ORIGINAL PAPER
Newborn Screening Healthcare Information System Based
on Service-Oriented Architecture
Sung-Huai Hsieh&Sheau-Ling Hsieh&Yin-Hsiu Chien& Yung-Ching Weng&Kai-Ping Hsu&Chi-Huang Chen& Chien-Ming Tu&Zhenyu Wang&Feipei Lai
Received: 9 December 2008 / Accepted: 11 February 2009 / Published online: 24 March 2009 # Springer Science + Business Media, LLC 2009
Abstract In this paper, we established a newborn screening system under the HL7/Web Services frameworks. We rebuilt the NTUH Newborn Screening Laboratory’s original stand-alone architecture, having various heterogeneous systems operating individually, and restructured it into a Service-Oriented Architecture (SOA), distributed platform for further integrity and enhancements of sample collections, testing, diagnoses, evaluations, treatments or follow-up services, screening database management, as well as collaboration, communication among hospitals; decision supports and improving screening accuracy over the Taiwan neonatal systems are also addressed. In addition, the new system not only integrates the newborn screening procedures among phlebotomy clinics, referral hospitals, as well as the newborn screening center in Taiwan, but also introduces new models of screening procedures for the associated, medical practitioners. Furthermore, it reduces the burden of manual operations, especially the reporting services, those were
heavily dependent upon previously. The new system can accelerate the whole procedures effectively and efficiently. It improves the accuracy and the reliability of the screening by ensuring the quality control during the processing as well. Keywords Newborn screening information system . HL7 . Service-oriented architecture . Web services
Introduction
Today, the newborn screening systems are recognized as complex entities. The primary components are more than sample collections, testing, diagnoses and treatments. The follow-up care or services, cost-effectiveness evaluations, hospitals or laboratories’ communications, screening data-base management, as well as parents or public education are also involving [1, 22]. At present, the newborn DOI 10.1007/s10916-009-9265-x
S.-H. Hsieh
:
S.-L. Hsieh:
Y.-C. Weng:
F. Lai Information Systems Office,National Taiwan University Hospital, Taipei, Taiwan
S.-H. Hsieh
:
Y.-C. Weng:
K.-P. Hsu:
C.-M. Tu:
F. Lai Department of Computer Science and Information Engineering, National Taiwan University,Taipei, Taiwan C.-H. Chen
:
F. LaiDepartment of Electrical Engineering, National Taiwan University,
Taipei, Taiwan S.-L. Hsieh
Network and Computer Centre, National Chiao Tung University, Hsin Chu, Taiwan
F. Lai
Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University,
Taipei, Taiwan Y.-H. Chien
Department of Medical Genetics, National Taiwan University Hospital, Taipei, Taiwan
Z. Wang
Computing Laboratory, Oxford University, Oxford, UK
S.-H. Hsieh (*)
Information Department, National Taiwan University Hospital, No.7, Chung-San South Road,
Taipei, Taiwan
screening process is independent upon systems imple-mented separately in the affiliates of Department of Health in Taiwan. It is important to integrate the ongoing processes and expansion efforts so that the children can attain complete, qualified health care. Moreover, screening cut-offs have significant effects on the quality control of the program. A general tool of sensitivity and specificity setting of cut-offs has been exceptional competing demands [2–4]. Background
Newborn screening programs for severe metabolic disorders, that hinder an infant’s normal physical or mental develop-ment, are well established. These metabolic disorders can be addressed by effective therapies at the early stages. If not diagnosed or treated in time, acute encephalopathy crises occur in most patients during infancy or early childhood.
New and refined screening methodologies based on the modern tandem mass spectrometry (MS/MS) of metabolites have been developed for routine deployment [58, 59]. It allows simultaneous analyses of multi-compounds in a high-throughput process. The functional endpoint of the metabolic cycles, offering a precise snapshot of the current metabolic state, can be detected, analyzed in a single, small blood sample that is collected during the first few days of life. Over the last two decades, this technology has been applied to newborn screening because it is open to population-wide testing for a large number of disorders of fatty acid, organic acid and amino acid metabolism. In addition, the technique is amenable to a broad range of compounds and inherently eliminates the need for extensive sample preparation. The fragmentation capabilities of MS/ MS also provide critical information for structural elucida-tion of unknown compounds [45].
The National Taiwan University Hospital (NTUH) initiated the newborn screening research in 1981. It has carried on the nation’s newborn screening tasks for metabolic diseases since July, 1985. The earliest NTUH newborn screening information systems started operations in October, 1987. At beginning, the NTUH Laboratory Information System (LIS, an outsourcing system) interfaced with the Newborn Screening Machines manually, stand-alone to collect specimens datasets, labeling, and schedul-ing. The systems only utilized computers to manage data access, storage as well as printing capabilities. As the medical technologies advancing, the hospital has purchased new, modern screening equipments, yet thus resulting in the raw data format incompatibility between the new and the legacy systems. In addition, further new statistic, evaluation testing data are required. Therefore, in 1994, NTUH LIS enhanced its applications. Thereupon, the refined screening system, emerging of Windows and Internet, contributed and enabled parents to access the infant’s screening results
through web sites as well as the interactive voice response (IVR) systems. It is a breakthrough nationwide.
At present, NTUH newborn screening laboratory pro-vides medical service to approximately half of the nation’s newborns for inborn errors of metabolism. For instance, in year 2006, Taiwan had a total of 204,459 newborns; among them 90,367 had their screening tests done at NTUH. Moreover, in Taiwan, the screening rate of newborn’s inherited metabolic diseases has improved from 6.4% in 1984 to reach over 99.9% in the past few years. At present, the screening program executed in Taiwan, all newborns are tested for 23 inherited metabolic disorders [57].
Problems
Currently, the NTUH Newborn Screening Healthcare Information Systems has problems that need to be solved. After examined and investigation, they can be categorized in the following four aspects:
1) Screening Process: NTUH newborn screening labora-tory (the national newborn screening center) presides over the screening processes more than 500 public and private medical institutions (including phlebotomy clinics and referral hospitals). The screening results from delivery and medical records tracking among hospitals are accomplished via mails or facsimiles, which is extremely high-cost and time-consuming. 2) System Integration: The existing screening
informa-tion systems are developed or established on various heterogeneous systems, including databases such as Access, dBase, Oracle, and operating systems such as DOS, Windows 98, Windows 2003, Windows XP, and more, which causing difficulties in exchanging medical information among the systems.
3) Quality Control: The abnormal screening results occasionally induced by apparatus or reagent failures during the experiments. The system may process the results and is unable to distinguish the proper accuracy. 4) Screening Accuracy and Analysis: As the newborn screening program is applied nationwide in Taiwan, the use of Tandem Mass Spectrometry (MS/MS) in newborn screening analyzing becomes increasing im-portant. At present, while dealing with the MS/MS newborn screening cases, the system is only capable of storing the screening results and partially the abnormal metabolites, yet not capable of efficiently conducting research on all the examined metabolites.
To deal with and conquer the above problems, in July of 2006, the Department of Genetic Medicine and the Information Systems Office initiated a joint project of a new generation Newborn Screening Healthcare Information System (NSHIS) in NTUH.
Related work
The newborn screening program represents one of the major advances in child health of the past century. The program is a system that must function within geographic, economic, and political constraints, and which must smoothly integrate sample collections, laboratory analyses, follow-up diagnoses and treatments [7].
In the United States (US), there are approximately 4 million births annually [8] ,the newborn screening program has been carried out in all fifty states since the 1970s [6–9]. However, the programs are state-run, and decisions are left to the individual states regarding the conditions to be screened for, the mechanism for confirmatory testing, and financing of the programs [10–12]. Each state in the US requires screening tests, but the specific tests performed vary among the states [14–19]. For example, the Georgia Newborn Screening Program has educational and monitoring mechanisms in place to remain watchful for any signs or symptoms of disorders in their patients [13]. Moreover, it can raise screening’s
cost-effectiveness, quality, and oversight [20, 21]. In addition, in order to continuously evaluate and improve the program quality, a comprehensive, real time, national data reporting system, or database must be universally accepted [22–24].
In Europe, the number of disorders screened for by MS/ MS ranges from two disorders (PKU and MCADD (medium-chain acyl-CoA dehydrogenase deficiency)) in some countries to 20 in others [29]. Thus, the disorders chosen to be included in European newborn screening programs differ considerably. For examples, 20 conditions are screened for in Austria, over 15 conditions in Belgium and Denmark. In Portugal and Poland, around ten con-ditions are screened for. In Germany, the health authorities decided in 2005, the number of disorders to be detected limits to 10. In Great Britain, only MCADD is screened and planned to cover 100% of the newborns in the near future. PKU and MCADD are examined in Switzerland. The rationale behind inclusion or exclusion of a respective disorder screen for is far from clear in most cases [29].
Asia Pacific newborn screening program demographics indicate that all countries in the region with an infant mortality rate (IMR) of less than 10/1,000 live births have achieved better than 90% screening coverage of their newborn population. Actually, most countries are approach-ing full coverage, although the number of conditions screened varies widely [25–27]. However, in the develop-ing countries, Nepal and India are of particular interest of developing and inquiring Congenital Hypothyroidism (CH) programs. CH is the universal important condition; usually it is the first condition considered for screening [18,28].
Newborn screening for genetic disease has been an enormously successful public health effort, and the number of disorders on screening panels has been increasing [27–29].
In general, disorders that benefit from screening are difficult to detect without systematic screening, have clearly benefi-cial treatments available, and have inexpensive and accurate screening tests [33].
In the following sections of the paper, we first elaborate the design of the overall NSHIS architecture. Detailed descrip-tions of the system components, integration mechanism, as well as the workflow process are illustrated. In Section “Scenario and implementation”, a comprehensive NSHIS
services with scenarios and data flow sequences are provided. The performance evaluations are presented and discussed in Section“Performance evaluations”. Finally, the
paper concludes in Section “Conclusion” with discussion
embedded in Section“Discussion”.
Design and implementation
A Service Oriented Architecture (SOA) represents the current pinnacle of interoperability, in which resources on a network are available as individual, loosely-coupled and independent services [41–44]. As Service Oriented Architecture matures, an efficient approach for the integration of Web services over heterogeneous systems, e.g. NSHIS, is required. Apparently, SOA is a desirable and inevitable solution.
System architecture
The overall architecture of the NTUH NSHIS is depicted in Fig. 1. In the diagram, it contains three major components, i.e., the front-end module, the middleware module, and the back-end services including database servers. The front-end module handles user interfaces via browsers. It establishes the user sessions in the Session Services. The services validate users’ authentications as well as authorizations. The middleware module, i.e., HL7 Middleware Framework as indicated in the diagram, glues the front-end services and the back-end facilities together. It provides communication and connectivity via SOA (Web Services) mechanism. The HL7 embedded XML formatted data is implemented in the Framework for data exchanges among the modules.
Further illustrations of the individual modules are provided as the following. In the architecture, for user friendly browsing interfaces, we adopt web based services. The Portal Servers and the Web User Interface Services, i.e., Web-session and Win-session Servers, are introduced. The Servers generate web-based pages, constructing dynamic web URL linkages [5], direct to Newborn Screening Healthcare Information System (NSHIS) components as well as their ancillary Sub-systems (NSHIS Server). The portal site requires login a process. In addition, we enhance the login mechanism using public key infrastructure based on Smart Client [36] according to the National Healthcare Insurance Card requirements in Taiwan.
The Win-session Servers and Web-session Servers inte-grate the system authentication and authorization facilities via Simple Object Access Protocol (SOAP) communication mechanism. The Single Sign on Service (SSOS) is enabled [38]. The State-session Servers store the user’s web session
status variables for analyzing user logic and validation. The HL7 Framework is the Middleware Integration Engine of the NSHIS architecture. It supports message management, routing, mapping, and database access. Detailed information about the processing of each message is also automatically logged by the Engine. Moreover, the Engine glues not only the NSHIS applications but the NTU HIS systems (e.g., Outpatient, Inpatient Information Systems, etc) together. The HL7 Middleware accesses HL7 messages, embedded in XML format, over the SOAP protocol [37,39].
In order to achieve the data consistency, we introduce a Data Exchange Server that only receives the message sending from the HL7 Middleware. While Data Exchange Server receiving messages, it will perform the data synchronization among NSHIS, patient demographic data in HIS, patient radiology information orders to RIS database or laboratory orders to LIS database. This data exchange processing can ensure and maintain data integrity over the NTUH systems [40].
To increase the performance of the NTUH NSHIS, a cluster of identical servers is deployed and dispatched dynamically by introducing Layer 4 Switches and Layer 2 Switches. All the servers are configured running under load balancing as well as failover modes to ensure the system’s availability and concurrency. The firewalls are also installed to enhance the security of the architecture.
The NSHIS system is accessible by all authorized screening program professionals, hospitals enabling the unique identification of babies, screening samples and
results. The authorized users can be doctors, medical staff, administrative personnel as well as parents. The screening hospitals include the national newborn screening center, referral hospitals, and phlebotomy clinics as depicted at the left hand side of Fig.1. In other words, these members can access the subsystems of NSHIS proceed with duty-related services following the newborn screening procedures after authentication and authorization by the Session Services. In addition, the medical staff can conduct mathematical and statistical analyses on the MS/MS metabolites’ concentra-tion resided in databases. During the screening procedures, all the operations of database transactions and data exchanges among systems are based on the HL7 Middle-ware Framework standards.
Software components
The four-tier NSHIS infrastructure software modules are described and depicted clearly in Fig. 2. At the upper portion of the diagram, it shows the NSHIS services categorized into Windows Applications and Web Applica-tions. These Applications provide NSHIS Services or Subsystems, e.g., Specimen Collection Subsystem, Sample Screening Subsystem, Test Result Tracking Subsystem, Diagnosis Confirmatory Subsystem, etc. In addition, in the Web Applications, SSOS, User Interface (UI), Session Management for authentication and authorization are included. All these services reside in Portal Servers, NSHIS Servers, State-session Servers, Web-session and Win-session Servers covered in the previous section.
The middle portions of the diagram consist of the HL7 Middleware modules. The modules handle message man-agement, routing, mapping, database access as well as L4 Switch L2 Switch Legacy system Web session Servers Win session Servers NSHIS Database HL7 Middleware Web Service (SOAP) .WSDL.DISCO Data Exchange Server State session Servers NSHIS Server Web Service UDDI XML Portal Servers Smart Client Firewall National Newborn Screening Center Referral Hospital Phlebotomy Clinic HIS Database Legacy Database Web Service front -end back-end middleware
Fig. 1 The overall architecture of the NTUH newborn screening HIS
connectivity among NSHIS components. Furthermore, a XML/HL7 message library (HL7 Library) is implemented in the module. Initially, in the XML/HL7 message, the HL7 Library generates all the fields of the message. It is expensive spatially and temporally. Later, we enhance the Library by creating fields dynamically as needed [54]. Moreover, the HL7 Transfer Engine exchanges or maps HL7 messages between NSHIS (implemented in C#) and RIS (implemented in Java). The lower portions of the diagram contain Data Exchange modules handle multi-database integrations as well as redirections to the third party outsourcing systems, i.e., RIS with PACS, LIS, etc.
The modulized architecture apparently induces the NSHIS software components reusability and resources sharing effec-tively and efficiently. The approaches also make the NSHIS developing and deploying sophisticated technically as well as financially.
NTUH simultaneously holds the responsibilities of a national newborn screening center, phlebotomy clinic, and referral hospital; hence its subsystems include all proce-dures of a newborn screening process, from the very first phlebotomy to the eventual diagnoses [56,57]. The whole Newborn screening process flowchart and their corre-sponding subsystems’ functionalities, relationships as well as roles or members’ involvements are illustrated in the following section.
Workflow process
In Fig.3, it elaborates an integrated workflow process of NSHIS in NTUH. The process can be categorized into four Sub-Systems; the functionalities of each Subsystem are
included in the diagram. The involving roles, their relation-ships, and activities are indicated as well.
1) Specimen Collection Subsystem: initially, the phlebot-omy clinics collect specimens, register the infants’ demographic data online (i.e., his/her mother’s name, date of birth, weight, etc), print out individual barcode label, and place the label onto the phlebotomy filter paper. Afterwards, the clinics deliver the newborns’ phlebotomy filter papers to the NTUH national newborn screening center daily via post mail. As soon as the center receives the specimens, laboratory serial numbers are assigned. Meanwhile, the newborns’ information are entered into the database through barcode scanning or input manually, thus, completing the initial sample registration. The phlebotomy clinic can track the status of the samples through the utilization of the subsystem, which may indicate as “received” or “not yet delivered,” and the results of the Primary Examination may be included as well. 2) Sample Screening Subsystem: secondly, screening
technicians place the specimens into the experimental apparatus, i.e., MS/MS, in order to analyze the blood compound and to obtain the raw data file of the concentration. The screened data are examined primar-ily by the subsystem to check whether the metabolites are within the normal range. If the result comes out negative, the case is directly stored into the database and issued a report; parents and phlebotomy clinics can review the status online later. This completes the newborn screening procedures. If the result turns out to be positive, the case forwards to the Diagnosis
UI Layout
NSHIS Web Application
User Interface Engine Utility Engine
HL7 Middleware
Data Exchange Engine
NSHIS NS Database XML Doc XML-HL7 Engine HL7 Library Segment Component Subcomponent Field Return Message Field Description Error Warning Input Message Required HL7 Field HL7/XML/SOAP Old System Web services Web services Authentication Authorization Printing Modules Specimen Collection Subsystem Sample Screening Subsystem Test Result Tracking Subsystem Diagnosis Confirmation Subsystem Ancillary Analysis Subsystem Report Modules Web services
Fig. 2 NSHIS infrastructure software modules
Confirmation Subsystem for further confirmations, dietary therapy or treatments. However, if the result comes out at borderline, concerned instrumental misjudg-ments or errors, the specimen will undergo a second examination; a re-test file is generated. The case returns to the Specimen Collection Subsystem on the following day. In addition, during the process, the subsystem simulta-neously creates a list of suspected positive case reports. The reports assist doctors and technicians to interpret and track the cases later in the Test Result Tracking Subsystem for the Public Health Bureau in Taiwan. 3) Test Result Tracking Subsystem: based on the primary
test results, the suspected positive cases are interrogat-ed, verified and confirmed by doctors. The cases return to the original phlebotomy clinic. The phlebotomy clinic is responsible for tracking and recall specimens. The specimens will be delivered to the national newborn screening center for Second Examination in the Specimen Collection Subsystem. If the result returned turns out to be positive again, the case will forward to the Diagnosis Confirmation Subsystem for further confirmations and treatments. In addition, the subsystem will issue an e-mail to notify the original phlebotomy clinic and the referral hospital as well. The parents of the newborns and associated clinics will be able to track the results online within three days. 4) Diagnosis Confirmation Subsystem: by this stage, the
referral hospital is in charge of tracing the positive cases. The hospital provides preliminary precaution advices and comprehensive confirmation diagnoses for
the cases. The subsystem also fills out doctor-approved diagnoses and a complete test report. The report will be delivered to the national newborn screening center, public health bureau, and the original phlebotomy clinic via e-mail. This completes the whole newborn screening procedures.
Through the cooperation and the coordination among these subsystems, the NSHIS systems provide an integrated, sophisticated, cost-effective, efficient, nationwide screening process for medical specialties in Taiwan. At present, the examined items within the NSHIS includes Congenital Adrenal Hyperplasia, Glucose-6-phosphate Dehydrogenase deficiency, Galactosemia, Congenital Hypothyroidism, Fabry disease, Pompe disease and other metabolic disorders detected using Tandem Mass Spectrometry.
Scenario and implementation
In this section, a scenario illustrates the collaborating, interoperability among NSHIS modules as well as the components in NTUH HIS as below.
For example, a newborn screening program professional examines screened data or procedures. The professional logins the NTUH Portal page, as shown at the left hand side of Fig.4, from the NTUH Intranet initially; after validation, he/she enters into the NTUH HIS main page. While selecting NSHIS category, as indicated at the upper right Fig. 3 Integrated workflow
pro-cess in newborn screening healthcare information system
corner of the Figure, the user enters the home page of the NSHIS. Meanwhile, the Specimen Collection Subsystem web page pops up automatically, as depicted at the lower right corner. The Single Sign-On Solution enables the users accessing, navigating different systems among NTUH HIS without multiple or further validation. A newborn’s demo-graphic data are displayed.
The NSHIS system is accessible by all authorized screening program professionals, hospitals enabling unique-ly identify babies’ information, screening samples and results. The authorized users can be doctors, medical staff, administrative personnel as well as parents. The screening
hospitals include the national newborn screening center, referral hospitals, as well as phlebotomy clinics. In other words, these members can access the subsystems of NSHIS following the newborn screening procedures after authen-tication and authorization. In addition, the portal site provides the menu access control dynamically. It means if a user has no access right to operate a function in the NSHIS Subsystems, the function link is not visible. For example, parents can only review their newborns’ demo-graphic data and screening results.
The sequence diagram of the scenario is depicted in Fig.5. The SOA HIS has integrated NSHIS, Inpatient, Outpatient Fig. 4 Snapshots of a secure,
data exchanges applied HL7 in NSHIS Portal Server Web/Win Server SGNSIS Server State Server HL7
Middleware DatabaseNSHIS Data Ex
Server MS/MS
Spectrometry HIS
Browse to Portal Authentication
RetACK
Validate account Response validated result Get Session Key
Compare Page ID Return Page ID result To NSHIS page
Respond Session Key
Data Exchange HL7 Msg over SOAP DB operation DB Response Exchange to HIS Exchange response HL7 Ack over SOAP Exchange response
Return to Portal Log Out
Experiment Raw Data
Fig. 5 The sequence diagram of a secure, data exchanges applied HL7 in NSHIS
Information Systems, RIS with PACS, LIS, etc. under the .NET environment. At beginning, the requests and responses are exchanged for validation via authentication, authorization by generated a session key. After the session established, HL7 Middleware over SOAP (Simple Object Access Protocol) activates the NSHIS Specimen Collect Subsystem functions (NSHIS Server) and retrieves the neonatal demo-graphic data (HIS) as well as the screening data (NSHIS Database). Meanwhile, the data are delivered, displayed on the web page via HL7 Middleware and Data Exchange Server subsequently. The data exchanged are HL7 standards, embedded in eXtensible Markup Language (XML) formats. Requests and responses among the associated components in the scenario are clearly indicated in Fig.5.
Performance evaluations
NTUH Newborn Screening Laboratory has officially launched NSHIS on May 14, 2007, dealing with around 500
newborn screening cases daily. Currently, the NSHIS servers conduct both NTUH administrative activities and Newborn Screening Applications, Subsystems as depicted in Fig.2.
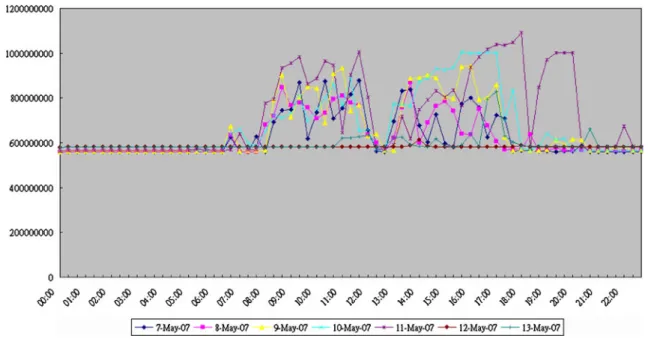
To pair quantitative data, the servers CPU and memory usages, from two different time periods are collected in a week aggregated by half-hour daily. Before NSHIS Appli-cations launched, between 05/07/2007 and 05/13/2007, are shown in Figs.6and 8. Similarly, between 07/07/2008 and 07/13/2008, after NSHIS Applications were activated and running stable over a year, the corresponding diagrams are presented in Figs. 7,8,9respectively.
In the diagrams, between 05/07/2007 and 05/13/2007, on average, during the hospital’s regular hours: 8:00 am and 6:00 pm, the servers CPU usage reaches 2.5% at most diurnally without the NSHIS Applications. The memory utilization is under 30% (on average, it reaches 1.2 GB approximately with total memory 4 GB) daily. In these two Figures, after the hospital operating hours, the testing NSHIS Applications are taking place; the CPU and memory utilizations remain active.
Fig. 7 The CPU utilization of NSHIS servers with NSHIS applications (07/07/2008~07/13/ 2008)
Fig. 6 The CPU utilization of servers without NSHIS applica-tions (05/07/2007~05/13/2007)
Accordingly, between 07/07/2008 and 07/13/2008, on average, the servers CPU usage reaches 6% at most diurnally while the NSHIS Applications are operating. The memory utilization is under 62.5% (on average, it reaches 2.5 GB approximately with total memory 4 GB) daily.
Conclusion
In this paper, we achieved a successful development under the HL7/Web service framework. We rebuilt NTUH Newborn Screening Laboratory’s original stand-alone
ar-chitecture and various heterogeneous systems, restructured it into a Service-oriented architecture (SOA). Our new system not only integrates the newborn screening proce-dures among phlebotomy clinics, referral hospitals, and the newborn screening center, but also models a new working flow for the screening team. Furthermore, it reduces the burden of manual operation, speeds up the whole process and improves the accuracy of work, by thus ensuring the newborn screening procedure’s quality control.
The NTUH NSHIS has been designed from the ground up to be an available, robust, reliable, secure, interoperable, and service-oriented architecture. Moreover, the system is Fig. 8 The memory utilization of WebUI server (05/07/2007~05/13/2007)
an innovation designed to address the continuously chang-ing and demandchang-ing nature of today’s newborn screenchang-ing services in Taiwan. In addition, it presents a solution to perform challenges imposed by heavy messaging traffic, among phlebotomy clinics, referral hospitals, and the newborn screening center, that is threatening the viability of Web-Services (.NET) implementations. As a result, the capital expenditures are controlled and the return on invest-ment is shorter.
The NSHIS programs in Taiwan are continuing to refine and expand their Newborn Screening Services. There are many research projects ongoing, for instances, the partic-ular interests are: 1) Congenital adrenal hyperplasia; 2) Glucose-6-phosphate dehydrogenase deficiency; 3) Galac-tosemia; 4) Congenital hypothyroidism; 5) Fabry disease; 6) Pompe disease.
The assessment investigation enables NSHIS to under-stand the screening practitioners’ satisfaction. In general, the system receives 72% of satisfaction under accessibility. Subsequently, the system is positive according to the available facilities. NTUH NSHIS establishes the principles and directions to guide the planning, development and delivery of Taiwan specialized genetics services for the years to come.
Discussion
NSHIS is a complete newborn laboratory information management system. The features include specimen receiv-ing, demographic barcode entry, specimen trackreceiv-ing, testing results entry, screening database management, quality control analyses as well as web integration. The program is able to improve the quality of care and well-being of newborns.
The NTUH newborn screening Program has educational and monitoring mechanisms in place to prevent and investigate any possible problems. However, it is still critical for health care providers to remain watchful for any signs or symptoms of these disorders in their patients. Any signs or symptoms of a disorder should be followed up immediately. The possibility of a disorder should not be ruled out solely on the basis of the newborn screening test result. A newborn screening result should not be considered diagnostic, and cannot replace the individualized evaluation and diagnosis of an infant by a well-trained, knowledgeable healthcare provider. Undoubtedly, the timely delivery of complete and accurate information can enhance the oppor-tunities to immunize the newborns.
The purpose of a screening test is to sort out apparently healthy individuals who have a disease from those who probably do not. However, screening programs are, by nature, imperfect. In setting cutoffs, a balance must be struck between time, money, anxiety caused by false
positives, and an acceptable number of missed cases. On one hand, laboratory advances in tandem mass spectrom-etry make it possible to screen newborns for many rate inborn errors of metabolism. This raises many policy issues including screening’s cost-effectiveness, ethics, quality, and oversight. On the other hand, new techniques in genetics surveillance have facilitated an improved public health approach to the detection of, and interventions offered for, a range of important genetic conditions. Over the past ten years, scientific advances associated with genetic have been increasing at an explosive rate. This has meant that an increasing number of diagnostic, predictive and carrier tests are available, for instance, leaning towards data mining technologies.
In the system, in order to keep the favorite links for the NSHIS users, we can log users’ behavior, and obtain users popular function links by adapting LRU (Least Recent Used) algorithm [32]. These links will be collected and implemented as“my favorite”. Therefore, users can quickly retrieve the links they frequently operate. In addition, the pre-fetched links can be cached in advance to improve screening performance. The approach can be achieved using Web 2.0-based technologies [52,53]. The techniques empower users to customize their experiences more effectively. Furthermore, the system lacks of sophisticated billing and charging applications currently. In the near future, the system can collaborate with vendors, for example Google Health [51], to offer Personal Health Records on the Web since birth.
NSHIS provides a wide range of facilities for presenta-tion and interrogapresenta-tion for MS/MS raw data [34, 35]. In addition, for a selected sample, the spectrum or chromato-gram can be viewed or interrogated. Visual panning over the chromatograms for whole plate is also possible [30,31,
46, 47]. Together, leading technology, total solutions and comprehensive support services are brought to Taiwan newborn screening. This expedites the evaluation process and simplifies the identification of anomalies [48–50].
Moreover, Machine learning techniques offer an obvious and promising approach to examine high dimensional data [60]. The Newborn Screening Analysis System [55] utilizes machine learning techniques, i.e., SVM (Support Vector Machine), and mining knowledge to construct the classifi-cation models [61–63] for metabolic disorders screening and diagnosis. The models possess high discriminatory power. In addition, the system has been developed based upon middleware, SOA technologies, i.e., Web Services .NET [64–66]. It can integrate diverse platforms, database as well as further merging, extending into NSHIS System to ensure the accuracy and the reliability of the screening processes. Acknowledgment The authors would like to acknowledge members of the Pediatrics and Medical Genetics Office, the Information Systems Office at NTUH for their kindly assistance.
References
1. Therrell, B.L., US newborn screening policy dilemmas for the twenty-first century. Mol. Genet. Metab. 74, 64–74 (2001). doi:10.1006/mgme.2001.3238
2. Ohkubo, S., Shimozawa, K., Matsumoto, M., Kitagawa, T., Analysis of blood spot 17α-hydroxyprogesterone concentration in premature infants -proposal for cut-off limits in screening for congenital adrenal hyperplasia-. Acta. Paediatr. Jpn. 34, 126–133 (1992)
3. John, A., Age-dependent cutoff values in screening newborns for Hypothyroidism. Clin. Biochem. 37(9), 791–797 (2004) 4. Lindner, M., et al., Neonatal screen for glutaric aciduria type I:
Strategies to proceed. J. Inherit. Metab. Dis. 29, 378–382 (2006). doi:10.1007/s10545-006-0284-1
5. Weng, Y.–C., Hsieh, S. –L., Hsieh, S. –H., Lai, F., Design and Enhance a Dynamic Healthcare Portal Site. Web Intelligence and Intelligent Agent Technology Workshops, 2007 IEEE/WIC/ACM International Conferences, pp. 173–176, 2007.
6. Stoddard, J.J., Farrell, P.M., State-to-state variations in newborn screening policies. Arch. Pediatr. Adolesc. Med. 151, 561–564 (1997) 7. Expanded Newborn Screening using Tandem Mass Spectrometry Financial, Ethical, Legal and Social Issues (FELSI),http://www. newbornscreening.info.
8.http://www.susps.org/overview/birthrates.html,“U.S. Birth Rates and Population Growth.
9.http://content.healthaffairs.org/cgi/content/abstract/26/2/559? ck=nckas of 08/09/2008.
10.http://content.healthaffairs.org, Newborn Screening: Current Sta-tus, Pamela H. Arn.
11.http://www.nlm.nih.gov/, Medical Encyclopedia: Newborn screen-ing tests.
12.http://www.drgreene.org, Newborn Screening Tests.
13. Georgia Newborn Screening Manual for Metabolic Diseases and Hemoglobinopathies. Georgia Department of Human Resources: Division of Public Health, 2007.
14.http://www.slh.wisc.edu/wps/scm/connect/extranet/newborn, Wis-consin Newborn Screening Laboratory.
15. Wisconsin Newborn Screening Laboratory Newsletter, No. 63, “2006 Testing Summary”, July 2007.
16.http://www.foxnews.com,“States Doubled Number of Newborns Tested for Genetic Diseases” as of 07/11/2006
17.http://www.healthsystem.virgina.edu,“Newborn Screening Tests”, University of Virginia Health System, as of 10/23/2007
18.http://www.kidshealth.org/parent/system/medical/newborn_scree ning_tests.htmlas of 27/08/2008
19.http://www.marchofdimes.com/printable Articles/298_834.asp as of 10/23/2007, Testing the newborn for Metabolic Birth Defects, March of Dimes
20. Hinman, A.R., Eichwald, J., Linzer, D., Saarlas, K. N., Integrating child health information systems. Am. J. Public Health 95(11), 1923–1927 (2006). doi:10.2105/AJPH.2004.051466
21.http://las.perkinelmer.com, Wallac LifeCycle for Neonatal Screen-ing–Design Principles–USA
22. Therrell, B.L., Hannon, W.H., National evaluation of US newborn screening system components. Ment. Retard. Dev. Disabil. Res. Rev. 12, 236–245 (2006). doi:10.1002/mrdd.20124
23. National Newborn Screening Information System, NNSIS,http:// www2.uthscsa.edu/nnsis
24. NNSGRC, http://genes-r-us.uthscsa.edu/resources/newborn/00/ 2000report.pdf a) National Newborn Screening and Genetics Resources Center
25.http://www.chw.edu.au/prof/services/newborn
26. Padilla, C.D., Therrell, B.L., Newborn screening in the Asia Pacific region. J. Inherit. Metab. Dis. 30, 490–506 (2007). doi:10.1007/s10545-007-0687-7
27.http://www.chw.edu.au/prof/services/newborn
28.http://www.health.vic.gov.au/genetics/strategy.htmGenetics Serv-ices Strategy for Victoria 2005–2009
29. Bodamer, O.A., Hoffmann, G.F., Lindner, M., Expanded newborn screening in Europe 2007. J. Inherit. Metab. Dis. 30, 439–444 (2007). doi:10.1007/s10545-007-0666-z
30.http://www.atlab.com/Neomate.phpas of 08/30/2008. 31.http://www.perkinelmer.com/as of 08/28/2008.
32. Liu, N., Marenco, L., Miller, P.L., ResourceLog: An Embeddable Tool for Dynamically Monitoring the Usage of Web-Based Bioscience Resources. J. Am. Med. Inform. Assoc. 2006 13(4), 432–437 (2006). doi:10.1197/jamia.M2013
33. Sean, A., McGhee, E. Stiehm, R., Cowan, M., Krogstad, P. McCabe, E. R. B., “Two-tiered universal newborn screening strategy for severe combined immunodeficiency”, Nov. 2, 2005;
http: www.sciencedirect.com
34. Webster, D., Quality performance of newborn screening systems: Strategies for improvement. J. Inherit. Meta. Dis. 30, 576–584 (2007) 35. Olgemoller, B., et al., Screening for congenital adrenal hyperpla-sia: adjustment of 17-Hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J. Clin. Endocrinol. Metab. 88(12), 5790–5794 (2003). doi:10.1210/ jc.2002-021732
36. Microsoft Developer Network (MSDN), http://msdn.microsoft. com/en-us/library/aa480482.aspxas of 02/09/2008.
37. Ko, L. F., Lin, J. C., et al., “HL7 middleware framework for healthcare information system”, IEEE Healthcare, pp 152–156, 2006. 38. Hsieh, S. L., Feipei Lai, S. H., Hsieh et al., “An Integrated Healthcare Enterprise Information Portal and Healthcare Informa-tion System Framework”. IEEE EMBC, pp 4731–4734, 2006. 39. Hsieh, S. H., Hsieh, S. L., Weng, Y. C., et al.,“Middleware based
Inpatient Healthcare Information System”, Bioinformatics and Bioengineering, BIBE 2007, Proceedings of the 7th IEEE International Conference, pp. 1230–1234, 2007.
40. Yang, T. H., Cheng, P. H., et al., “A Scalable Multi-tier Architecture for the National Taiwan University Hospital Infor-mation System based on HL7 Standard”, CBMS August 2006, Proceedings of the 19th IEEE Symposium on Computer-Based Medical Systems, pp. 99–104, 2006.
41. Pierce, M., Fox, G., Youn, C., Mock, S., Mueller, K., Balsoy, O., Interoperable Web Services for Computational Portals. Proceed-ings of Supercomputing 2002, Baltimore.
42. Murray, M., “Strategies for the successful implementation of workflow systems within healthcare: a cross case comparison”, System Sciences, 2003. Proceedings of the 36th Annual Hawaii International Conference, pp. 10, 2003.
43. Bunge, R., Chung, S., Endicott-Popovsky, B., McLane, D.,“An operational framework for service oriented architecture network security”, Hawaii International Conference on System Sciences, Proceedings of the 41st Annual, pp. 312–312, 2008.
44. Lewis, G. A., Morris, E., Simanta, S., et al., “Common Misconceptions about Service-Oriented Architecture”, Commercial-off-the-Shelf (COTS)-Based Software Systems, ICCBSS‘07, Sixth International IEEE Conference, pp. 123–130, 2007
45. Millington, D. S., Tandem Mass Spectrometry in Clinical Diagnosis. 46. Pinheiro, M., Oliveira, J. L., Santos, M. A. S., Rocha, H., Cardoso, M. L., Vilarinho, L.,“NeoScreen: A software application for MS/MS newborn screening analysis”, in Biological and Medical Data Analysis (ISBMDA'2004), Lecture Notes in Computer Science - Volume 3337, Barcelona, Spain, 2004. 47. Pinheiro, M., Oliveira, J.L., Santos, M.A.S., Rocha, H., Cardoso,
M.L., Vilarinho, L., A computer-based solution for screening of inherited metabolic diseases. J. Inherit. Metab. Dis 27(1), 4 (2004). abstract
48. Michael, J., Maranda, PhD, Brian Gugerty, DNS, MS, RN.“CISIES: An Informatics Measurement Instrument”.http://cisevaluation.com/
49. Hurley, A.C., Lancaster, D., Hayes, J., Wilson-Chase, C., Bane, A., Griffin, M., Warden, V., Duffy, M.E., Poon, E.G., Gandhi, T.K., The medication administration system—nurses assessment of satisfaction (MAS—NAS) scale. J. Nurs. Scholarsh. 38, 298–300 (2006). doi:10.1111/j.1547-5069.2006.00117.x
50. Staggers, N., Kobus, D., Brown, C., Nurses’ Evaluations of a Novel Design for an Electronic Medication Administration Record. Comput. Inform. Nurs. 25, 67–75 (2007). doi:10.1097/ 01.NCN.0000263981.38801.be
51.www.google.com/healthas of 08/30/2008
52. Knights, M.,“Web 2.0”, Communications Engineer, 5(1), 30–35, 2007. 53. Schroth, C., Janner, T.,“Web 2.0 and SOA: Converging concepts enabling the internet of services”. IT Professional 9(3), 36–41 (2007) 54. Ping, X.-O., Ko, L.-F., Shang, R.-J., Lai, F., et al., “Dynamic messages creation method for HL7 based healthcare information system”, 9th International Conference on e-Health Networking, Application and Services, June 2007, pp. 150–155.
55. Hsieh, S.-H., Hsieh, S.-L., et al.,“Web services based newborn screen-ing system with support vector machines”, ICITA 2008, June, 2008. 56. Tu, C. -M., Chang, H. -Y., Tang, M. -Y., Lai, F., et al.,“The design
and implementation of a next generation information system for newborn Screening”, HEALTHCOM 2007, June, 2007.
57. Tu, C.-M., The new generation of information system for newborn screening—A case study of National Taiwan University Hospital. Dept. of Computer Science and Information Engineering, National Taiwan University, Taiwan, Master Thesis, June (2007)
58. Chace, D.H., Kalas, T.A., Naylor, E.W., Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin. Chem. 49, 1797–1817 (2003). doi:10.1373/ clinchem.2003.022178
59. Expanded Newborn Screening using Tandem Mass Spectrometry Financial, Ethical, Legal and Social Issues (FELSI),http://www. newbornscreening.info.
60. Michie, D., Spiegelhalter, D. J., Taylor, C. C., Campbell, J., “Machine learning, neural and statistical classification”, 1995. 61. Cortes, C., Vapnik, V., Support-vector network, 1995.
62. Ward, J.J., McGuffin, L.J., Buxton, B.F., Jones, D.T., Secondary structure prediction with support vector machine. Bioinformatics 19, 1650–1655 (2003). doi:10.1093/bioinformatics/btg223
63. Chen, P. H., Fan, R. E., Lin, C. J., “A Study on SMO-type Decomposition Methods for Support Vector Machines,” January 2005.
64. Papazoglou, M.P., van den Heuvel, W.-J., Service-oriented architectures: approaches, technologies and research issues. VLDB J 16(3), 389–415 (2007). doi:10.1007/s00778-007-0044-3
65. Mike, P., Papazoglou,“Service -Oriented Computing: Concepts, Characteristics and Directions”, Proceedings of the Fourth International Conference on Web Information Systems Engineer-ing, p. 3, December 10–12, 2003.
66. Krafzig, D., Banke, K., Slama, D., Enterprise SOA: Service Oriented Architecture Best Practices. Prentice-Hall, Englewood Cliffs (2005)