissue-specific ectopic expression of rasd1 can be induced in dexamethasone (DEX) treated larvae.
(E-F) Percentages of rasd1 ectopic expression induction rate
Figure 7………..…….………..…..….…….………..…….…………..………...……31 (A-C) Dexamethasone (DEX) treatment induced ectopic rasd1 expression in the choroid plexus
(D-E) Dexamethasone (DEX) injection induced ectopic rasd1 expression
Figure 8………..…….………..…..….…….………..…….…………..……...…..……32 (A-D) Effects of dexamethasone (DEX) treatment in earlier embryonic stages Figure 9………..…….………..…..….…….………..…….…………..………...……33
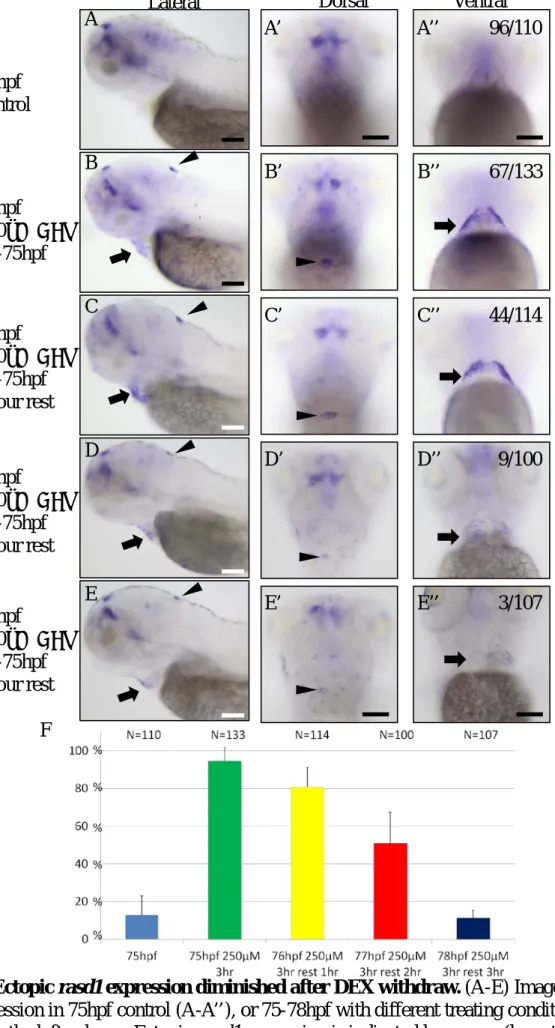
(A-E) Ectopic rasd1 expression diminished after DEX withdrawal (F) Percentages of rasd1 ectopic expression induction rate
Figure 10………..…….………..…..….…….………..…….…………..………...……34 (A-C) CRISPR-Cas9-mediated rab6bb knockout line generation
中文摘要
Neuropilins 是 Class III Semaphorins 的穿膜受體蛋白,並且在軸突導向中扮演 著重要的角色。Class III Semaphorins (Sema3s)通常參與在排斥性軸突導向,並且 其機制已經有被許多研究報導過。然而,Sema3s 也同時參與在吸引性軸突導向,
其機制目前仍尚未明朗。Sema3D 為一種 Sema3s,並且表現在 3 到 4 天斑馬魚的 腳間核(Interpeduncular nucleus)。同個時間點,Sema3D 的受體 Nrp1a 表現在斑馬 魚左韁核(Left habenula)。抑制 Nrp1a 表現將造成左韁核的軸突被導向腳間核的量 減少,其肇因於缺少 Nrp1a 的軸突無法被 Sema3D 所吸引。過去的實驗室成員發 現 rasd1 以及 rab6bb 兩個 small GTPases 與 nrp1a 共同表現在第 4 天斑馬魚左韁 核內。各別以 Morpholino knock down 兩個 small GTPases 將造成與 nrp1a 的 Morphants 相似的軸突導向缺失。我接收了 rasd1 突變種並且製造了 rab6bb 突變 種,兩個突變種都是經由 CRISPR/Cas9 技術製造。我的分析資料顯示與野生種比 較,rasd1 突變種並沒有明顯的軸突導向缺失。此外,我發現 Dexamethasone 可引 起特定組織表現 rasd1,但韁核內生性 rasd1 表現並不受到 Dexamethasone 影響。
除了 rasd1 突變種外,我使用 CRISPR/Cas9 製造了一隻在生殖細胞中帶有目標位 置突變的 rab6bb 基因的斑馬魚。突變 rab6bb 基因的製造將可幫助我們了解蛋白 質 Rab6bb 在細胞內中的的功能。
關鍵字: rasd1, rab6bb, dexamethasone, CRISPR/Cas9, 斑馬魚
Abstract
Neuropilins are transmembrane proteins and act as the receptors of Class III Semaphorins in axonal target finding process. Class III Semaphorins (Sema3s) usually play a role in repulsive axon guidance events and the mechanism has been well-studied.
However, Sema3s also play roles in attractive axon guidance events with unknown mechanism. Sema3D is expressed in interpeduncular nucleus (IPN), and Nrp1a is expressed in left habenula (LHA) at 3dpf and 4dpf. Disruption of nrp1a displays LHA axon projection defect due to loss of Nrp1a-mediated axon attraction by Sema3D.
Previously our laboratory had identified rasd1 and rab6bb that are coexpressed with nrp1a in zebrafish LHA at 4dpf. Their morphants also display similar LHA axonal
projection defect as nrp1a morphants do. I have adopted rasd1 and generated rab6bb mutants that were created with the CRISPR/Cas9 system. My analyses indicated that there was no significant difference in volume and morphology between rasd1 mutants and WT. In addition, I found that tissue-specific ectopic rasd1 expression can be induced in choroid plexus in the hindbrain and hematocyte-like cells by dexamethasone treatment, but the rasd1 expression in HA neurons is not influenced. Besides to rasd1 knockout mutants, one F0 fish has been confirmed to carry rab6bb mutation in the germline cells at the targeted location. The generation of rab6bb mutant fish will be valuable to understand the roles of zygotic rab6bb beyond its basic cellular function as a protein.
Key words: rasd1, rab6bb, dexamethasone, CRISPR/Cas9, zebrafish
Chapter 1 Introduction
1.1 Dorsal diencephalon conduction system: the habenula- interpeduncular circuit
Habenula is one of the highly conserved structures in vertebrate brains, and is involved in pain, emotion, motivation, light-dark cycle, and reward process (Fakhoury and López, 2014; Hikosaka, 2010a; Shelton et al., 2012). It mainly transmits information or signals from the limbic system to the interpeduncular nucleus (IPN) in the midbrain (Fakhoury and López, 2014; Hikosaka, 2010b). In mammals, the habenular complex contains medial and lateral habenula. In zebrafish, the habenular complex contains dorsal and ventral parts that show homology to the medial and lateral habenula, respectively, in mammals (Amo et al., 2010). The medial hebunula mainly conducts signal to IPN, the lateral habenula mainly conducts signal to both IPN and raphe nuclei (Bianco and Wilson, 2009; Hikosaka, 2010b; Shelton et al., 2012). In zebrafish, habenula is involved in the determination of winner or loser in a fight (Chou et al., 2016), and the motivation of fear and anxiety (Jesuthasan, 2012). Disruption of habenula causes enhanced anxiety and defect in avoidance (Lee et al., 2010).
Utilizing zebrafish as the model, several genetic regulations of habenula development have been studied. Nodal signaling plays an important role in habenular asymmetry determination at 18 to 24hpf, and left-side bias Fgf8 is expressed at 24hpf (Aizawa, 2013; Taylor et al., 2010). Nodal signaling and Fgf8 guide the parapineal migration to left side and encourage neuron stem cells in habenula to start generating neural precursors (Aizawa, 2013). Asymmetric generation of habenula seems to be due to the activity of Notch signaling that represses the generation of neuron stem cells (Aizawa, 2013; Taylor et al., 2010). The identity and location of habenula progenitors
havebula neurons nevertheless are largely unknown. The habenula starts stretching its axons to IPN at as early as 32hpf and reaches the midbrain at 57hpf (Beretta et al., 2012), and its axon encircles IPN afterwards. In addition, genes like nrp1a (Kuan et al., 2007) and daam1a (Colombo et al., 2013) were found to show left-right asymmetric expression in or near habenula and regulate habenula axon development.
1.2 Molecules that involve in habenular axonal target recognition Previous study by Kuan et al. showed that Semaphorin 3D (Sema3D), one of the Class III Semaphorins, plays a role in attracting Neuropilin 1a (Nrp1a)-positive habenular axons to their targets in the IPN (Kuan et al., 2007).
Class III Semaphorins usually play roles in axon guidance as a repulsive signal and have been well studied. Semaphorin3A (Sema3A) is the most well-studied repulsive signal in axon guidance. When a Nrp1-positive neuron faces Sema3A, Sema3A binds to Neuropilin-1 and increases local intracellular cGMP concentration (Pasterkamp, 2012;
Shelly et al., 2011). cGMP activates phosphodiesterase 4 (PDE4) through activating protein kinase G (PKG) and decreases cAMP level (Pasterkamp, 2012; Shelly et al., 2011). Lower cAMP level causes lower protein kinase A (PKA) activity and decreases the level of PKA-dependent protein phosphorylation in serine/threonine-protein kinase 11(STK11) and glycogen synthase kinase 3β (GSK3β) that regulate cytoskeletal rearrangement. (Pasterkamp, 2012; Shelly et al., 2011)
As a Class III Semaphorin, Sema3D is thought to be involved in repulsive axon guidance in the same manner as other family members usually did (Song et al., 1998).
Indeed, previous studies showed that Sema3D is mainly involved in repulsive axon guidance events that are mediated by different Neuropilins (Liu et al., 2004; Wolman et al., 2004), but during HA-IPN circuit development in zebrafish larvae Sema3D was
found to function as attractants (Kuan et al., 2007). In brief, in the zebrafish larvae, Sema3D is expressed in dorsal IPN (dIPN), and Neuropilin-1a (Nrp1a) expressed in LHA at 2dpf to 4dpf. Loss of Nrp1a shows axon guidance defect of LHA. It is due to the loss of Nrp1a-mediated axon attraction to their target. (Kuan et al., 2007). This observation suggested that Neuropilins and their downstream molecules play important roles in mediatory of attractive or repulsive axon guidance of Sema3D. However how Nrp1a mediates attractive axon guidance is completely unknown.
1.3 Sema3D-Nrp1a signaling pathway in axon attraction
Sema3D plays a role in attractive axon guidance in the formation of other neural connection system in larval zebrafish brain as well (Kuan et al., 2007; Wolman et al., 2004). Previous study shows that Nrp1a mediates axon guidance that is repulsed by Sema3D in the nucleus of the anterior commissure, on the other hand, both Nrp1a and Nrp2b mediate axon guidance that is attracted by Sema3D in the medial longitudinal fasciculus (MLF) (Wolman et al., 2004). The attractive role of Sema3D in both HA-IPN circuit and MLF development in zebrafish larvae. In order to understand the mechanism of Class III Semaphorins as an attractive axon guidance cues, a systematic approach to uncover more players that involve in Sema3D-Nrp1a-mediated attractive axon guidance is needed.
In order to find the downstream effectors that are involved in Nrp1a-mediated HA axon attraction, previous members in our laboratory started to screen for several small GTPases that have been shown to regulate cytoskeleton dynamics during axon path finding and cell proliferation. The results showed that rasd1 and rab6bb are coexpressed with nrp1a in zebrafish LHA at 4dpf (Fig.1). To know whether they are involved in LHA axon guidance, morpholinos (MOs) knocking down rasd1or rab6bb
had been performed, their morphants showed similar habenula axon projection defects that was observed in nrp1a morphants (Kuan et al., 2007).
1.4 Function and expression control of rasd1
Reports have raised some phenocopy issues between the morphants and knockout mutants (Kok et al., 2015; Rossi et al., 2015), the generation of rasd1 and rab6bb knockout lines therefore were conducted to further verify the roles of rasd1 and rab6bb during HA axonal target finding process.
Rasd1 belongs to the Ras superfamily of small GTPase and was first uncovered being over-expressed in mouse AtT-20 cells, an adrenocorticotropic hormone (ACTH) secreting cell line, with dexamethasone (DEX) treatment (Kemppainen and Behrend, 1998). Further study shows that Rasd1 is involved in glutamate-NMDA neurotoxicity in primary cortical neurons (Fig.1-A) (Chen et al., 2013). When glutamate-NMDA binds to its receptor, calcium inflow triggers neuronal nitric oxide synthase (nNOS) through calmodulin and S-nitrosylation of cysteine-11of Rasd1 which leads to the binding of Rasd1 to the peripheral benzodiazepine receptor associated protein 7 (PAP7) (Cheah et al., 2006). PAP7-Rasd1 complex interacts with the divalent metal transporter 1 (DMT1) which causes ferrous (Fe2+) import and eventually the death of the primary cortical neurons (Cheah et al., 2006; Chen et al., 2013).
Rasd1 also was found to play a role in the development of ESR1-positivec breast cancer cells. Estrogen receptor 1 (ESR1) that is overexpressed in around 70% invasive breast cancer cells is downregulated by RASD1 through upregulating microRNA-375 (miR-375) which is shown to be able to inhibit the expression of RASD1 in ESR1- positive breast cancer cells (Fig.1-B) (Simonini et al., 2010). It forms a potential positive feedback loop that enhances ESR1 expression in ESR1-positive breast cancer
cells.
Rasd1 functions as a guanylyl nucleotide exchange factor (GEF) of Giα that inhibits adenylyl cyclase (AC) (Graham et al., 2004). Rasd1 is induced by dexamethasone treatment or hypertonic stress inhibits AC activity by activating Giα, and it causes lower cAMP level in Att-20 cells (Fig.1-C) (Greenwood et al., 2016).
Lower cAMP level causes lower protein kinase A (PKA) activity, and decreases phosphorylation level of cAMP response element binding protein (CREB) that plays a role in neuronal plasticity (Greenwood et al., 2016; Kandel, 2012). These evidences suggest that Rasd1 potentially regulates neuronal plasticity via inhibiting cAMP level.
However whether Rasd1 plays a role in Nrp1a-mediated HA axonal target recognition has never been explored before.
1-5 Rab6b is Rab6bb homology and regulates retrograde transport from Golgi to ER
Rab6bb is a member of the Rab subfamilly of small GTPases that is involved in cellular vesicle formation, trafficking, and membrane fusion (Stenmark, 2009; Zerial and McBride, 2001). Rab6b is Rab6bb homology has been proposed to regulate the retrograde transport from Golgi to ER in human neuronal cells (Wanschers et al., 2007), and loss of Rab6b causes growth retardation in mouse (MGI, http://www.informatics.jax.org/). Golgi is a major membrane donor of exocytic vehicles.
Endocytosis and exocytosis play a role in axon development and mediated by Rab subfamily GTPases (Tojima and Kamiguchi, 2015). Rab6b mediates membrane dynamics at Golgi suggests that Rab6bb possibly regulates exocytosis leading to axon growth in LHA.
In this thesis project, my goal is to examine the function of Rasd1 and Rab6bb in
HA development and explore the expression regulation of rasd1 upon dexamethasone (DEX) treatment. My analyses showed that rasd1 may not play a strong role in LHA axonal target recognition process, because the axonal project volumes showed no distinguishable difference in wildtype (WT) and rasd1 mutants. Interestingly, I found that in zebrafish embryos or larvae, tissue-specific ectopic rasd1 expression can be induced by DEX treatment, whereas the rasd1 expression in the habenular neurons was unaffected. I further confirmed that one of the ectopic rasd1 expression tissues located in the hindbrain is the choroid plexus (Jiao et al., 2011). To study the role of rab6bb in HA axonal development, I have started to generate rab6bb mutant by CRISPR/Cas9 system. One F0 fish is confirmed to carry mutation in the germline cells at the targeted location. The role of rab6bb in LHA axonal targeting process will be examined once the line is established.
Chapter 2 Material and Method
2.1 Primer and guiding RNA sequences
PCR product
name template primer1(5’ to 3’) primer2(5’ to 3’)
rasd1 293bp genomic DNA
CATCCCGGCGAAAAACTGCT
GCAGATGACACCTCTTTTATT GCAT
rasd1 121bp genomic
DNA CTCAATGGTCGGCGTGTACT
rab6bb 443bp
genomic
DNA AGCAGAGTTTACGAGGGACG CCGACTTGTTTGACGGGGAG rab6bb
139bp
genomic DNA
GGTGCCACCTGTACATATTAA TCC
GGCAAACCCAAATACTCACC G
T7-rab6bb sgRNA
pCRII-(T7- rabb6bb sgRNA)
CGAATTGCAACGATTTAGGTG AAGCACCGACTCGGTGCC
clu cDNA for cloning
3-4dpf zebrafish cDNA
TCCATCTTCATAAACGGCCA TGTAGCGACCCTGAATGTGA A
2.2 Zebrafish strains and maintenance
AB strain zebrafish were purchased from Taiwan Zebrafish Core Facility in Academic Sinica (http://icob.sinica.edu.tw/tzcas/). CRISPR/Cas9-mediated rasd1 mutant zebrafish were generated by ZGENEBIO BIOTECH INC. (Taiwan). All embryos and fishes were maintained and bred at 28℃ with automatic control for a 14- hours light, 10-hours dark cycle.
2.3 Whole mount RNA in situ hybridization
DIG-labeled rasd1 RNA probe was generated from linearized pCMV-SPORT6.1- rasd1 (ZGC MGC:65909, cut by NotI) utilizing SP6 RNA polymerase (Roche
10810274001) and Dig RNA labeling mix (Roche 11277073910). FITC-labeled clu RNA was generated form linearized pCRII-clu (PCR with 5’- TCCATCTTCATAAACGGCCA-3’ and 5’-TGTAGCGACCCTGAATGTGAA-3’
primers and cloned into pCRII vector (Invitrogen, 25-0069), cut by BamHI) utilizing T7 RNA polymerase (Roche 10881767001) and FITC RNA labeling mix (Roche 11685619910). Whole mount RNA in situ hybridization was carried out as described in Wu, Wen et al. 2015 (Wu et al., 2015).
2.4 Microinjection
Capillaries needles were made by micropipette puller (Sutter Instrument Co., P97) and were cut to appropriate size before they were used. The clone for making rasd1Δ181-
265 RNA was generated form pCMV-SPORT6.1-rasd1 (ZGC MGC:65909) by PCR with T7, SP6, 5’-AACGTGGATCAGTAGAGTGAGCCA-3’, and 5’- TGGCTCACTCTACTGATCCACGTT-3’ primers. rasd1Δ181-265 was cloned into pCRII vector (Invitrogen, 25-0069). Caped sense rasd1Δ181-265 RNA was generated utilizing mMESSAGE mMACHINE T7 Transcription Kit (Ambion, AM1344). rasd1 morpholino 5’-CCGACGGACTCATTTTCTTGATCAT-3’ was purchased from GENE TOOLS. For microinjection, approximately 300ng of mRNA or 10ng of MO in 1nL were diluted with DEPC treated water (Bio Basic Canada INC., DD 1005. 500ML) containing 0.005% phenol red. For ventricular zone injection, approximately 25 pmole dexamethasone (SIGMA, D2915-100MG), 40ng dextran 568 were diluted with PBS containing 0.005% phenol red. Then they were injected by pressure of nitrogen gas with microinjector (NARISHIGE, IM300 microinjector). All embryos were incubated in embryo water (30mg PTU (SIGMA, P7629-10G) and 0.3g sea salt/L distilled water) at 28℃, 14-hours light, 10-hourse dark cycle with growth chamber (CHANG KUANG,
CK-68E).
2.5 Whole mount immunofluorescence staining
Zebrafish larvae were fixed 16-24 hours with 4% PFA (paraformaldehyde, SIGMA, P6148-500G) in PBS (BIOMAN Scientific CO., PBS101000) at 4℃. Fixed larvae were washed and dehydrated with methanol (MERCK, 1.07018.2511) and stored in 10%
DMSO (SIGMA, D8418-100ML) in methanol at -20℃ more than 16 hours. Fixed larvae were rehydrated with following series: 75% methanol in PBST (PBS and 1%
tween20, tween20 was purchased from SIGMA, P7949-100ML), 50% methanol in PBST, 25% methanol in PBST and additional PBST washes 6 times. Each step was 5 minutes. Rehydrated larvae were treated with 10μg/mL protease K(Roche , 03115879001) in PBST 22 minutes, and re-fixed with 4% PFA in PBS 15 minutes at room temperature. Re-fixed larvae were washed with PBSTX (PBS and 0.1% TritonX- 100, TritonX-100 was purchased from SIGMA, T8787-100ML) 6 minutes 5 times, and were blocked in blocking buffer (2.5% goat serum, 2% BSA, and 1.2% DMSO in PBSTX) for 1 hour. The larvae were incubated with 1:200 rabbit anti-Lov (custom- made by Kuan laboratory) 16-24 hours in blocking buffer at room temperature. The larvae were washed 4 times with PBSTX for 60 minutes each and were incubated in 1:200 goat anti-rabbit(Fab)2 AlexaF568 16-24 hours in blocking buffer in dark at room temperature. The larvae were washed 3 times with PBSTX for 45 minutes each and were stored in 4% PFA in PBS in dark at 4℃.
2.6
rab6bb mutant generationrab6bb mutant was generated by CRISPR/Cas9 system. Cas9 protein was dissolved to 2000ng/μL in 20% glycerol and stored at -80℃ for stock. pCRII-(T7-
rabb6bb sgRNA) was modified from the plasmid containing guiding RNA sequence and 5’-CGTGACTTTGGGAACCCTTTA-3’ spacer that was purchased from Integrated Bio LTD. (Taiwan). rabb6bb sgRNA was generated from PCR product utilizing Ambion MEGAscript® T7 Transcription Kit (Ambion, AM1334). For microinjection, 150ng/μL Cas9 protein, 300ng/μL rabb6bb sgRNA and 0.005% phenol red were dissolved in DEPC treated H2O, and incubated 5 minute at room temperature before microinjection.
2.7 T7 Endonuclease I assay
PCR products that were generated with rab6bb 443bp or rab6bb 139bp primer pairs were purified with ethanol precipitation and resolved in 10μL 1.1X NEBuffer2 (New England BioLabs INC., B7002S). Steps of denaturation and reannealing were carried out as the description in NEB T7 Endonuclease I (New England BioLabs INC., M0302L) manual. 0.5μL T7 Endonuclease I was added in 4.5μL PCR product and incubated in 37℃ for 30 minutes. 1μL 250mM EDTA was added in the mixture to inactivate T7 Endonuclease I activity. DNA fragment was separated with 6% PAGE.
2.8 Restriction enzyme genotyping
PCR products of rasd1 293bp and rasd1 121bp were generated with above- mentioned primers. PCR product was purified with ethanol precipitation and resolved in 10μL ddH2O. 5μL cleaned PCR product was added 0.25μL Hpy188III (New England BioLabs INC., R0622S), 1μL 10XCutSmart® Buffer, and 3.75μL ddH2O, and incubated in 37℃ for 60 minutes. DNA fragment was separated with 6% PAGE.
2.9 Dexamethasone treatment
Dexamethasone (SIGMA, D2915-100MG) was dissolved to 2.5mM with sterile
double distilled water before use. About twenty of 1 to 4dpf zebrafish larvae were transfer into 2mL tube with 500μL embryo water. 100 to 20μL embryo water was removed (dependent on final dexamethasone concentration), and 100 to 20μL 2.5mM dexamethasone solution was added. Equal ddH2O was added as a control. All larvae were incubated at 28℃ within growth chamber. The larvae were fixed with 4% PFA in PBS after 3 hours dexamethasone treatment.
In withdrawing tests, the larvae were washed with fresh embryo water twice after 3 hours dexamethasone treatment. The larvae were also fixed with 4% PFA in PBS after dexamethasone withdrawal time.
Chapter 3 Result
3-1
rasd1 mutants do not show significant axon projection defect inhabenula
rasd1 is expressed in LHA in 3dpf and 4dpf larvae (Fig.2), and LHA neurons
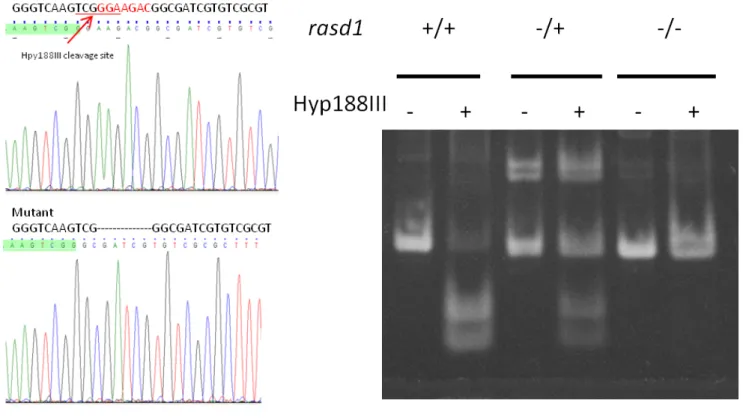
project their axons to dorsal IPN at 4dpf. To determine whether rasd1 is involved in LHA axon projection, rasd1 mutant was generated by CRISPR/Cas9 system. Mutant rasd1 carries 7-base deletion in exon1, and the deletion causes frame shift mutation
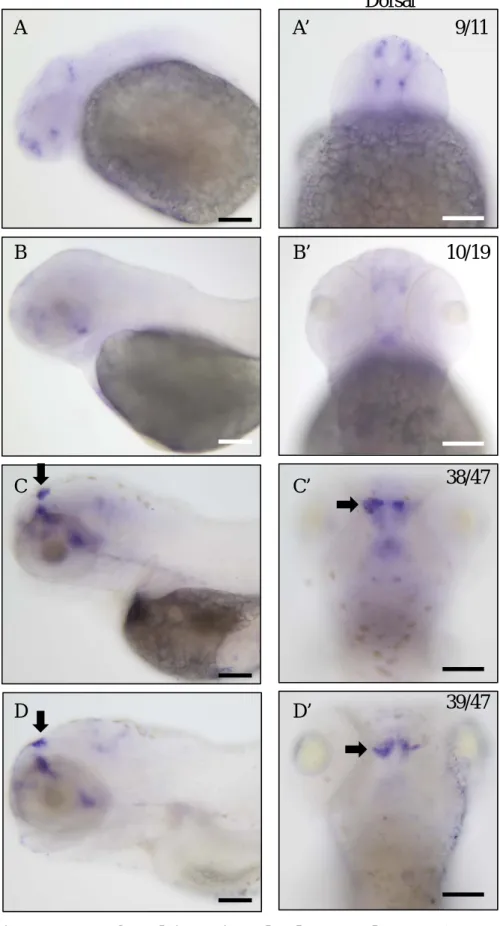
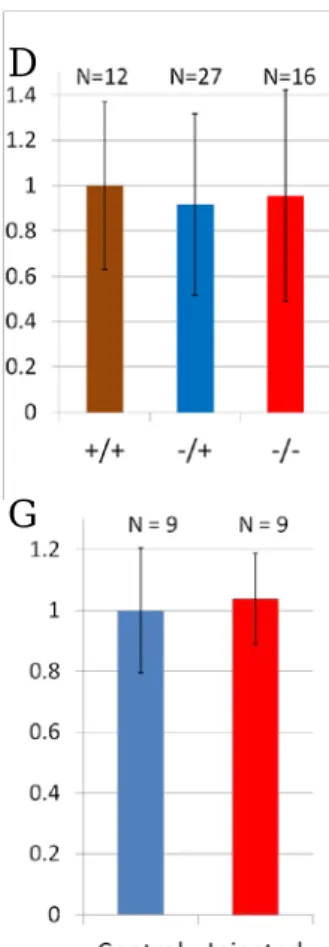
leading to an immature stop codon introduction (Fig.3). Anti-Leftover (Lov) staining that labels LHA neurons and their axons is used for detecting habenula LHA axon projection into the IPN in zerafish larval brain (Gamse et al., 2005). Anti-Lov staining shows that there are no significant difference in habenula axon projection between rasd1-/- larvae and their WT siblings (Fig.4, A-D).
To block rasd1 signal, we designed rasd1Δ181-265 that is assumed as a dominant negative form and injected it into zebrafish embryos (Fig.4-H). The larvae that are injected with rasd1Δ181-265 RNA still do not show significant difference between injected larvae and no-injection control (Fig.4, E-G).
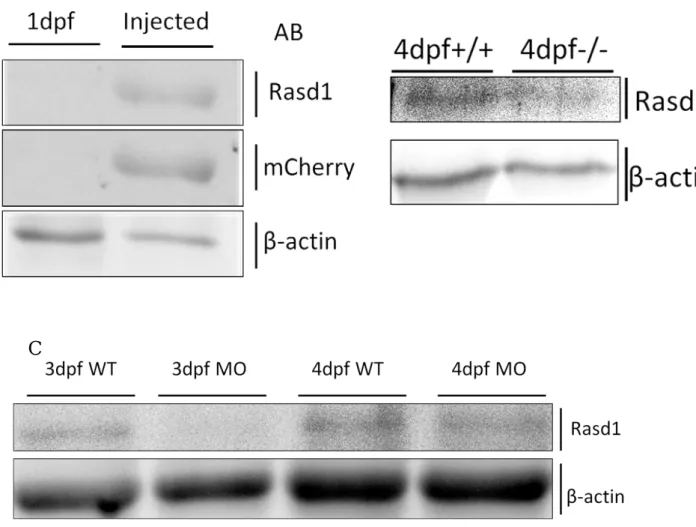
In Western blot analysis, I found the anti-Rasd1 antibodies generated in our laboratory could recognize over-expressed mCherry-tagged Rasd1, but it was hard to detect endogenous Rasd1 (Fig.5, A-B). Western blot result also indicated rasd1 MO is able to reduce Rasd1 expression in 3dpf larvae, and less effect in 4dpf larvae (Fig.5, C).
3-2 Ectopic rasd1 is induced in choroid plexus and hematocyte- like cells
Previous works show ectopic rasd1 expression is induced by DEX treatment in
Kemppainen and Behrend, 1998). To understand whether rasd1 expression in habenula is also induced by DEX treatment in zebrafish larvae, 3dpf larvae were treated by DEX.
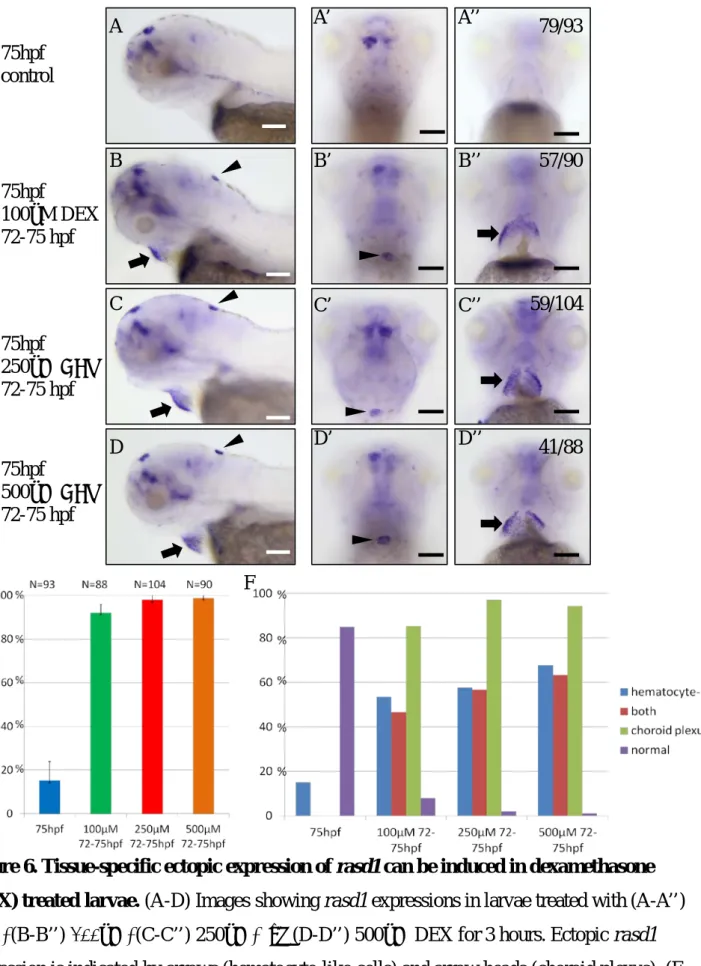
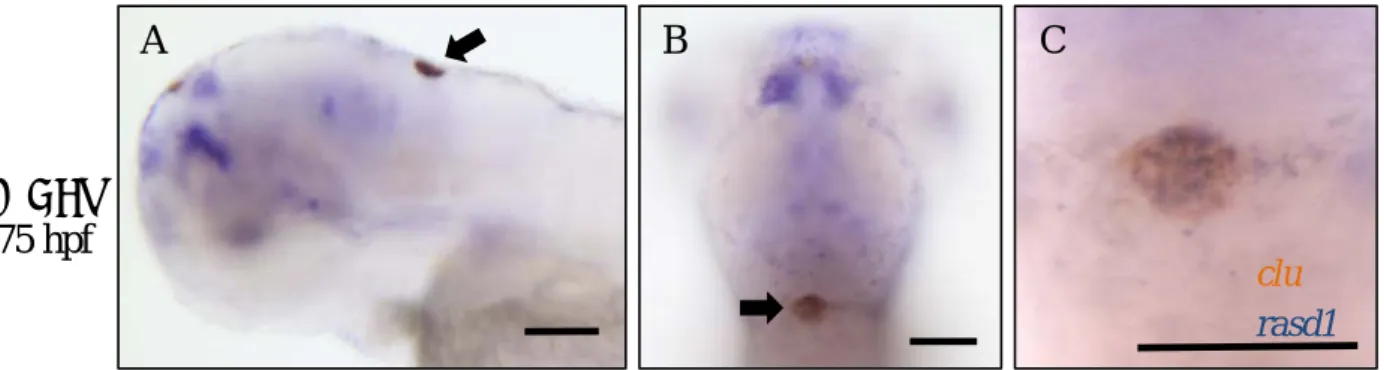
Ectopic rasd1 is not induced in HA, but in cells in hindbrain and cells surrounding the hatching gland region (Fig.6, A-D), and ectopic rasd1is induced in almost all of larvae by 100μM to 500μM DEX treatment. Comparing to control, ectopic rasd1 expression is significantly induced in choroid plexus (CP) and hematocyte-like cells by 100μM to 500μM DEX treatment (Fig.6-E). Two-color in situ hybridization of rasd1 and clusterin (clu) confirms the co-expression of rasd1 and clu in CP (Fig.7, A-C).P-value of ectopic rasd1 induction rate in the CP is 0.035 between 100μM and 250μM DEX treatment, and is 0.620 between 250μM, and 500μM DEX treatment (Fig.6-F). It suggests that 250μM and 500μM DEX treatment have similar efficiency to induce ectopic rasd1 expression in CP.
Ectopic rasd1 is only induced in the surface of larvae by DEX treatment. Because DEX may not pass through the epidermis efficiently, I injected DEX into ventricular zone. Ectopic rasd1 expression is induced by DEX injection in 3dpf larvae, and the regions of ectopic rasd1 expression are similar to it in 3dpf larvae that are soaked in DEX (Fig.7, D-E). The resembling expression pattern suggests that DEX is able to infiltrate the epidermis.
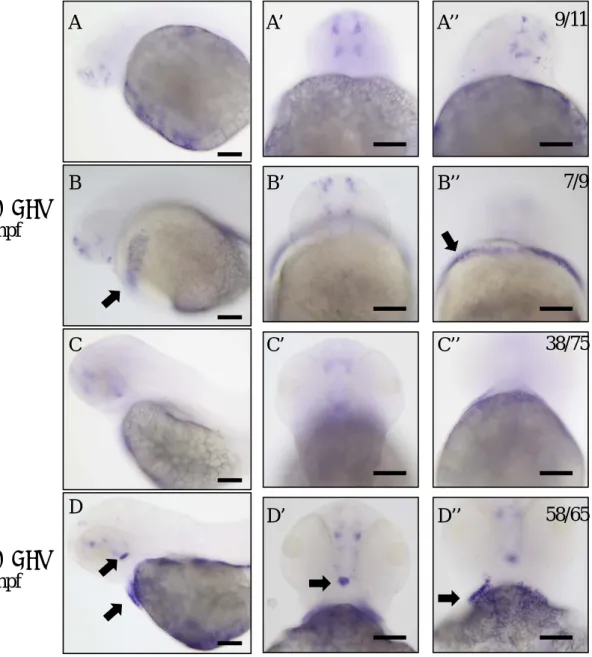
To prove whether rasd1 is induced in habenula in 1dpf and 2dpf larvae with DEX treatment, we treated 1dpf and 2dpf larvae with 250μM DEX. DEX treatment induces ectopic rasd1expression in hematocyte-like cells in 1dpf and 2dpf larvae, and does not induce rasd1 expression in habenula (Fig.8).
Ectopic rasd1 expression is induced in CP and hematocyte-like cells in 3dpf and 4dpf larvae, and is only induced in hematocyte-like cells in 1dpf embryos. Ectopic rasd1 expression in CP is only induced in 10% of 2dpf embryos. DEX treatment does
not induce rasd1 expression in habenula in 1dpf and 2dpf embryos, and does not influence rasd1 expression in habenula in 3dpf and 4dpf larvae. It suggests that rasd1 expression in habenula is regulated by genetic programing.
3-3 Efficiency of ectopic rasd1 expression induced by DEX is decreased gradually after DEX withdrawal
Effects of most of drugs decrease gradually after the treatment is terminated (Al Katheeri et al., 2004; Grady et al., 2010). To confirm whether ectopic rasd1 expression is induced by DEX treatment, we treated the larvae with 250μM DEX 3 hours and washed with fresh embryo water. Ectopic rasd1 expression significantly decreases in 1 hour withdrawal and less ectopic rasd1 expression is observed in 2 hours withdrawal (Fig.9). Finally, ectopic rasd1 expression resumes to almost normal level in 3 hours after DEX withdrawal.
3-4
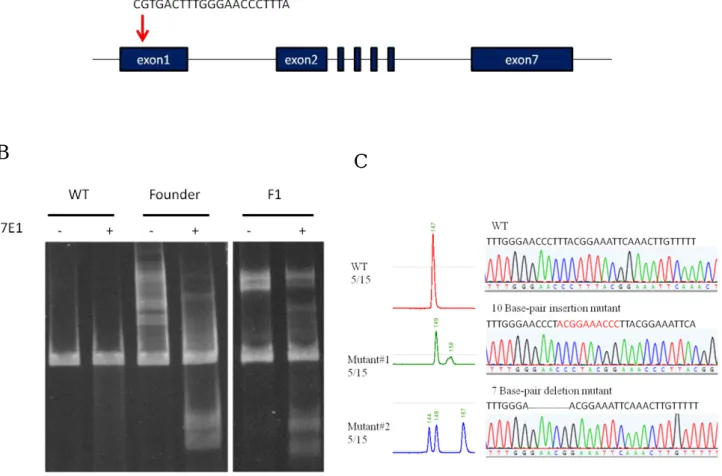
rab6bb mutants are generated by CRISPR/Cas9 systemTo study the role of rab6bb in LHA axonal targeting process, I stared to generate rab6bb mutant with CRISPR/Cas9 system. Diagram in Fig.10 indicates the sgRNA
target site in exon1 of rab6bb (Fig.10-A). The target site is 30 base-pair downstream to the 5' end of the open reading frame. rab6bb DNA fragments of the adult fishes that has been injected were cut by T7E1. T7E1 assay indicates that one of the F0 and its descendants carry mutated rab6bb alleles (Fig.10-B). Capillary electrophoresis and sequencing show that five F1 carry 10-base-pair mutation and another five F1 carry 7- base-pair dleltion in fifteen F1 (Fig.10-C). rab6bb mutant is going to be utilized to confirm the role of rab6bb in LHA axon guidance once the line is established.
Chapter 4 Discussions and Conclusions
In this Master thesis project, the function of zygotic rasd1 and the mRNA expression regulation of rasd1 have been investigated. Although results from previous studies in our laboratory demonstrated that rasd1 and nrp1a morphants show similar LHA axon projection defect, the axon projecting onto the IPN seemed to be no difference between the rasd1 knockout fish and their WT siblings. Because previous works show mutants and morphants of several genes exhibit different phenotypes (Kok et al., 2015; Rossi et al., 2015). The discrepancy of the mutants and morphants could be caused by off-target effect of rasd1 MOs, or genetic compensation. LC-MS/MS or DNA microarray assay should be applied to further understand the discrepancy between the rasd1 morphants and mutants.
To block Rasd1 function, I injected rasd1Δ181-265 RNA which shall express dominant negative (DN) Rasd1 in embryos (Guo and Fang, 2006; Suzuki et al., 2006).
The injected larvae and WT control do not show significant difference in LHA projection volume and morphology. The reasons why the LHA axon projection pattern was unaffected in the rasd1Δ181-265 injected larvae could be that the truncated Rasd1 is able to block the signal cascade via Rasd1 but Rasd1 does not play a role in LHA axon targeting process. Alternatively, the rasd1Δ181-265 mRNAs injection did not express the DN Rasd1 as I had expected.
Even though Western blot could not indicate whether Rasd1 remains in rasd1 mutants, if rasd1 is involved in LHA axon target finding, minimum variation in LHA projection pattern formation may be cause by two possibilities. First possibility: Rasd1 is still expressed in zebrafish because of gene duplication. Undefined duplicated rasd1 in zebrafish genome is expressed and complements mutant rasd1. Undefined paralog of
able to be disrupted by rasd1 MOs. Nevertheless, this is unlikely the cause because 99%
of the Danio rerio genome sequences has been verified (ZFIN.org). Second possibility:
MOs knock-down causes disruption of the Rasd1 protein function, but an unknown protein complements the function of mutated Rasd1. This unknown protein has similar function as Rasd1, or it plays downstream to Rasd1 pathway and is up-regulated, so that the requirement of Rasd1 is bypassed.
Rasd1 was first uncovered as a downstream response gene to DEX treatment. DEX treatment induces rasd1 expression in several types of mouse cells (Greenwood et al., 2016; Kemppainen and Behrend, 1998). In my DEX treating experiments, rasd1 expression in the larval habenula does not elevated in three different DEX treating conditions (100, 250, 500uM). DEX is a type of steroid medication which activates glucocorticoid receptor (Pandit et al., 2002). Under normal physiological conditions, glucocorticoid is released by stress stimulation (Popoli et al., 2012). It suggests glucocorticoid may increase in blood because of environmental change. rasd1 expression in habenula is not influenced by DEX treatment. This suggests that rasd1 expression in habenula is programmed by genetic algorithm. Interestingly, I found ectopic rasd1 expression is induced by DEX treatment in the larval CP and hematocyte- like cells. Double in situ hybridization of rasd1 and clusterin verified the ectopic rasd1 expression in the CP. CP is a major secretory tissue that produces cerebrospinal fluid (CSF) which protects neuron and cleans waste in the vertebrate brain (Lun et al., 2015).
It forms blood-CSF barrier (Lun et al., 2015) and plays a role in neurogenesis (Johansson, 2014). Previous studies also exhibit that stress enhances specific genes expression in CP (Martinho et al., 2012; Sathyanesan et al., 2012), but how CP influences CSF under stress is still unclear. DEX treatment induces ectopic rasd1 expression in CP suggesting that Rasd1 may play a role in the stress response process of
CP. Investigation of rasd1 in CP should help us to know how CP responses to stress.
Previous study exhibits DEX treatment induces ectopic rasd1 expression via glucocorticoid receptor (GR) and signal transducer and activator of transcription 5b (STAT5b) in rat (Lellis-Santos et al., 2012), but nr3c1 and stat5b is widely expressed in zebrafish larvae. DEX treatment only induces ectopic rasd1 expresion in CP and hematocyte-like cells in zebrafish. I propose three possibilities. First and second possibilities: because there are three GR isoforms and three STAT5b isoforms in zebrafish, rasd1 expression is induced by DEX only in two GR or STAT5b isoforms, or one of the GR or STAT5b isoforms functions as a dominate negative form and inhibits rasd1 expression in other tissue. Third possibility: tissue-specific transcription factor
and GR cooperatively enhance rasd1 expression in specific tissue under DEX treatment.
Rab6b is Rab6bb homology and is proposed to mediate the retrograde transport from Golgi to ER in human neuronal cells (Wanschers et al., 2007). Rab subfamily small GTPases localize on the surface of intracellular organelles and control membrane traffic (Brumell and Scidmore, 2007). If Rab6bb is involved in targeting process, mutated rab6bb will impact exocytosis or endocytosis. rab6ba is the paralog of rab6bb may be able to compensate mutated rab6bb. However, our pervious lab members found rab6ba is not expressed in LHA in 4dpf zebrafish larvae. The different expression regions of rab6bb and rab6ba should help us to understand the role of rab6bb in Nrp1a- mediated LHA axon path finding without severe whole body phenotype.
References
Aizawa, H., 2013. Habenula and the asymmetric development of the vertebrate brain.
Anat Sci Int 88, 1-9.
Al Katheeri, N.A., Wasfi, I.A., Lambert, M., Saeed, A., 2004. Pharmacokinetics and pharmacodynamics of dexamethasone after intravenous administration in camels:
effect of dose. Vet Res Commun 28, 525-542.
Amo, R., Aizawa, H., Takahoko, M., Kobayashi, M., Takahashi, R., Aoki, T., Okamoto, H., 2010. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci 30, 1566-1574.
Beretta, C.A., Dross, N., Guiterrez-Triana, J.A., Ryu, S., Carl, M., 2012. Habenula circuit development: past, present, and future. Front Neurosci 6, 51.
Bianco, I.H., Wilson, S.W., 2009. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci 364, 1005- 1020.
Brumell, J.H., Scidmore, M.A., 2007. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev 71, 636-652.
Cheah, J.H., Kim, S.F., Hester, L.D., Clancy, K.W., Patterson, S.E., Papadopoulos, V., Snyder, S.H., 2006. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 51, 431-440.
Chen, Y., Khan, R.S., Cwanger, A., Song, Y., Steenstra, C., Bang, S., Cheah, J.H., Dunaief, J., Shindler, K.S., Snyder, S.H., Kim, S.F., 2013. Dexras1, a Small GTPase, Is Required for Glutamate-NMDA Neurotoxicity. Journal of Neuroscience 33, 3582-3587.
Chou, M.Y., Amo, R., Kinoshita, M., Cherng, B.W., Shimazaki, H., Agetsuma, M.,
2016. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science 352, 87-90.
Colombo, A., Palma, K., Armijo, L., Mione, M., Signore, I.A., Morales, C., Guerrero, N., Meynard, M.M., Perez, R., Suazo, J., Marcelain, K., Briones, L., Haartel, S., Wilson, S.W., Concha, M.L., 2013. Daam1a mediates asymmetric habenular morphogenesis by regulating dendritic and axonal outgrowth. Development 140, 3997-4007.
Fakhoury, M., López, S.D., 2014. The Role of Habenula in Motivation and Reward.
Advances in Neuroscience Volume 2014, 6.
Gamse, J.T., Kuan, Y.S., Macurak, M., Brosamle, C., Thisse, B., Thisse, C., Halpern, M.E., 2005. Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development 132, 4869-4881.
Grady, J.A., Davis, E.G., Kukanich, B., Sherck, A.B., 2010. Pharmacokinetics and pharmacodynamics of dexamethasone after oral administration in apparently healthy horses. Am J Vet Res 71, 831-839.
Graham, T.E., Qiao, Z., Dorin, R.I., 2004. Dexras1 inhibits adenylyl cyclase. Biochem Biophys Res Commun 316, 307-312.
Greenwood, M.P., Greenwood, M., Mecawi, A.S., Antunes-Rodrigues, J., Paton, J.F., Murphy, D., 2016. Rasd1, a small G protein with a big role in the hypothalamic response to neuronal activation. Mol Brain 9, 1.
Guo, W., Fang, B.L., 2006. Dominant negative Ras as an anticancer agent. Cancer Biol Ther 5, 1492-1493.
Hikosaka, O., 2010a. The habenula: from stress evasion to value-based decision-making.
Nat Rev Neurosci 11, 503-513.
Hikosaka, O., 2010b. The habenula: from stress evasion to value-based decision-making.
Nature Reviews Neuroscience 11, 503-513.
Jesuthasan, S., 2012. Fear, anxiety, and control in the zebrafish. Dev Neurobiol 72, 395- 403.
Jiao, S., Dai, W., Lu, L., Liu, Y.Z., Zhou, J.F., Li, Y., Korzh, V., Duan, C.M., 2011. The Conserved Clusterin Gene Is Expressed in the Developing Choroid Plexus Under the Regulation of Notch But Not IGF Signaling in Zebrafish. Endocrinology 152, 1860-1871.
Johansson, P.A., 2014. The choroid plexuses and their impact on developmental neurogenesis. Front Neurosci-Switz 8.
Kandel, E.R., 2012. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain 5, 14.
Kemppainen, R.J., Behrend, E.N., 1998. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem 273, 3129-3131.
Kok, F.O., Shin, M., Ni, C.W., Gupta, A., Grosse, A.S., van Impel, A., Kirchmaier, B.C., Peterson-Maduro, J., Kourkoulis, G., Male, I., DeSantis, D.F., Sheppard-Tindell, S., Ebarasi, L., Betsholtz, C., Schulte-Merker, S., Wolfe, S.A., Lawson, N.D., 2015. Reverse genetic screening reveals poor correlation between morpholino- induced and mutant phenotypes in zebrafish. Dev Cell 32, 97-108.
Kuan, Y.S., Yu, H.H., Moens, C.B., Halpern, M.E., 2007. Neuropilin asymmetry mediates a left-right difference in habenular connectivity. Development 134, 857- 865.
Lee, A., Mathuru, A.S., Teh, C., Kibat, C., Korzh, V., Penney, T.B., Jesuthasan, S., 2010.
The habenula prevents helpless behavior in larval zebrafish. Curr Biol 20, 2211- 2216.
Lellis-Santos, C., Sakamoto, L.H., Bromati, C.R., Nogueira, T.C., Leite, A.R.,
Yamanaka, T.S., Kinote, A., Anhe, G.F., Bordin, S., 2012. The regulation of Rasd1 expression by glucocorticoids and prolactin controls peripartum maternal insulin secretion. Endocrinology 153, 3668-3678.
Liu, Y., Berndt, J., Su, F., Tawarayama, H., Shoji, W., Kuwada, J.Y., Halloran, M.C., 2004. Semaphorin3D guides retinal axons along the dorsoventral axis of the tectum. J Neurosci 24, 310-318.
Lun, M.P., Monuki, E.S., Lehtinen, M.K., 2015. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci 16, 445-457.
Martinho, A., Goncalves, I., Costa, M., Santos, C.R., 2012. Stress and glucocorticoids increase transthyretin expression in rat choroid plexus via mineralocorticoid and glucocorticoid receptors. J Mol Neurosci 48, 1-13.
Pandit, S., Geissler, W., Harris, G., Sitlani, A., 2002. Allosteric effects of dexamethasone and RU486 on glucocorticoid receptor-DNA interactions. J Biol Chem 277, 1538-1543.
Pasterkamp, R.J., 2012. Getting neural circuits into shape with semaphorins. Nature Reviews Neuroscience 13, 605-618.
Popoli, M., Yan, Z., McEwen, B.S., Sanacora, G., 2012. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13, 22-37.
Rossi, A., Kontarakis, Z., Gerri, C., Nolte, H., Holper, S., Kruger, M., Stainier, D.Y., 2015. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230-233.
Sathyanesan, M., Girgenti, M.J., Banasr, M., Stone, K., Bruce, C., Guilchicek, E., Wilczak-Havill, K., Nairn, A., Williams, K., Sass, S., Duman, J.G., Newton, S.S., 2012. A molecular characterization of the choroid plexus and stress-induced gene
regulation. Transl Psychiatry 2, e139.
Shelly, M., Cancedda, L., Lim, B.K., Popescu, A.T., Cheng, P.L., Gao, H., Poo, M.M., 2011. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron 71, 433-446.
Shelton, L., Becerra, L., Borsook, D., 2012. Unmasking the mysteries of the habenula in pain and analgesia. Prog Neurobiol 96, 208-219.
Simonini, P.D.R., Breiling, A., Gupta, N., Malekpour, M., Youns, M., Omranipour, R., Malekpour, F., Volinia, S., Croce, C.M., Najmabadi, H., Diederichs, S., Sahin, O., Mayer, D., Lyko, F., Hoheisel, J.D., Riazalhosseini, Y., 2010. Epigenetically Deregulated microRNA-375 Is Involved in a Positive Feedback Loop with Estrogen Receptor alpha in Breast Cancer Cells. Cancer Res 70, 9175-9184.
Song, H., Ming, G., He, Z., Lehmann, M., McKerracher, L., Tessier-Lavigne, M., Poo, M., 1998. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281, 1515-1518.
Stenmark, H., 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Bio 10, 513-525.
Suzuki, H., Kuzumaki, S., Nakagawa, K., Takimoto, M., Shichinohe, T., 2006.
Suppressive effect of modified dominant negative RAS mutant on human cancer by gene transfer with non-viral vector. Cancer Biol Ther 5, 1487-1491.
Taylor, R.W., Hsieh, Y.W., Gamse, J.T., Chuang, C.F., 2010. Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development. Development 137, 681-691.
Tojima, T., Kamiguchi, H., 2015. Exocytic and endocytic membrane trafficking in axon development. Dev Growth Differ 57, 291-304.
Wanschers, B.F., van de Vorstenbosch, R., Schlager, M.A., Splinter, D., Akhmanova, A.,
Hoogenraad, C.C., Wieringa, B., Fransen, J.A., 2007. A role for the Rab6B Bicaudal-D1 interaction in retrograde transport in neuronal cells. Exp Cell Res 313, 3408-3420.
Wolman, M.A., Liu, Y., Tawarayama, H., Shoji, W., Halloran, M.C., 2004. Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J Neurosci 24, 8428-8435.
Wu, B.T., Wen, S.H., Hwang, S.P.L., Huang, C.J., Kuan, Y.S., 2015. Control of Wnt5b secretion by Wntless modulates chondrogenic cell proliferation through fine- tuning fgf3 expression. J Cell Sci 128, 2328-2339.
Zerial, M., McBride, H., 2001. Rab proteins as membrane organizers. Nat Rev Mol Cell Bio 2, 107-117.
Figures
A B C
Figure 1. Rasd1 function and mRNA expression pattern. (A) Rasd1 mediates ferrous homeostasis in mouse cortical neurons. (Figure is modified from Cheah, Kim et al. 2006) (B) Rasd1 inhibits ESR1 expression in human breast cancer cells (Figure is modified form Wu, Liu et al. 2011) (C) Rasd1 regulates CREB phosphorylation via control PKA activity in Att-20 cells.
(Figure is modified form Greenwood, Greenwood et al. 2016) (D-F) rasd1 (in D) and rab6bb (in E) is coexpressed with nrp1a (in F) in 4dpf larval habenula. Arrows indicate the location of habenula. All images are dorsal views.
D E F
rasd1 rab6bb nrp1a
Figure 2. Expression patterns of rasd1 in various developmental stages. (A-D) Images showing mRNA staining of rasd1 in (A and A’) 24hpf, (B and B’) 48hpf, (C and C’)72hpf, and (D and D’) 96hpf. rasd1 expression level is significant increase in habenula and telencephalon from 72hpf to 96hpf. Arrows indicate the location of habenula. (A-D): Lateral views. (A’-D’):
Dorsal views. Scale bar:100μm.
24hpf
48hpf
72hpf
Lateral Dorsal
96hpf
A
B
C
D
B’
A’
C’
D’
9/11
10/19
38/47
39/47
Figure 3. CRISPR-Cas9-mediated rasd1 knockout line generation. (A) Sequencing result indicates a 7 base-pair deletion in exon1. This 7 base-pair deletion causes Hpy188III cleavage site loss and immature stop mutation. (B) Restriction enzyme test confirms Hpy188III cleavage site loss. DNA fragments are separated in 6% PAGE. (C) The schematic diagram
demonstrating the truncated Rasd1 predicted to be expressed in the rasd1 mutants.
A B
C
Figure 4. Loss- and gain-of-Rasd1 functional analyses. (A-C) Images showing anti-Lov antibody staining in the IPN. WT (in A), rasd1 heterozygous and homozygous mutant (in B and C) do not show significant difference in IPN projection morphology. (D) IPN projection
volumes from images in (A-C). (E-F) Images showing IPN of rasd1Δ181-265RNA injected larvae.
(G) Quantitative result of the IPN from (E and F) showing no significant difference in their morphology. (H) The diagram of Rasd1Δ181-265, truncated Rasd1 remains 180 amino-acid and C- terminal has been deleted. Scale bar:10μm.
A B C
D
E F
G
H
Lov
Lov
Figure 5. Rasd1 antibodies can detect overexpressed Rasd1. (A) Western blot result from Rasd1-mCherry RNA-injected embryo lysates indicates that anti-mCherry labeled band can be detected by anti-Rasd1 produced in our laboratory. The anti-b-actin was used as loading control.
(B) Western blot result from 4dpf total larval lysates indicated the endogenous Rasd1 level is hardly detected by our anti-Rasd1 antibodies. (C) Western blot result indicated that knocking down rasd1 expression by antisense morpholino (MO) could reduce Rasd1 expression in 3dpf injected larvae, and it showed less effect in 4dpf.
A B
C
75hpf control
Lateral Dorsal Ventral
75hpf
100μM DEX 72-75 hpf
75hpf
250μM DEX 72-75 hpf
75hpf
500μM DEX 72-75 hpf
Figure 6. Tissue-specific ectopic expression of rasd1 can be induced in dexamethasone (DEX) treated larvae. (A-D) Images showing rasd1 expressions in larvae treated with (A-A’’) 0μM, (B-B’’) 100μM, (C-C’’) 250μM, and (D-D’’) 500μM DEX for 3 hours. Ectopic rasd1 expression is indicated by arrows (hematocyte-like cells) and arrow heads (choroid plexus). (E- F) Statistical results showing the percentages of rasd1 ectopic expression induction rate (E), and distribution of expression regions (F). (A-D): Lateral views. (A’-D’): Dorsal views. (A’’- D’’): Ventral views. Scale bar:100μm.
E F
A
B
C
D
A’ A’’
B’ B’’
C’ C’’
D’ D’’
79/93
57/90
59/104
41/88
Figure 7. Dexamethasone (DEX) treatment induced ectopic rasd1 expression in the
choroid plexus. (A-C) Images showing double mRNA in situ hybridization results of rasd1 and clusterin (clu) expressions in the DEX treated 75hpf larvae. Arrows indicate the CP. (D-D’’) PBS-injected larvae or (E-E’’) DEX-injected larvae. (A, D-E): Lateral views. (B, D’-E’):
Dorsal views. (D’’-E’’): Ventral views. (C): Enlarged dorsal view. Scale bar:100μm.
clu rasd1
A B C
Lateral Dorsal
75hpf
250μM DEX at 72-75 hpf
Enlarged
75hpf
PBS injected at 72 hpf
75hpf
25 pmole DEX injected at 72 hpf
Lateral Dorsal Ventral
D’ D’’
E’ E’’
D
E
6/14
23/31
Figure 8. Effects of dexamethasone (DEX) treatment in earlier embryonic stages. (A-D) rasd1 expression in 27hpf (A-A’’) control or DEX treated (B-B’’) embryos, or in 51hpf (C-C’’) control or DEX treated (D-D’’) larvae incubated in 250μM DEX for 3hrs. Ectopic rasd1
expression is indicated by arrows. (A-D): Lateral views. (A’-D’): Dorsal views. (A’’-D’’):
Ventral views. Scale bar:100μm.
Lateral Dorsal Ventral
27hpf
27hpf
250μM DEX 24-27hpf
51hpf
51hpf
250μM DEX 48-51hpf
A A’ A’’
B
C
D
B’ B’’
C’ C’’
D’ D’’
9/11
7/9
38/75
58/65
Figure 9. Ectopic rasd1 expression diminished after DEX withdraw. (A-E) Images showing rasd1 expression in 75hpf control (A-A’’), or 75-78hpf with different treating conditions
indicated in the left column. Ectopic rasd1 expression is indicated by arrows (hematocyte-like cells) and arrow heads (choroid plexus). (F) Statistical chart showing the percentage of ectopic rasd1 expression induction rates. Ectopic rasd1 expression induction rate decreases gradually after the treatment is terminated. (A-E): Lateral views. (A’-E’): Dorsal views. (A’’-E’’): Ventral views. Scale bar:100μm.
75hpf control
Lateral Dorsal Ventral
75hpf
250μM DEX 72-75hpf
76hpf
250μM DEX 72-75hpf 1hour rest
77hpf
250μM DEX 72-75hpf 2hour rest
78hpf
250μM DEX 72-75hpf 3hour rest
F
96/110
67/133
44/114
9/100
3/107
A A’ A’’
B B’ B’’
C C’ C’’
D D’ D’’
E E’ E’’
Figure 10. CRISPR-Cas9-mediated rab6bb knockout line generation. (A) The diagram of rab6bb locus in zebrafish genome. CRISPR/Cas9 target site is indicated by arrow. (B) rab6bb PCR products contain target site are cut by T7E1. DNA fragments are separated in 6% PAGE.
(C) Snapshop showing the sequencing results from the F1 embryos produced by the F0 fish.
B
A
C