1780
Acta Cryst. (1984). C40, 1780-1781
Reinvestigation of the Structure of Potassium Pyrosulfite,
K 2 8 2 0 5BY I - C H I A C H E N AND Y U W A N G *
Department of Chemistry, National Taiwan University, Taipei, Taiwan
( R e c e i v e d 31 M a r c h 1984; accepted 17 July 1984) Abstract. M r -- 444.63, monoclinic, P21/m, a = 6.921 (1), b = 6.160 (1), c = 7.537 (1)A, f l = 102.79(1) °, V = 3 1 3 . 3 3 A 3, Z = 2 , D m = 2 . 3 6 , D x = 2.356 g cm -3, 2(Mo Kct) = 0.7093/k, /~ = 20.94 cm -~, F ( 0 0 0 ) = 188, T = 298 K, R = 0.040 for 780 observed reflections. The molecule contains a plane symmetry ( S - S - O ) and a long S - S bond [2.2194(9)/k] between the thionite and thionate groups. The S - O distances are 1.4870 (8),/~ in the thionite group and 1.453 (1) and 1.4602 (8),/~ in the thionate group. A comparison with other compounds containing the $205 z- ion is made.
Introduction. The structure of the title compound has been reported by Lindqvist & M6rtsell (1957) using film data. They showed that the molecule has an unusual- ly long S - S bond [2.209 (7),/~1. We are particularly interested in different types of S - S bond. In order to obtain more accurate parameters we have rein- vestigated the structure with diffractometer data.
Experimental. Crystal 0.13 × 0.13 x 0.3 mm, CAD-4 diffractometer, unit cell: 17 reflections, 20 range 40 to 50 °. D m by flotation (CHCI3/CHBr3). No absorption correction. 20max=60 ° ( - 9 < h < 9 , k < 8 , l < 1 0 ) . Three standard reflections monitored every 0 . 5 h ; variation 2%. 992 unique reflections, 780 observed with I _> o(/). Atoms refined anisotropically, function minimized Y.W(Fo--Fc)2; R = 0 . 0 4 0 , R w = 0 . 0 2 3 , S = 1.54; weighting scheme from counting statistics.
(A/a)max=O.07. Peaks on final Ap map +0.5 to
- 0 . 6 e ,~-3. Atomic scattering factors from
International Tables for X-ray Crystallography (1974). Programs from N R C C SDP PDP-11 package (Gabe & Lee, 1981).
Discussion. The atomic coordinates and equivalent isotropic temperature factors are listed in Table 1.t Our data confirm the previous work (Lindqvist & M6rtsell,
* To whom correspondence should be addressed.
"I" Lists of anisotropic thermal parameters and structure factors have been deposited with the British Library Lending Division as Supplementary Publication No. SUP 39625 (I0 pp.). Copies may be obtained through The Executive Secretary, International Union of Crystallography, 5 Abbey Square, Chester CHI 2HU, England.
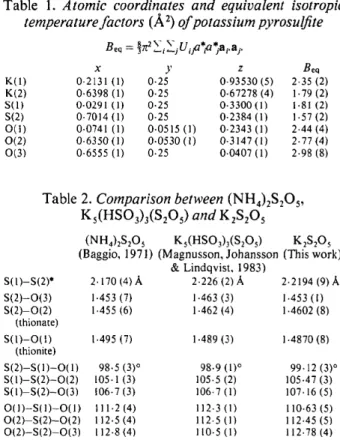
Table 1. Atomic coordinates and equivalent isotropic temperature factors (,~2) of potassium pyrosulfite
Beq = ~ ~i,....jt.J ifl.~ t jai.a j.
x y z Beq K(I) 0.2131 (1) 0.25 0.93530 (5) 2.35 (2) K(2) 0.6398 (1) 0.25 0-67278 (4) 1.79 (2) S(I) 0.0291 (1) 0.25 0"3300 (1) 1-81 (2) S(2) 0"7014 (1) 0"25 0"2384 (1) 1.57 (2) 0(1) 0.0741 (1) 0"0515 (1) 0.2343 (1) 2.44 (4) 0(2) 0"6350(1) 0.0530(1) 0"3147(1) 2.77(4) 0(3) 0"6555 (1) 0.25 0.0407 (1) 2"98 (8)
Table 2. Comparison between (NH4)2S205, Ks(HSO3)3(S2Os) and KzS205 S(1)-S(2)* S(2)-O(3) S(2)-0(2) (thionate) s(1)-o(i) (thionite) S(2)-S( I ) - 0 ( I ) S(I)-S(2)-0(2) S(I)-S(2)-0(3) O(1)-S(1)-O(1) 0 ( 2 ) - S ( 2 ) - 0 ( 2 ) 0(2)-S(2)-0(3) * Atoms (NH4)2S205 Ks(HSO3)3(S2Os) K2S205 (Baggio, 1971) (Magnusson, Johansson (This work)
& Lindqvist, 1983) 2.170 (4) ./~ 2.226 (2)/~ 2.2194 (9)/~, 1.453 (7) 1.463 (3) 1.453 (1) 1.455 (6) 1.462 (4) 1.4602 (8) 1.495 (7) 1.489 (3) 1.4870 (8) 98.5 (3) ° 98.9 (1) ° 99.12 (3) ° 105.1 (3) 105.5 (2) 105.47 (3) 106.7 (3) 106.7 (1) 107.16 (5) I 11.2 (4) 112.3 (1) 110.63 (5) 112.5 (4) 112.5 (1) 112.45 (5) 112.8 (4) 110.5 (1) 112.78 (4)
labeled according to this work.
1957), but the S - S bond is slightly longer. The S - S value of 2.2194 (9),/~ is significantly longer than the S - S single bond (2.046-2.052 A) (Coppens, Yang, Blessing, Cooper & Larsen, 1977), but slightly shorter than that in ZnS204.py [2.386 (2) A] (Kiers & Vos, 1978). The bond length of S - O in the thionite group is 1.4870 (8)/~ which is longer than the cor- responding values in the thionate group 1.4602 (8), 1.453(1)/k. A comparison of Ks(HSO3)3(S2Os), (NH4)2S205 and K2SzO 5 is given in Table 2.
The authors would like to express their appreciation for the support of this work from the National Science Council for both the research grant and the use of the CAD-4 diffractometer.
I-CHIA C H E N A N D YU W A N G 1781
References
BAGGIO, S. (1971). Acta Cryst. B27, 517-522.
COPPENS, P., YANG, Y. W., BLESSING, R. H., COOPER, W. F. & LARSEN, F. K. (1977). J. Am. Chem. Soc. 99, 760-766. GABE, E.J. & LEE, F. L. (1981).Acta Cryst. A37, C339.
International Tables for X-ray Crystallography (1974). Vol. IV.
Birmingham: Kynoch Press.
KIERS, C. T. & VOS, A. (1978). Acta Cryst. B34, 1499-1504. LINDQVIST, I. & MORTSELL, M. (1957). Acta Cryst. 10, 406-409.
MAGNUSSON, A., JOHANSSON, L. & LINDQVIST, O. (1983). Acta Cryst. C39, 819-822.
Acta Cryst. (1984). C40, 1781-1785
Structure, Deformation Density and Atomic Charges in Potassium Hydrogenperoxo-
monosulfate Monohydrate, KHSOs.H20
BY E. O.
SCHLEMPER,
R. C. THOMPSON, C. KAY FAIR AND F. K. RossDepartment of Chemistry, University of Missouri, Columbia, MO 6 5 211, USA
AND E. H. APPELMAN AND L. J. BASILE
Department of Chemistry, Argonne National Laboratory, Argonne, IL, USA
(Received 9 January 1984; accepted 20 July 1984)
Abstract. M r = 170.2, monoclinic, C2/c, a =
18.226 (3), b - - 7.662 (1), c = 7.420 (5)A, f l = 90.83 (2) °, V = 1036.1 (8) A 3, Z = 8, D x =
2.182 (1)g cm -3 , Mo Kct, ,I,=0.7107,/t, / t = 13.5 cm -1, F(000) = 608, T = 130 (2) K, R = 0.023, 4144 independent reflections. Sulfur has tetrahedral coordination with the average sulfate-type S - O dis- tance of 1.445 (5)A, the peroxo S - O distance of 1.634 (1) A, the peroxo O - O distance of 1.463 (1) A and the S - O - O - H torsion angle of 87 (1) °. Electron deformation density maps reveal the expected bonding features and lone-pair density. Atomic charge cal- culations are presented and compared with theoretical calculations for $20 2-.
Introduetlon. Although potassium hydrogenperoxo-
monosulfate is sold in an impure form commercially, pure salts of peroxomonosulfate have not been pre- viously available. We have developed a procedure involving hydrolysis of K2S208 that affords highly pure KHSOs.H20. This product yielded crystals suitable for the first determination of the structure of the HSO~- ion. An earlier X-ray study (Kyrki & Lappalainen, 1965) reported an orthorhombic unit cell but did not include a structure determination.
Because of the difference in chemistry of oxidation- reduction in comparison with H202 [i.e. a tendency for peroxide-bond breakage in HSO~- upon oxidation (Thompson, 1981)], we were interested not only in comparison of the O - O distance with that in $20 2- and in H20 2 but also a comparison of deformation density with that observed for H20 2 (Savariault & Lehmann, 1980), for SO 2- (Kirfel & Will, 1980, 1981), and for $2032- (Elerman, Bats & Fuess, 1983).
0108-2701/84/I 11781-05501.50
Experimental. Concentrated solution of sodium peroxo-
disulfate was converted to the acid by passage through a column of Dowex 50X8 cation-exchange resin in the hydrogen form. The solution was concentrated in a rotary evaporator and hydrolyzed by heating at 323 K until no peroxodisulfate was detected. The solution was then neutralized to pH 3.5 by addition of K H C O 3 in an ice bath, filtered and freeze-dried. The residue was redissolved in a small quantity of water at room temperature and filtered. The filtrate was chilled in an ice bath and the crystals that formed were filtered, washed with a small quantity of absolute ethanol and dried between sheets of filter paper. Iodometric analysis indicated a bulk composition approximating KHSOs.- H20. Plate-like crystal; ~0.07 x 0.23 x 0.27 mm; Enraf-Nonius CAD-4 diffractometer; T maintained by constant flow of N 2 gas; 24 reflections, 0 = 9-15 °, used for cell dimensions; empirical ~,-scan absorption (range of transmission = 0 . 8 3 - 1 . 0 0 , average = 0.95); graphite monochromator; max. 2 0 = 89°; hkl and hkl
( 2 0 = 3-89°), hkl and hkl (20 = 2-27.5°); 3 standard reflections every 3600 s (~ 1% variation, no correction); 5842 reflections measured; 0--20 scan method; scan speed to obtain 2% statistics to a maximum scan time of 120 s; 4144 unique; R l n t = 0 . 0 2 4 (measures agree- ment of the lower-angle data); 933 reflections with
Fo 2 < 2.0tr(Fo 2) considered unobserved; structure solved with M U L T A N (Germain, Main & Woolfson, 1971) and difference Fourier syntheses; refinement minimized
~ w ( I F o l - IFcl)2; 85 variables (all positional parameters and anisotropic temperature factors except isotropic for hydrogen atoms); R = 0 . 0 2 3 ; w R =
0.030; S = 1.21; w -1 = [O.counting2 + (O.03Fo2)2]/4Fo2;
max. A/tr in last cycle = 0.20; max. difference density © 1984 International Union of Crystallography