行政院國家科學委員會專題研究計畫 成果報告
精神分裂症患者血中脯胺酸濃度與脯胺酸脫氫脢基因型的
相關研究
計畫類別: 個別型計畫 計畫編號: NSC92-2314-B-002-292- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學醫學院精神科 計畫主持人: 劉智民 共同主持人: 胡海國 計畫參與人員: 劉玉麗, 劉絮愷, 黃宗正, 謝明憲 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 11 月 23 日
中英文摘要:
關鍵詞: 精神分裂症, 脯胺酸去氫脢基因, 脯胺酸, 定基因型, 高壓液相色層分 析 精神分裂症是一重大慢性的神經精神疾病,其病因目前仍不清楚,但確定 有明顯的遺傳因素。全基因體掃描發現在第 22 對染色體長臂 11-12 區域 (22q11-12) 與精神分裂症有連鎖證據,過去本研究團隊在台灣的精神分裂病人樣 本也發現類似的連鎖證據。而在第 22 對染色體長臂 11 區域有微小刪除 (22q11 microdeletion)的病人身上,有 25%-31%符合 DSM-IV 的精神分裂症診斷標準, 約為正常人的 20-30 倍。而精神分裂症病人約有 2%帶有 22q11 微小刪除的異常, 約為正常人的 80 倍。脯胺酸去氫脢基因 (proline dehydrogenase gene; PRODH) 位 於第 22 對染色體長臂 11 區微小刪除區域的近絲端 1.5 百萬鹼基對中,是精神分 裂症表現於 22q11 微小刪除染色體異常的重要區域。最近 Liu 等人的研究(2002) 顯示脯胺酸去氫脢基因中數個單核甘酸變異 (single nucleotide polymorphism)組 成的單套基因型 (haplotype) 在精神分裂症的家族中有顯著的連鎖不平衡 (linkage dysequibrium) 的證據,本研究團隊以台灣的精神分裂症病人與對照組的 初步研究結果也顯示類似的結果。最近 Jacquet 等人的研究(2002)顯示某些帶有 此基因 exon 特定變異的精神分裂症病人,其血中的脯胺酸濃度顯著高於正常濃 度,但其樣本數較少,且只限於少數罕見的 exon 變異。至於血中脯胺酸濃度與 精神分裂症相關的脯胺酸去氫脢基因的特定基因型是否有相關性,則有待探討。 本研究收集了 100 位精神分裂症病人及 112 位正常對照的 DNA 及血漿,以 限制脢片段長度變異 (restriction fragment length polymorphism, RFLP)及直接定 序(direct sequencing)的方法定出此基因 4 個單核甘酸變異及 1 個 insertion/deletion 變異,同時以高壓液相色層分析 (high performance liquid chromatography, HPLC) 的方法測量個案血中脯胺酸的濃度。結果顯示(1)在精神分裂症病人與正常對照 組間,其血中脯胺酸的濃度沒有顯著差異;(2)脯胺酸脫氫脢基因的 5 個變異中, DGCR6 insertion/deletion, 及 PRODH757 這兩個變異在精神分裂症病人與正常對 照組間有顯著的差異;(3)血中脯胺酸的濃度與此基因的五個變異無關;(4)病人 組的持續性注意力(continuous performance task) 與 PRODH2026 及 PRODH1766 有顯著相關。Keywords: Schizophrenia, proline dehydrogenase gene (PRODH), proline, genotyping, high performance liquid chromatography (HPLC)
Schizophrenia is a major and chronic neuropsychiatric disorder. The etiology is still unknown. The genetic factor plays an important role in the pathogenesis. Previous genome-wide scan studies revealed suggestive linkage evidence of schizophrenia to chromosome 22q11-12. Our research team also replicated this finding in Taiwanese schizophrenic families. About 25-31% of the patients with 22q microdeletion met DSM-IV diagnostic criteria for schizophrenia, 20-30 times higher prevalence than normal population. About 2% of the schizophrenic patients had the 22q11 microdeletion abnormality, 80 times higher than normal population. Therefore, the 22q11 region may harbor genes of susceptibility for schizophrenia. Proline dehydrogenase gene (PRODH) is located in the proximal 1.5 Mb of the 22q11 deletion region, a critical region for the expression of schizophrenic phenotype in this syndrome. Liu et al. (2002) revealed a specific haplotype determined by several single neucleotide polymorphisms (SNPs) of PRODH was significantly preferentially transmitted to the schizophrenic descendants using the parent-offspring trios sample. The preliminary result of our research team using Taiwanese schizophrenic case-control sample also revealed positive results with this gene. Jacquet et al (2002) revealed the plasma proline level of some schizophrenic patients with specific exon mutations of PRODH was significantly higher than normal. The result is limited due to small sample size and rare exon mutations. Whether the plasma proline level correlates with the specific polymorphism of PRODH or not remained to be determined.
We collected the DNA and plasma of 100 schizophrenic patients and 112 normal controls this year. We genotyped four SNPs and 1 insertion/deletion polymorphism over the PRODH region reported to be positive results in our preliminary result using the methods of restriction fragment length polymorphism (RFLP) and direct sequencing. The plasma proline level was measured by high performance liquid chromatography (HPLC).
Our results showed (1) there was no significant difference in the plasma proline level between schizophrenic patients and normal controls; (2) there were significant difference in genotype and allele distribution in two polymorphisms (DGCRinsertion/deletion, PRODH757) between the two groups (3) there was no significant difference in proline level among difference genotypes of the PRODH polymorphisms; (4) there were significant differences in the continuous performance task (CPT) among the different genotypes of PRODH1945 and PRODH1766 in the schizophrenic patients.
研究目的
The specific aims of this project are (1) to prove the significant difference of specific polymorphisms of PRODH between schizophrenic patients and normal controls; (2) to explore the difference of proline plasma level between schizophrenic patients and normal controls; (3) to clarify the correlation between the plasma proline level and the specific polymorphisms of PRODH.
文獻探討
A few genome-wide scans of schizophrenia have been published for the decade and many chromosome regions showed suggestive evidences for linkage, including chromosome 1q21-q22, 1q31-q42, 2p22-q21, 4q24-q32, 6p24-p22, 6q16-q23, 8p24-p21, 10p14-p13, 13q14-q32, 15q13-q14, 22q11-q13 (Coon et al., 1994; Shaw et al., 1998; Levinson et al.1998; Blouin et al., 1998; Kaufmann et al.1998; Faraone et al., 1998; Rees et al., 1999; Williams et al., 1999; Hovatta et al., 1999; Brzustowicz et al., 2000). Among these candidate regions, meta-analysis of whole genome linkage scans of schizophrenia found the strongest evidence for susceptibility loci on 8p (p<2*10-4), 13q (p<7*10-5), and 22q (p<9*10-5) (Badner and Gershon, 2002).
A relative high frequency of severe mental illness was found in patients with 22q11 microdeletion. Specifically, Two independent studies have reported that 25-31% of patients with the 22q11 microdeletion met diagnostic criteria for schizophrenia or schizoaffective disorder (Pulver et al 1994; Murphy et al. 1999). Although the microdeletion occurs in the population at a rate of 0.025%, it has been found in 2% of adult schizophrenic patients (Karayiorgou et al. 1995) and in 6% of cases with childhood onset schizophrenia (Usiskin et al. 1999). These studies collectively suggest that the morbid risk of schizophrenia for a patient with a 22q11 microdeletion may be approximately 20-30 times the general population risk of 1%, and that the rate of 22q11 microdeletion in schizophrenia, although relatively low, may be approximately 80 times the estimated general population rate. The chromosome regions may harbor susceptibility genes for schizophrenia.
Recent reports revealed the positive evidences of the susceptibility genes for schizophrenia on the 22q11 microdeletion region, including PRODH/DGCR6 (Liu et al. 2002a), and two membrane-associated proteins KIAA1292 and NOGO-R (Liu et al. 2002b). Among these candidate genes, PRODH/DGCR6 received much attention for further researches. In the study of PRODH/DGCR6 region (Liu et al.
2002a), the delineation of associated region extended from exon 11 of PRODH to the ins/del polymorphism of DGCR6 exon 1. They found two independent risk genetic patterns in the region; one is the 3-SNP risk haplotype, PRODH*1766/1945/2026 2-2-1 haplotype; another pseudogene-like variants mostly in exon 11.
PRODH is involved in the degradation of the amino acid proline. It catalyzes the conversion of proline to pyroline-5-carboxylate (P5C). PRODH was mapped to 22q11.2, a region previously implicated in type I hyperprolinemia in a case of CATCH 22 syndrome (Campbell et al. 1997). Northern blot showed that the gene is expressed in human lung, skeletal muscle, and brain, to a lesser extent in heart and kidney, and weakly in liver, placenta, and pancreas (Campbell et al. 1997).
Recently, it was reported type I hyperprolinemia was present in a subset of schizophrenic patients (Jacquet et al. 2002). This study revealed 1 of 63 unrelated schizophrenic patients and her affected sibling had a heteozygous deletion of the entire PRODH gene, associated with hyperprolinemia. In addition, two heterozygous PRODH missense mutations (Leu441ÆPro and Leu289ÆMet) detected in 3 of 63 schizophrenic patients but in none among 68 controls, were also associated with increased plasma proline levels. Segregation analysis within the two families harboring respectively the PRODH deletion and the Leu441ÆPro mutation showed that the presence of a second PRODH neucleotide variation (Arg453ÆCys and Arg431ÆHis) resulted in higher levels of prolinemia. The complex interaction between genotypes of PRODH and plasma proline level needs further exploration. The study suggests a possible correlation between schizophrenia and alteration of the proline pathway. However, prolinemia was not systematically assessed in this study. Quantifying precisely the magnitude of the association between hyperprolinemia and schizophrenia will require systematic measurement of plasma proline levels in a large population of patients and controls.
The family sample we collected previously had joined an international collaboration study organized by Gill et al (1996) entitled as "Schizophrenia Collaborative Linkage Group", which reported suggestive linkage evidence to D22S278 (p=0.001) on chromosome 22q12. We also tested the association between the SNPs of PRODH and schizophrenia in our case-control sample, of which consisted 124 schizophrenic patients and 112 normal controls. The result revealed significant results were found on the SNPs PRODH*757 (p=0.006), PRODH*1766 (p=0.049), PRODH*1945 (p=0.004), DGCR6 Ins/del (p=0.042) when compared the allele frequency of these SNPs between schizophrenia and normal controls. Our preliminary results partially replicate the association
between PRODH and genetic susceptibility for schizophrenia, which reported in the previous report (Liu et al.2002a), in Taiwanese sample.
Based upon our positive results on this gene, it’s time to study the correlation between the genotypes of PRODH and plasma proline level in schizophrenic patients to further validate the hypothesis that the proline pathway may be involved in the pathogenesis of schizophrenia.
研究方法
Sample
We collected the DNA and plasma of 100 schizophrenic patients from the psychiatric inpatient ward and outpatient clinic of National Taiwan University Hospital and 112 normal controls from hospital staff and community sample. All the individuals were screened for the presence of conditions influencing the total concentration of proline in human plasma, such as bone disease (Gasser et al. 1979; Smith 1980), tumor (Powles et al. 1976; Kelleher and Smith 1982), chronic uremia (Dubovsky et al. 1968), and alcoholic liver disease (Rojkind 1990). After obtaining inform consents, all individuals met the screen criteria were drawn about 10 cc blood from the antecubital vein in the morning after overnight fasting because the plasma proline level is subjected to diurnal variation. They all received test for continuous performance task (CPT).
Genotyping methods of SNPs of PRODH
1. Plasma and DNA purification
After obtaining the whole blood sample, separation of the plasma and blood cells will be performed immediately. The plasma will be stored in the -20℃ refrigerator as soon as possible for further HPLC analysis of proline level. The blood cells will be used for purification of DNA. We will use DNA purification kits (Gentra™) for the better quality of DNA based upon our previous experiences.
2. Genotyping methods of SNPs of PRODH
The SNPs of PRODH1945, PRODH1766, and PRODH757 will be genotyped by the method of PCR-based RFLP and the SNPs of PRODH2026 and DGCR6Ins/del will be determined by direct sequencing.
Determination of Total Proline in Human Plasma
Chemicals:
Chemicals for serum proline analysis will be purchased in analytical reagent grades unless stated otherwise. DPS-Cl will be purchased from the distributor in Taiwan of Dojindo Laboratories (Kumamoto, Japan). Proline, hydroxyproline and o-phthalaldehyde (OPA) will be obtained from Wako (Osaka, Japan), and the nipecotic acid (internal standard) from Tokyo Kasei (Tokyo, Japan). Deionized distill water will be used in all the solution preparations. Organic solvents will be from Merck (Germany). Bond Elut C18 (100 mg/ml) from Waters (Varian, CA,
U.S.A.) will be conditioned with 2 ml of methanol followed by 2 ml of water and 0.4 ml of borate buffer (0.1M, pH8.5).
Plasma Sample Preparation:
All patients participated in this project will be asked for written consents following the IRB issued by the National Taiwan University hospital. Patients will be fasted overnight and taken 5 ml of blood in EDTA anticoagulant-containing glass tubes the next morning. The blood samples will be processed immediately or stored at -70℃ until used.
Instrumental Conditions:
A Nova-Pak C18 column (150 x 3.9 mm i.d., 4 um; Waters) will be connected with a TSK Guardgel ODS-80 Tm (15 x 3.2 mm i.d.; Tosoh, Tokyo, Japan) as a guard column. The gradient system will be using (A) phosphate buffer (1 mM, pH 7) and (B) acetonitrile at 25℃. The elution program will be a linear gradient from 12 to 20 % of B for 15 min, followed by a stepwise increase in B to 80% to wash the column for 5 min. The flow rate will be 1 ml/min. The fluorescence intensity will be monitored at excitation and emission wavelengths of 315 and 385 nm, respectively. The vacuum suction of Bond Elut C18 column will be carried out using GL-SPE manifold kit (GL Science, Tokyo, Japan) under a vacuum of 5 inches Hg.
Analytical Procedure:
To human serum (10 ul) will be added concentrated hydrochloric acid (100 ul) and nipecotic acid solution (2 mM, 100 ul), as an internal standard (IS). The mixture will be hydrolyzed at 120℃ for 16 hrs in a screw-capped vial using a heating block and then neutralized with sodium carbonate (2M, 300 ul). The mixture (100 ul) will be then transferred to a vial and mixed with borate buffer (0.1M, pH 8.5, 300 ul) and OPA (4% in acetonitrile-borate buffer (0.1M, pH8.5, 300 ul) (1:1, v/v), 100 ul). After standing for 3 min at room temperature, 200 ul of the OPA-treated mixture will be passed through a Bond Elut C18 column. The
column will be washed twice with borate buffer 400 ul each time. The effluent of the OPA-treated mixture and the washings will be mixed. An aliquot (300 ul) of the mixture will be used for the derivatization reaction with DPS-Cl (1.2 mM in acetone, 500 ul) at 30℃ for 10 min. The reaction mixture will be mixed well with dichloromethane (0.8 ml) and then centrifuged (500x g) for 10 min. An aliquot (20 ul) of the aqueous layer will be subjected to HPLC.
Precision and Recoveries:
The within-day and day-to-day precision of the method will be evaluated using serum with 10 replicates assays in one day for the former and assays in five consecutive days for the latter. These values will be collected and calculated for the average and the standard deviations.
The recovery rate will be carried out with duplicate assays by adding IS solution containing various amounts of standard proline and hydroxyproline to serum. The recoveries of a range of mean will be calculated respectively.
Linearities and Detection Limits:
The linearity will be studied over wide ranges of concentrations of proline and hydroxyproline (ranging from 10 uM to 100 mM each). The peak area to IS ratio will be linear in the ranges investigated. The regression equations of serum analysis at the concentration ranges will be calculated. The equation and the regression coefficient will be obtained from the calculation. The detection limits will be presented for both proline and hydroxyproline.
Statistics Analysis
The comparison of demographic data including age, education, and body mass index (BMI) between the two groups was performed using t-test; the comparison of sex using Chi-square test. The correlation between proline level and other variables was calculated using Pearson correlation. The comparison of proline level between the two groups was performed using multiple regression test with proline level as dependent variable, group and body mass index as inpendent variables. The genotype-wise and allele-type wise comparison between the two groups was performed using Chi-square test and Fisher-exact test. The comparison of proline level and continuous
performance task (CPT) between different genotypes was performed using Kruskal-Wallis test.
Result
education, and body mass index between the two groups. The schizophrenic patients showed significant lower education level and higher BMI. Table 2. showed the Pearson correlation between proline level and age, dprime index of undegraded CPT and degraded CPT, and BMI. The proline level showed mild (R=0.14) but significant correlation with BMI. The mean level of plasma proline of schizophrenic group was 90.4μmole/l and that of control group 92.7μmole/l. There was no significant difference in plasma proline level between the two groups, even after controlling the possible confounding factor of BMI through multiple regression analysis. There was not any case or control whose plasma proline level attained the criterion of
hyperprolinemia , that is above 290μmole/l.
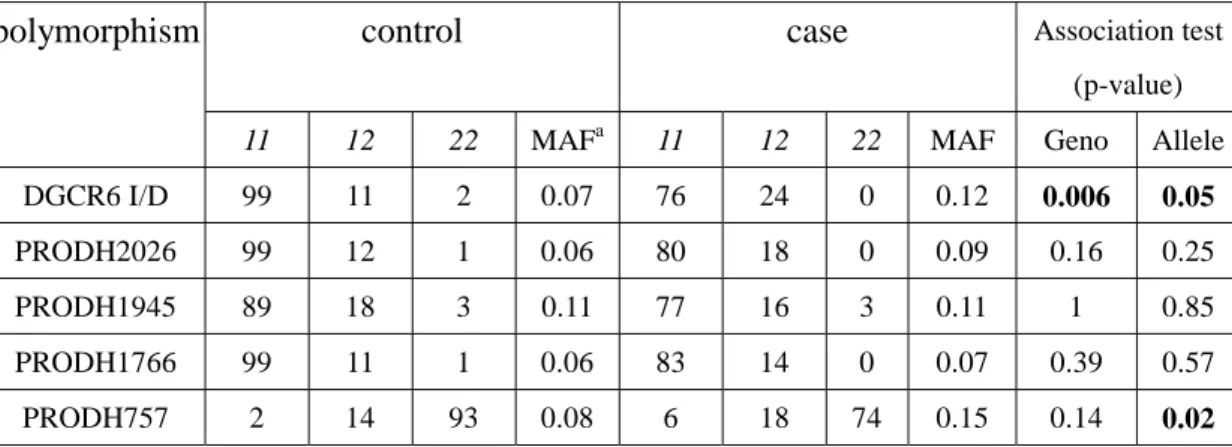
Table 3 showed the genotype-wise and allele-wise comparison of the five PRODH/DGCR6 polymorphisms between case and control. There were significant allelic and genotypic difference in DGCR6 I/D and allelic difference in PRODH757 between the two groups.
Table 4 showed the quantitative phenotype comparison among the genotypes of five polymorphisms. There was no significant difference in plasma proline level in different genotypes of the five polymorphisms. However, significant differences in dprime of degraded CPT were found in the genotypes of PRODH 1945 and PRODH 1766.
Our results showed significant association of PRODH/DGCR6 locus in
schizophrenia, which replicated our previous study. However, we tried to clarify the association between PRODH genotype and plasma proline level and the association was non-significant. We found there was no significant difference of plasma level between schizophrenia and controls and cannot replicate the finding of Jacquet et al. 2002, which reported hyperprolinemia in schizophrenic patients. The plasma proline level may be influenced by other genes than PRODH gene, eg. high-level expression of a homologous proline dehydrogenase (PRODH1 or HsPOX1) in peripheral tissues, or by other environmental factors our study didn’t define.
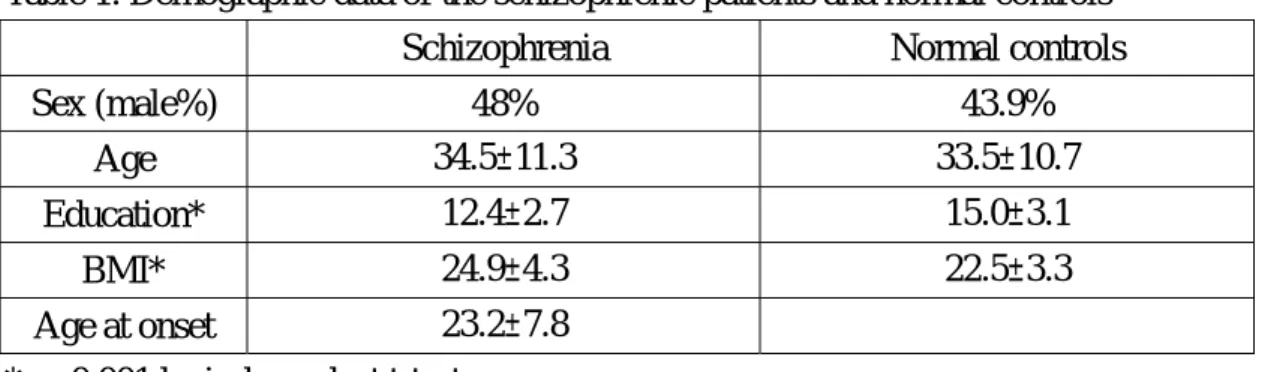
Table 1. Demographic data of the schizophrenic patients and normal controls
Schizophrenia Normal controls
Sex (male%) 48% 43.9% Age 34.5±11.3 33.5±10.7 Education* 12.4±2.7 15.0±3.1 BMI* 24.9±4.3 22.5±3.3 Age at onset 23.2±7.8 * p<0.001 by independent t-test
Table 2. The Pearson correlation between proline level and age, dprime index of undegraded (zdprime) and degraded CPT (mzdprime), and BMI
Age Dprime Mzdprime BMI
Proline level Pearson r -0.02 0.09 0.03 0.14* p<0.05
Table 3. The genotype distribution and allele-wise and genotype-wise comparison of the five polymorphisms between case and control.
control case Association test (p-value)
polymorphism
11 12 22 MAFa 11 12 22 MAF Geno Allele
DGCR6 I/D 99 11 2 0.07 76 24 0 0.12 0.006 0.05
PRODH2026 99 12 1 0.06 80 18 0 0.09 0.16 0.25
PRODH1945 89 18 3 0.11 77 16 3 0.11 1 0.85
PRODH1766 99 11 1 0.06 83 14 0 0.07 0.39 0.57
PRODH757 2 14 93 0.08 6 18 74 0.15 0.14 0.02
aMAF: minor allele frequency
Table 4. Quantitative phenotype comparison among the genotypes of five polymorphisms
Phenotype SNPs (p-value)
Case
DGCR6 PRO2026 PRO1945 PRO1766 PRO757
D’ of undegraded CPT 0.98 0.46 0.82 0.25 0.72
D’ of degraded CPT 0.55 0.02 0.53 0.003 0.78
Proline level 0.86 0.70 0.78 0.33 0.69
Control
DGCR6 PRO2026 PRO1945 PRO1766 PRO757
D’ of undegraded CPT 0.11 0.17 0.69 0.42 0.98
D’ of degraded CPT 0.98 0.75 0.99 0.34 0.05
Proline level 0.75 0.94 0.97 0.93 0.67
Reference:
Badner JA and Gershon ES. 2002. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 7: 405-411.
Usiskin SJ, Nicolson R,Krasnewich DM, et al. 1999. J Am Acad Child Adolesc Psyhciatry. 38:1536-1543.
Karayiorgou M, Morris MA, Morrow B, et al. 1995. Proc Natl Acad Sci USA. 92: 7612-7616..
Murphy KC, Jones LA, Owen MJ. 1999. Gigh rates of schizophrenia in adults with velo-cardio-facila syndrome. Arch Gen Psyhciatry 56:940-945.
Pulver AE, Nestadt G, et al. 1994b. Psyhotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis 182:476-478. Blouin JL et al. 1998 Nature Genet. 20: 70
Brzustowicz LM, Hodgkinson KA, Chow EWC, Honer WG, Bassett AS. 2000. Location of a major susceptibility locus for familiar schizophrenia on chromosome 1q21-q22. Science 288:678-682.
Coon H et al. 1994 Am. J. Med. Genet. Neuropsychiatr. Genet. 54:59 Faraone SV et al. 1998 Am. J. Med. Genet. Neuropsychiatr. Genet. 81: 290. Hovatta I, Varilo T, Suvisaari J, Terwilliger TD, Olikainen V, Arajarvi R, Juvonen H,
Kokko-Sahin M-L, Vaisanen L, Mannila H, Lonngvist J, and Peltonen L. 1999. A genome-wide search for schizophrenia genes in an isolated Finnish
subpopulation suggesting multiple susceptibility loci. Am J Hum Genet 65:1114-1124.
Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD, Meyer J, Zambuto CT, Schmitt K, Matise TC, Friedman JMH, Hampe C, Lee H, Shore D, Wynne D, Faraone SV, Tsuang MT, Cloninger R. 1998. NIMH Genetics Initiative Millennium Schizophrenia Consortium: Linkage analysis of African-American pedigrees. Am J Med Genet (Neuropsychiatr Genet) 81:282-289.
Levinson DF et al. 1998 Am. J. Psychiatry 155, 741 Rees MI et al. 1999 Mol. Psychiatry 4:353
Schizophrenia Collaborative Linkage Group (Chromosome 22)(corrdinated by Gill M, Vallada H, Collier D): A combined analysis of D22S278 marker alleles in affected sib-pairs: support for a susceptibility locus for schizophrenia at 22q12. Am J Med Genet (Neuropsychiatric Genet) 67:40-45,1996.
Shaw SH et al. 1998 Am. J. Med. Genet. Neuropsychiatr. Genet. 81:364 Williams NM et al. 1999 Hum. Mol. Genet. 8:1729
Liu H, Heath SC, Sobin C, et al. 2002a. Genetic variation at the 22q11
PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci USA. 99: 3717-3722.
Liu H, Abecasis GR, Heath SC, et al. 2002b. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc Natl Acad Sci USA. 99:16859-16864.
Campbell HD, Webb GC, Young IG. 1997. A human holologue of the Drosophila melanogaster sluggish-A (proline oxidase) gene maps to 22q11.2, and is a candidate gene for type I hyperprolinemia. Hum Genet. 101: 69-74.
Jacquet H, Raux G, Thibaut F, et al. 2002. PRODH mutations and hyperprolinemia in a subset of schizophrenic patients. Hum Mol Genet 11: 2243-2249.
Gasser AB, Depierre D, Courvoisier B. 1979. Br J Cancer. 39:280-283. Smith R. 1980. Clin Sci. 59: 215-213.
Powles TJ, Rosset G, Leese CL, et al. 1976. Cancer 38: 2564-2566. Kelleher PC, Smith CJP. 1982. Clin Lab Med 2: 519-542.